Cardiolipin activates antigen-presenting cells via TLR2-PI3K-PKN1-AKT/p38-NF-kB signaling to prime antigen-specific naïve T cells in mice
Abstract
Mitochondrial defects and antimitochondrial cardiolipin (CL) antibodies are frequently detected in autoimmune disease patients. CL from dysregulated mitochondria activates various pattern recognition receptors, such as NLRP3. However, the mechanism by which mitochondrial CL activates APCs as a damage-associated molecular pattern to prime antigen-specific naïve T cells, which is crucial for T-cell-dependent anticardiolipin IgG antibody production in autoimmune diseases is unelucidated. Here, we show that CL increases the expression of costimulatory molecules in CD11c+ APCs both in vitro and in vivo. CL activates CD11c+ APCs via TLR2-PI3K-PKN1-AKT/p38MAPK-NF-κB signaling. CD11c+ APCs that have been activated by CL are sufficient to prime H-Y peptide-specific naïve CD4+ T cells and OVA-specific naïve CD8+ T cells. TLR2 is necessary for anti-CL IgG antibody responses in vivo. Intraperitoneal injection of CL does not activate CD11c+ APCs in CD14 KO mice to the same extent as in wild-type mice. CL binds to CD14 (Kd = 7 × 10−7 M). CD14, but not MD2, plays a role in NF-kB activation by CL, suggesting that CD14+ macrophages contribute to recognizing CL. In summary, CL activates signaling pathways in CD11c+ APCs through a mechanism similar to gram (+) bacteria and plays a crucial role in priming antigen-specific naïve T cells.
Introduction
Antiphospholipid (PL) syndrome is a systemic autoimmune disease (AD) marked by a clinical complex of coagulopathy and tissue damage in patients with anti-PL antibodies in their blood 1. High titers of anti-cardiolipin (CL) IgG antibodies have been detected in the sera of patients with anti-PL syndrome, and anti-CL antibodies contribute to the pathogenesis of anti-PL syndrome 2. Activation of both the innate immune system and auto-reactive T cells is responsible for tissue destruction in these patients.
In some patients with AD, dysregulated mitochondrial function is crucial for auto-reactive inflammatory responses 3. Damage-associated molecular patterns (DAMPs) from mitochondria induce inflammation via cytosolic pattern recognition receptors (PRRs, such as NLRP3 and TLR9) or cell surface PRRs (such as P2 × 7R and N-formyl peptide receptor) 4, 5. Mitochondrial oxidative stress, mitochondrial DNA (mtDNA), ATP, and CL initiate inflammatory responses by activating cytosolic NLRP3 inflammasomes or TLR9, along with the subsequent activation of caspase-1 and proinflammatory cytokine cascades 3, 6, 7. In addition, many mtDAMPs are also released from necrotic cells and activate immune cells in tissue microenvironments via PRRs on the surface of innate immune cells 5, 8. For example, N-formyl peptide (NFP) receptors are responsible for chemotaxis and activation of neutrophils in response to extracellular NFP released from mitochondria 5. Indeed, mitochondria have been implicated in systemic inflammatory response syndrome 9, a condition with trauma that mimics sepsis but occurs in the absence of any detectable infectious agent 10. It appears that mitochondrial debris, released from traumatized tissues, are immunostimulatory in this condition 7. In patients with femur fractures, the plasma levels of mtDNA were much higher than in healthy volunteers and were crucial for the proinflammatory pathogenesis 11. Oxidized mtDNAs have also been detected in the synovial fluid of rheumatoid arthritis patients and were immunostimulatory 7, 9, 12.
Among the mitochondrial components, CL is a highly conserved phospholipid present in most bacteria, fungi, and parasites 13. CL thus qualifies as a pathogen-associated molecular pattern (PAMP) according to the model proposed by Janeway 14. In addition, as mitochondria are intracellular organelles that are not normally found outside uninjured cells, mitochondrial molecules may also qualify as endogenous DAMPs 15. In mammals, CL only exists in the mitochondria and constitutes approximately 20% of the total lipid in mitochondrial membranes 16. Because CL is a major cell wall component of Gram-positive bacteria 17 and, evolutionarily, mitochondria are symbiotic alpha-proteobacteria 18, CL might activate PRR-mediated signaling pathways in APCs to prime naïve T cells and induce anti-CL antibody responses. Studies elucidating mechanisms by which CL activates APCs and subsequently antigen-specific naive T cells are crucial to obtain an understanding of the pathogenesis of ADs 9.
APCs play a central role in recognizing both pathogens and tissue injury through PAMPs 14 and DAMPs 15. APCs such as CD11c+ cells that are normally quiescent must become activated to present the appropriate activation signals to naïve T cells 15, 19. Although several studies have delineated the roles of CL in activating the innate immune system, no reports have described the essential signaling pathways in APCs that are activated by CL to prime antigen-specific naïve T cells. In this study, CL activated TLR2-PI3K-PKN1-AKT/p38 MAPK-NF-kB signaling in CD11c+ APCs to activate antigen-specific naïve T cell and anti-CL IgG responses.
Results
CL increases the expression of costimulatory molecules and the number of CD11c+ APCs
CL increased the surface expression of CD80, CD86, I-Ab, and CD40 on CD11c+ APCs derived from the BM of C57BL/6 (B6) mice in vitro (Fig. 1A and B, and Supporting Information Fig. 1). The percentage of CD11c+ APCs expressing costimulatory molecules increased in a manner dependent on the CL concentration (Fig. 1A, R2 = 0.99). In a replicate analysis, 50 μg/mL CL significantly increased the expression of costimulatory molecules and I-Ab on CD11c+ APCs (Fig. 1B). The percentage of BM-derived CD11c+ APCs from TLR2 KO mice expressing costimulatory molecules was significantly lower than cells from WT B6 mice (Fig. 1B, Supporting Information Fig. 1). However, higher CD40 expression was observed in TLR2 KO CD11c+ APCs than in WT CD11c+ APCs.

When we injected 50 μg of CL into the peritoneal cavity of each WT B6 mouse in vivo, the total number of peritoneal cells did not change (Fig. 2A). CL increased the number of CD11c+ cells in the peritoneal cavity (Fig. 2B). Interestingly, a significantly lower percentage of CD11c+ I-Ab +APCs was observed in WT B6 mice injected with LPS than in the CL-injected group (Supporting Information Fig. 2). A significantly higher percentage of activated CD11c+ APCs (I-Ab +, CD80+, CD86+, and CD40+) was also observed in the peritoneal cavity of mice in the CL group than in the PBS group (Fig. 2C).
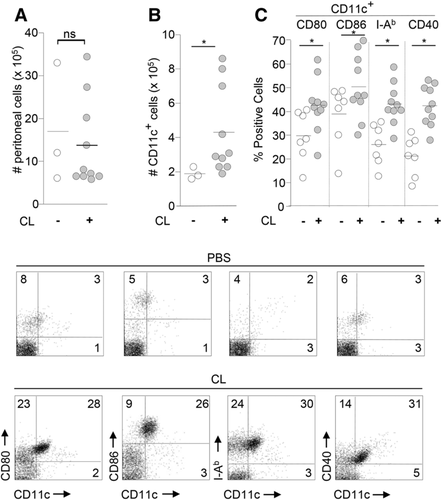
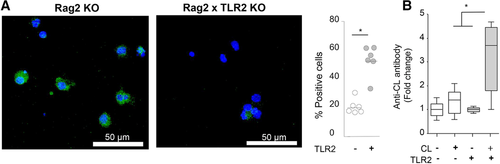
CL binds to TLR2 on CD11c+ APCs and induces anti-CL antibody production in the presence of TLR2
We investigated whether CL binds to TLR2 because various lipids activate the TLR2 pathway 20. CL bound to WT CD11c+ APCs, but not TLR2 KO CD11c+ APCs (Fig. 3A). The titers of anti-CL IgG antibodies were increased in WT mice in vivo upon immunization with 100 μg/mL CL, but not in TLR2 KO mice (Fig. 3B), suggesting that TLR2 is required for anti-CL IgG antibody responses in vivo.
Signaling pathways in BM-derived CD11c+ APCs that are activated by CL
We analyzed signal transduction pathways and the expression of transcription factors in CD11c+ APCs activated by CL. SB203580 (an inhibitor of p38 MAPK), LY294002 (an inhibitor of phosphatidylinositol 3-kinase (PI3K), casein kinase-2, proto-oncogene serine/threonine-protein kinase, and bromodomain and extra-terminal motif proteins (BET)) and SN50 (an inhibitor of nuclear translocation of NF-κB, STAT, AP-1, and NFAT4) significantly inhibited CL-mediated upregulation of costimulatory molecule expression in BM-derived CD11c+ APCs (Fig. 4A).
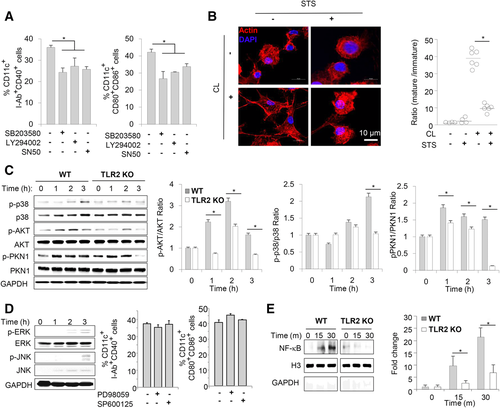
As the migration of CD11c+ APCs to regional lymph nodes is required for priming antigen-specific naïve T cells, we tested whether CL might induce cytoskeletal rearrangements in CD11c+ APCs. Indeed, CL induced cytoskeletal rearrangements (Fig. 4B). Staurosporin (STS), an inhibitor of various protein kinases, including PKC, PKA, and PTK of p60 v-src, inhibited the CL-induced cytoskeletal rearrangements in CD11c+ APCs (Fig. 4B). The ratio of dendrite+ mature CD11c+ APCs to dendrite− immature CD11c+ APCs was significantly higher in the CL group than in other groups (Fig. 4B, right panel).
CL induced the phosphorylation of p38 MAPK, AKT, and PKN1 in a time-dependent manner (Fig. 4C). Phosphorylation of these molecules was significantly reduced in TLR2 KO CD11c+ APCs compared to WT CD11c+ APCs (Fig. 4C). Although CL also increased the phosphorylation of ERK and JNK (Fig. 4D, left panel), treatment with a MEK1/2 inhibitor (PD98059, upstream of ERK) or a JNK inhibitor (SP600125) did not decrease the expression of the costimulatory molecules that was upregulated by CL (Fig. 4D, right panel).
Nuclear extracts from CL-stimulated CD11c+ APCs showed greater amounts of NF-κB in the nuclear fraction (Fig. 4E), indicating that CL induced the nuclear translocation of NF-κB. In the absence of TLR2, CL-induced NF-κB translocation to the nucleus of CD11c+ APCs was markedly attenuated (Fig. 4E).
CL functions as an adjuvant to prime antigen-specific T cells
In both Marilyn mice (Fig. 5A) and OT-1 mice (Fig. 5B), the H-Y peptide or OVA alone increased the number of effector/memory T cells in draining LN. However, mice injected with CL+H-Y peptide or CL+OVA displayed significantly increased CD44 and CD69 expression and decreased CD62L expression on T cells compared to the control groups (Fig. 5 and Supporting Information Fig. 3).
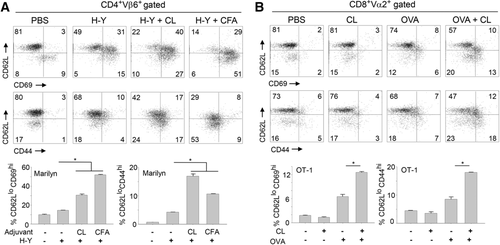
TLR2 is essential for the adjuvant effect of CL on activating OVA-specific CD8+ T cells
WT B6 mice or TLR2 KO mice were immunized with OVA (or PBS) and/or CL. IFN-γ production and CD8+ T-cell proliferation were analyzed by FACS after lymphocytes were stimulated with OVA (or SIINFEKL for proliferation) in vitro (Fig. 6). CD8+ T cells from WT B6 mice immunized with CL+ OVA produced more IFN-γ than CD8+ T cells from WT B6 mice immunized with CL or OVA alone (upper panel of Fig. 6A and Supporting Information Fig. 4). When TLR2 KO B6 mice were immunized with CL + OVA, CD8+ T cells did not produce as much IFN-γ upon in vitro stimulation with OVA as CD8+ T cells from WT B6 mice immunized with CL + OVA (lower and right panels of Fig. 6A).
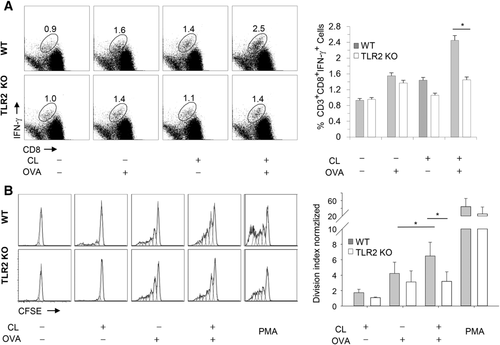
Likewise, CD8+ T cells from WT B6 mice immunized with CL + OVA exhibited greater proliferation than CD8+ T cells from WT B6 mice immunized with OVA alone upon in vitro stimulation (upper panel of Fig. 6B and Supporting Information Fig. 5). When TLR2 KO B6 mice were immunized with CL+ OVA, CD8+ T cells did not proliferate upon in vitro stimulation with SIINFEKL to the same extent as CD8+ T cells from WT B6 mice immunized with CL+ OVA (lower and right panel of Fig. 6B).
The CL-CD14 interaction plays a role in the activation of NF-kB and CD11c+ APCs
Based on the results of the Biacore analysis, CL bound to CD14 with an average Kd of 6.5 × 10−7 (Fig. 7A). In vivo, peritoneal CD11c+ APCs were activated by CL in WT mice to a greater extent than APCs from CD14 KO mice in vivo (Fig. 7B), suggesting that the activation of CD14+ peritoneal macrophage by CL enhances the activation of peritoneal CD11c+ APCs by CL. NF-kB reporter Chinese hamster ovary (CHO) cells showed that CD14, but not MD2, was also essential for NF-kB activation by CL (Fig. 7C and Supporting Information Fig. 6).

Cytotoxicity of CL
CL (50 μg/mL) did not exert cytotoxic effects more than medium control in vitro and in vivo (Supporting Information Fig. 7), suggesting that the activation of CD11c+ APCs by CL was not due to the release of DAMPs from necrotic cells.
Discussion
Immature CD11c+ cells and monocytes are recruited to injured tissues 21, 22. When APCs in local tissues are activated by PAMPs or DAMPs, CD11c+ APCs mature and migrate to draining LNs to prime naïve T cells 23. In addition, mature CD11c+ APCs express costimulatory molecules and cytokines that are different from immature CD11c+ APCs 23, 24. In this study, the CL injection increased the number of peritoneal CD11c+ cells. These cells might be monocytes that are recruited in response to CL and differentiated into functional CD11c+ APCs 21.
Inefficient clearance or increased apoptosis in tissues caused by genetic defects enhances the probability that patients will develop autoimmunity 25, 26. In these patients, apoptotic cells proceed to secondary necrosis and might release mtDAMPs that could activate APCs presenting self-peptides on MHC molecules 27. The necrotic tissues could potentially release up to 6 mg/mL CL into the microenvironment; thus, the amount of CL necessary to activate APCs is approximately 1–2 % of CL that is potentially released in a damaged tissue (see the Methods section for the calculation).
Lipids generate diverse immune responses, depending on the length of the acyl chains, the number of acyl chains, the type of polar head groups, and the number of unsaturated carbon bonds 28. The TLR2 and TLR4 pathways are activated by saturated fatty acids and inhibited by unsaturated fatty acids 29, 30. In addition, the bioactivity of LPS depends on the shape of the lipid A component 31. Conical lipid A is more active than cylindrical lipid A 31. Considering that various types of CL constitute mitochondrial membranes 32, certain types of CL might be inhibitory, just as certain types of LPS inhibit immune responses 31. In addition, the size, surface charge, and membrane fluidity of liposomes determine the ability of lipids to function as adjuvants in initiating immune responses 33, 34. According to Balasubramanian et al., CL dampens cytokine production in macrophages 35. These results might be due to differences in the CL used to produce liposomes and the type of CL liposomes generated. Balasubramanian et al. used large unilamellar vesicles (LUVs) composed of tetralineoyl-cardiolipin mixed with dioleoylphosphatidylcholine (DOPC) 35. The multilamellar dispersion composed of CL+ DOPC was extruded through polycarbonate membranes to generate LUVs in that study. These LUVs are comparable to small unilamellar vesicles comprising CL, which were sonicated without another lipid (DOPC) in the present study.
We are the first to reveal that mitochondrial CL binds to TLR2. In addition, TLR2 plays roles in IgG antibody production in response to immunization with CL in vivo. Compared with other isotypes (IgM and IgA), the IgG isotype of the anti-CL antibody is the most crucial isotype in the development of coagulopathies in patients with AD 36. Hence, TLR2-dependent anti-CL IgG antibody production might be crucial in the development of coagulopathies, such as in anti-PL syndrome. These results deepen our knowledge of molecular and cellular mechanisms underlying the presence of anti-CL IgG antibodies in many patients with autoimmune disorders who present with dysregulated mitochondrial function 9.
CL activated CD11c+ APCs, possibly via the TLR2-PI3K-PKN1-p38MAPK/AKT-NF-κB signaling pathway. Bacterial peptidoglycan also activates CD11c+ APCs via the TLR2-PI3K-AKT-AP-1 signaling pathway and enhances IL-6 production 37. MD2 is necessary for activating the TLR4 pathway in response to LPS stimulation 28 and for activating the TLR2 pathway following peptidoglycan or lipoteichoic acid stimulation 38. In this study, MD2 was not required for the CL-induced activation of NF-kB. In addition, in our previous report, necrotic cells activated NF-kB in the absence of MD2 39. Thus, bacterial contaminants in CL preparations were not likely to be responsible for the activation of the TLR2 pathway in the present study.
Although the inhibitors used in this study interact with various targets, several reports, and our data support the hypothesis that CL activates the TLR2-PI3K-AKT/PKN1 axis (Fig. 8). (1) PI3K and AKT are central regulators of activated CD11c+ cells 40, (2) activation of TLR2 transduces signals via the MAL-PI3K-AKT-NF-kB signaling pathway 41, and (3) the interaction of PI3K with MAL induces PI3K-dependent phosphorylation of AKT 41. However, we must determine the roles of other adapter molecules, such as BCAP in B cells, in future studies 42.
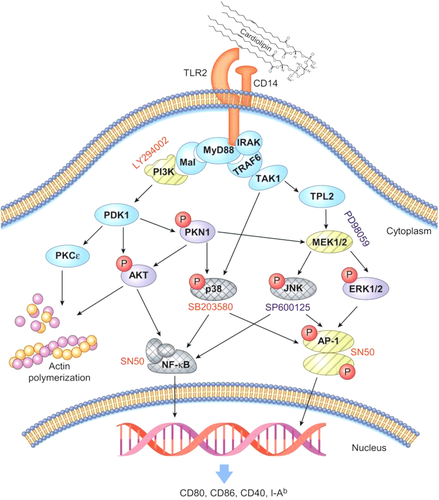
LY294002 inhibited the CL-induced increase in the expression of costimulatory molecules in CD11c+ APCs (Fig. 4). LY294002 inhibits various protein kinases (PKs), such as PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PIK3CG), casein kinase-2 (Csnk2), proto-oncogene serine/threonine-protein kinase (PIM1), and BET 43. PI3K phosphorylates p38 MAPK in response to stimulation with various TLR ligands 42, 44. CL bound to TLR2 and phosphorylated p38 in CD11c+ APCs, and SB203580 (a p38 inhibitor) inhibited the CL-induced increase in the expression of costimulatory molecules. Based on these findings, CL likely activates the TLR2-PI3K-p38 MAPK axis in CD11c+ APCs instead of signaling through PIM1, PIK3CG, CK2, or BET (Fig. 8). CL-induced activation of PIM1 should be examined in future studies, since PIM1 transmits signals via the CD180/MD1 complex, an unusual member of the TLR family 45. This finding might explain why TLR2 KO CD11c+ APCs upregulate CD40 expression to the same levels as WT CD11c+ cells in response to CL (Fig. 1B).
CL might phosphorylate PKN1 and AKT by activating PI3K 44, 46. Because PI3K also negatively regulates proinflammatory cytokine production in TLR-stimulated myeloid cells, additional studies using specific inhibitors are required 42. Furthermore, the role of PI3K in TLR signaling must be distinguished from its role in endocytosis because PI3K activation is crucial for the endocytosis of TLR2-ligand complexes 47. Eight PI3K isozymes induce the activation of PI3K-driven signaling pathways 48. A better understanding of the mechanisms by which CL-TLR2 interactions regulate different PI3K isozymes might assist in the elucidation of precise pathways downstream of TLR2.
CL increases the number of peritoneal CD11c+ APCs in vivo and induces actin polymerization, which is necessary for the migration of CD11c+ APCs to regional lymph nodes for priming naïve T cells (Figs. 2 and 4). Although STS inhibits a broad spectrum of protein kinases 49, several findings support the hypothesis that the TLR2-PI3K-PKCε/AKT pathway might play a crucial role in CL-mediated cytoskeletal rearrangements 50. PKCɛ is closely related to integrin signaling for cell migration 51. PI3K activates PKCε 52 and MARCKS, which plays a crucial role in cytoskeletal rearrangements and binds to actin polymers in a PKCε-dependent manner 53. In addition, CL-induced cytoskeletal rearrangements might play a role in the phagocytosis of cell debris by CD11c+ APCs 54.
PAMPs increase the expression of costimulatory molecules in CD11c+ APCs with the help of the transcription factors NF-kB, CREB, and AP-1 through MEK, p38, JNK, and ERK 55. NF-κB is an essential transcription factor in proinflammatory responses and is controlled by PI3K/AKT 56; moreover, SN50 inhibits the nuclear translocation of various transcription factors, including NF-κB, STAT, AP-1, and NFAT4. Based on the SN50-mediated inhibition experiments (Fig. 4) and TLR2-dependent nuclear translocation of NF-kB induced by CL (Fig. 4E), NF-kB activation might be required for the expression of costimulatory molecules in CD11c+ APCs in response to CL. AP-1 might not play a major role in this process, since neither a MEK inhibitor nor a JNK inhibitor inhibited the CL-induced upregulation of costimulatory molecule expression, although CL increased the levels of phosphorylated ERK and JNK (Fig. 4D).
In the draining lymph nodes, activated CD11c+ APCs prime naïve T cells, and the naïve T cells become effector/memory T cells 57. However, the expression levels of activation markers, such as I-Ab, CD80, CD86, and CD40, in CD11c+ APCs are not always correlated with the ability to prime antigen-specific naïve T cells 57. Sometimes, mature CD11c+ APCs induce tolerance 58. CD11c+ APCs activated by CL were sufficient to activate antigen-specific naïve CD4+ T cells and CD8+ T cells in vivo when tested using H-Y peptide-specific TCR transgenic Marilyn mice 59 and OVA-specific TCR transgenic OT-1 mice 60, respectively (Fig. 5).
In conclusion, our findings suggesting that CL activates CD11c+ APCs via TLR2-PI3K-PKN1-AKT/p38-NF-kB signaling pathways through a similar mechanism as gram (+) bacteria have theoretical implications for bridging the two main current models of innate immunity, DAMPs versus PAMPs. Exogenous PAMPs and endogenous DAMPs might be recognized by the innate immune system in a similar way without evolutionary discrimination 15 because both are signals that warn the innate immune systems that tissue homeostasis has been disrupted, for example, by an infection or sterile necrotic cell death. This observation might explain why DAMPs and PAMPs transmit alarm signals in similar ways, although further comparisons of the signaling pathways involved in these processes are required.
Materials and methods
This section was written in compliance with the Minimal Information about T Cell Assays (MIATA) guidelines (http://miataproject.org).
Reagents
CL was purchased from Avanti Polar Lipids (Cat #830012P, Alabaster, AL) and small, unilamellar vesicles (SUVs) were generated according to the manufacturer's protocols. Briefly, CL reconstituted in 100% ethanol was dried under a vacuum. Completely dry CL was then reconstituted in 1 mL PBS (10 mg/mL), and large multilamellar vesicles (LMVs) were formed by vigorous vortexing. LMV suspensions in PBS were sonicated in a bath-type sonicator (Branson, CT) for 15 min twice to generate SUVs. SUVs composed of CL in PBS were stored in a glass vial, which was rinsed with endotoxin removal solution (United States Biological, MA). Endotoxin levels in SUVs composed of CL were tested using the limulus assay (Thermo Fisher Scientific, MA) and were used in subsequent assays when endotoxin levels were < 0.2 EU/mL. 10-N-nonyl acridine orange (NAO) from Invitrogen (Carlsbad, CA) was solubilized in DMSO (50 mM). Cells were blocked with anti-mouse CD16/CD32 (Mouse BD Fc Block, clone 2.4G2, BD Pharmingen) for 30 min on ice before staining with antibodies for the FACS analysis. All antibodies were purchased from BD Pharmingen (San Diego, CA), unless stated otherwise. 7-Amino-actinomycin D (7AAD) and Annexin V-FITC (Sigma and Molecular Probes, Inc., Eugene, OR) were used to gate live cells for FACS. Antibodies used for immunoblotting were obtained from Cell Signaling (Danvers, MA), with the exception of an anti-GAPDH antibody purchased from Abfrontier (Seoul, Korea).
Mice
Rag2 KO mice, TLR2 KO mice and T-cell receptor (TCR, Vβ6) transgenic Rag2 KO Marilyn mice harboring monoclonal CD4+ T cells specific for the male antigen H-Y peptide presented by I-Ab were obtained from Taconic Farms, Inc. (Hudson, NY). Wild-type B6 mice and TCR (Vα2) transgenic OT-1 mice harboring monoclonal CD8+ T-cell populations specific for the OVA antigen presented by H-2Kb were procured from the Jackson Laboratory (Bar Harbor, ME). Rag2 × TLR2 KO mice were obtained by cross-breeding Rag2 KO mice and TLR2 KO mice. Mice were housed under SPF conditions, and the genotypes of mice were determined according to the manufacturer's protocols. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (SNU-1102-08-01).
CD11c+ antigen-presenting cells
CD11c+ APCs were generated from bone marrow that was flushed from the femurs and tibias of 6-to-8-week-old Rag2 KO mice (WT) or Rag2 × TLR2 double KO (TLR2 KO) mice, washed, and cultured in IMDM supplemented with 10% heat-inactivated FCS, 50 nM 2-mercaptoethanol (Invitrogen), 2 mM L-glutamine, antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin and 50 μg/mL gentamycin, WelGENE Inc., Korea), recombinant mouse GM-CSF (1.5–3 ng/mL, PeproTech, Rocky Hill, NJ) and mouse IL-4 (1.5 ng/mL, PeproTech, Rocky Hill, NJ). Half of the medium was replaced daily. For in vitro activation of CD11c+ APCs, various concentrations of CL were mixed together with CD11c+ APCs on day 6, and surface markers on CD11c+ APCs were analyzed on day 7 using FACS. The following antibodies were used for FACS: anti-mouse CD11c-APC (clone HL3), anti-mouse CD40-FITC (clone HM40-3), anti-mouse CD80-FITC (clone 16-10A1), anti-mouse CD86-PE (clone GL1, BioLegend), anti-mouse I-Ab-PE (clone AF6-120.1), and a rat IgG isotype control. The percentage of positive cells was determined by FACS (FACS Canto II, BD Biosciences, CA) after gating using the strategy shown in Supporting Information Fig. 1.
Activation of CD11c+ APCs by CL in vivo
For activation of CD11c+ APCs in vivo, 200 μL of CL (3–200 μg/mouse) or PBS as a negative control were intraperitoneally (i.p.) injected into B6 mice. Peritoneal cells were harvested after 18 h, and surface markers were analyzed by FACS after gating using the strategy shown in Supporting Information Fig. 2. The number of CD11c+ cells was calculated from the percentage of intraperitoneal CD11c+ cells and total number of peritoneal cells.
The binding of CL with TLR2
NAO (100 μM) and CL (33.5 μM) were mixed in PBS and conjugated at room temperature (RT) for 15 min, as previously described 61. CD11c+ APCs were incubated with the CL-NAO conjugate for 30 min at 4°C and washed with PBS six times. Cells were fixed with 4% paraformaldehyde for 20 minutes at RT. After washing with PBS, cells were adhered to microscope slides using cytospin (Shandon cytospin3, Cheshire, UK). The nucleus was stained by mounting the slides with FluoroshieldTM containing 4,6-diamidino-2-phenylindole (DAPI, ImmunoBioScience Corp., Mukilteo, WA) for 5 min at RT. Images were obtained using an Olympus confocal imaging system (FV1000, New Hyde Park, NY). The fluorescence intensity was digitally analyzed using ImageJ software (Center for Information Technology, National Institutes of Health, Bethesda, MD). We counted green fluorescence-positive cells in two randomly selected fields (40 × magnification) and analyzed slides from three biological replicates.
Anti-CL antibody responses
B6 mice or TLR2 KO B6 mice were immunized with CL or PBS (n = 12 each). Mice were i.p. injected with 150 μL of CL (50 μg) or PBS three times at weekly intervals. Sera were obtained 7 days after the last immunization, diluted 1:20, and subjected to an ELISA using an anti-CL ELISA Kit (Alpha Diagnostic International, San Antonio, TX). Fold changes were calculated by dividing the optical density (O.D.) from test groups by the mean O.D. from a PBS-immunized control group.
Inhibition of signaling pathways in CD11c+ APCs activated by CL
CD11c+ APCs from Rag2 KO or Rag2 × TLR2 KO mice were incubated with SB203580 (10 μM), LY294002 (5 μM), SN50 (20 μM), STS (40 nM), PD98059 (10 μM, Promega, Madison, WI) or SP600125 (10 nM, Sigma-Aldrich) for 30 minutes and then stimulated with PBS or CL for another 18 h. SB203580, LY294002, SN50, and staurosporin were purchased from Calbiochem (San Diego, CA).
Cytoskeletal rearrangement
CD11c+ APCs from Rag2 KO or Rag2 × TLR2 KO mice were treated with PBS or STS for 30 minutes and stimulated with CL (50 μg/mL) for another 18 h before staining. Cells were adhered to slides using cytospin (Shandon cytospin3, Cheshire, UK) and then fixed with 4% paraformaldehyde for 20 minutes at RT, followed by permeabilization with 0.25% Triton X-100 for 20 min. Cells were blocked with blocking buffer (5% normal goat serum and 1% BSA in PBS) for 30 min and then stained with Alexa Fluor 568-conjugated phalloidin (Invitrogen) for 30 min. Cellular nuclei were visualized with FluoroshieldTM containing DAPI (ImmunoBioScience Corp., Mukilteo, WA) after a 5 min incubation at RT. Images of the immunofluorescent staining were obtained using an Olympus confocal imaging system (FV1000). The number of CD11c+ APCs displaying cytoskeletal rearrangements (with dendritic, branch-like membrane protrusions characteristic of mature cells) was counted. In addition, the number of CD11c+ APCs lacking cytoskeletal rearrangements (showing microvilli, tiny finger-like plasma protrusions characteristic of immature cells) was also counted. The ratio (number of mature cells/number of immature cells) was determined after 2 independent investigators counted the cells in 2–3 randomly selected fields (40× magnification).
Western blotting
Cells were lysed in a modified RIPA lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS). Protein concentrations in cell lysates were determined using the Bradford assay (Bio-Rad, Hercules, CA). Ten micrograms of proteins from cell lysates were subjected to SDS-PAGE and then transferred to PVDF membranes, followed by blocking in 5% BSA-containing buffer. Primary antibodies, including anti-phospho-p38 (clone D3F9, Cell Signaling Technology), anti-p38 (polyclonal, Cell Signaling Technology), anti-phospho-AKT1 (ser473, clone D9E, Cell Signaling Technology), anti-pan AKT (clone C67E7, Cell Signaling Technology), anti-phospho-PKN1 (Thr774/Thr816, polyclonal, Cell Signaling Technology), anti-PKN1 (clone 49/PRK1, BD Transduction Laboratories), anti-phospho-Erk1/2 (clone D13.14.4E, Cell Signaling Technology), anti-histone H3 (clone D2B12, Cell Signaling Technology), anti-NF-κB p65 (clone L8F6, Cell Signaling Technology), and anti-GAPDH (polyclonal, AbFrontier) antibodies, were used. PVDF membranes were incubated with HRP-conjugated secondary anti-mouse IgG(H+L) (polyclonal, Thermo Scientific) or anti-rabbit IgG (polyclonal, Santa Cruz Biotechnology) after unbound primary antibodies were removed by washing. Images were obtained using a LAS system (GE Healthcare Life Sciences, Uppsala, Sweden) after incubation with an ECL reagent (Promega, Madison, WI).
NF-κB translocation
CD11c+ APCs from Rag2 KO or Rag2 × TLR2 KO mice were stimulated with CL for the indicated times, washed with ice-cold PBS, resuspended in hypotonic buffer A (10 mM HEPES/KOH, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT, and 0.5 mM PMSF, pH 7.9), and incubated for 15 min on ice. Cells were then lysed by adding 10% nonidet P (NP)-40 and centrifuged at 4°C. The supernatant containing the cytoplasmic fraction was collected. The pellet was resuspended in buffer B (50 mM HEPES/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% glycerol, 1 mM DTT, and 0.5 mM PMSF, pH 7.9) and incubated on ice for 20 min. After centrifugation, the supernatant containing the nuclear fraction was collected for a gel shift assay. Histone H3 and GAPDH were used as internal controls to normalize input proteins.
FACS analysis of T cells
Cells from OT-1 mice were stained with anti-CD3ε-APC(clone 145-2C11), anti-Vα2-FITC (clone B20.1) and anti-CD8-APC-Cy7 (clone 53–6.7) antibodies, and cells from Marilyn mice were stained with anti-Vβ6-FITC (clone RR4-7) and anti-CD4-APC antibodies (clone GK1.5). Anti-CD69-PE-Cy7 (clone H1.2F3), anti-CD62L-Percp-Cy5.5 (clone MEL-14, BioLegend, San Diego, CA), anti-CD44-PE (clone IM7) antibodies and an appropriate rat-anti-mouse IgG isotype control were used for staining. CD4+ Vβ6+ cells from Marilyn mice or CD8+ Vα2+ cells from OT-1 mice were gated for the FACS analysis (Supporting Information Fig. 3). The data were directly acquired after finishing the staining using a FACS Canto II (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Effector/memory phenotypes of antigen-specific T cells in response to CL in vivo
For in vivo T-cell activation, female Marilyn mice or OT-1 mice were injected with 200 μL of antigen alone (0.1 μg of H-Y peptide/mouse or 30 μg of OVA/mouse) or mixed with adjuvant (CFA, CL or PBS as a negative control) at the base of the tail. One hundred micrograms or 50 μg of CL were injected into each Marilyn or OT-1 mouse, respectively. Lymphocytes from inguinal and periaortic lymph nodes were isolated from Marilyn mice 2 days after injection or from OT-1 mice 7 days after injection. Increases in the numbers of effector/memory T cells upon immunization were analyzed by FACS after T cells were stained with anti-CD62L, anti-CD69, and anti-CD44 antibodies.
IFN-γ production of antigen-specific T cells in response to CL
WT B6 or TLR2 KO B6 mice were immunized with OVA (5 μg/mouse) ± CL (50 μg/mouse) at the base of the tail (n = 3 each) twice at 10 day intervals to evaluate IFN-γ production in T cells. Lymphocytes were purified from inguinal and periaortic lymph nodes 7 days after the last immunization. Lymph node cells obtained from the mice were counted using trypan blue staining and a microscope. The cell viability was more than 90%. All samples met minimum acceptance criteria of >80% viability. Lymphocytes were cocultured with CD11c+ APCs from WT B6 mice that had been pulsed with OVA for 18 h prior to the experiment. Cells were cultured in IMDM supplemented with 10% heat-inactivated FCS, 50 nM 2-mercaptoethanol (Invitrogen), 2 mM L-glutamine, and antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamycin, WelGENE Inc., Korea). After culturing the mixture of cells for 48 h, lymphocytes were incubated with Golgi plug (BD Pharmingen) for another 5 h and subjected to intracellular IFN-γ staining for the FACS analysis. Golgi-plugged cells were washed with staining buffer (BD Pharmingen), blocked with an anti-mouse CD16/CD32 antibody (Mouse BD Fc Block, clone 2.4G2, BD Pharmingen) and stained with anti-CD3ε-APC (clone 145-1C11, BD Pharmingen) and anti-CD8- APC-Cy7 (clone 53–6.7, BD Pharmingen) antibodies on ice for 30 min. Cells were suspended in Cytoperm/Cytofix solution (BD Pharmingen) after two washes and incubated on ice for 20 min. After blocking again with the anti-mouse CD16/CD32 antibody, cells were stained with an anti-IFN-γ-PE (clone XMG1.2, BD Pharmingen) antibody on ice for 30 min.
Lymphocyte proliferation assay
WT B6 mice or TLR2 KO mice were immunized with OVA (5 μg/mouse) + adjuvant (PBS or CL) at the base of the tail twice at 10 day intervals (n = 3 each). Lymphocytes were purified from inguinal/periaortic lymph nodes and splenocytes 7 days after the last immunization. Lymphocytes were purified by removing red blood cells using a 4-min incubation in RBC lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA, pH 7.2). Murine T cells were subsequently enriched by negative selection using a Pan T Cell Isolation MACS Kit containing biotin-conjugated monoclonal antibodies against CD11b, CD45R, DX5, and Ter-119 (Miltenyi Biotech, CA). The enriched cells (1 × 105) were stained with 1 μM CFSE in PBS containing 0.5% FCS for 15 min at 37°C. CFSE-stained lymphocytes (1 × 105) were coincubated for 18 h with CD11c+ APCs (1 × 104) that were pulsed with 1 μg/mL SIINFEKL peptide (Invivogen, CA). Cells were stained with an anti-CD8- APC-Cy7 antibody (clone 53–6.7, BD Pharmingen), and the division index of CD8+-gated T cells was calculated using FlowJo software (Tree Star Inc., Ashland, OR).
CL binding to CD14
CL binding to CD14 was measured using a Biacore T200 instrument (Uppsala, Sweden). Briefly, recombinant CD14 (Cellscience, 22.5 μg/μL) was immobilized to a CM5 sensor chip via amino coupling with a 7 min contact time at a flow rate of 5 μL/min. Untreated functional groups were blocked by the injection of 1 M ethanolamine-HCl (pH 8.0). Binding of CL (0, 18.8, 37.5, 75, or 150 μM) to the CD14-immobilized sensor chip was assessed at a flow rate of 70 μL/min in 0.5 M NaCl/PBS.
Activation of peritoneal cells from CD14 KO mice by CL
To observe the effects of CD14 in recognizing CL, two hundred microliters of CL (100 μg/mouse) or PBS were i.p. injected into WT B6 mice or CD14 KO mice to observe the ability of CD14 to recognize CL. Peritoneal cells were harvested after 18 h, and expression of CD11c, CD80, CD86, CD40, and I-Ab was analyzed by FACS. The MFI of costimulatory molecules on CD11c+ cells was calculated.
NF-kB reporter assay
The role of CD14 in recognizing CL was further analyzed in vitro using NF-κB reporter CHO cells carrying CD14 and MD2 (mutant or WT). CHO cells also carry a reporter gene for the membrane form of human CD25 under the control of an NF-κB responsive promoter. The NF-κB reporter CHO cells were kindly provided by Dr. Golenbock, DT (UMASS, Worsceter, MA) 62. NF-κB reporter CHO cells were transfected with pCEP4 (vector control) or pCEP4-CD14 (from Dr. Golenbock) to generate stable cell lines. NF-κB reporter CHO cells were maintained in Ham's F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Invitrogen), 400 U/mL of hygromycin (LC Laboratories, Woburn, MA), antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamycin, WelGENE Inc., Daegu, Korea) and 400 μg/mL of G418 (Invitrogen). NF-κB reporter CHO cells were incubated with various doses of CL for 14 hours. NF-κB activation was determined by FACS after cells were stained with an anti-human CD25-PE antibody. 7AAD− cells were gated (Supporting Information Fig. 6) and the percentage of activated cells was determined by normalizing the percentage of CD25+ cells in a test group to the percentage of CD25+ cells in a control group. Nonstimulated cells served as a control group.
Cell viability assay
The viability of CD11c+ APCs that had been treated with either CL or LPS for 18 h in vitro or in vivo was examined by FACS after cells were stained with 7AAD.
Calculations
The amounts of physiological CL that might be released from necrotic cells were estimated as described below. A single cell contains 2.2 ± 0.6 fmol of CL 63 (approximately 3 pg of CL/cell); thus, approximately 3 μg of CL is derived from 106 cells, considering that the molecular weight of tetraoleyl CL is approximately 1500 Da. As the cell diameter is approximately 10 μm, the volume of a cell is 524 μm3, and the volume of one million cells would be approximately one-half of a microliter. Thus, the maximal concentration of CL released into local tissues may be as high as 6 mg/mL. The 100 μg/mL of CL is thus approximately 1/60 of the potential maximum locally released concentration.
Statistics
Student's t-test was used for comparisons between groups. Data are presented as the means ± SEM of experiments performed in triplicate. Mean values are depicted as a single line between multiple circles.
Acknowledgments
J.A.C., T.J.K., H.J.M., Y.J.K., and H.K.Y. conducted the experiments. S.Y.S. conceived the study, analyzed the data, and wrote the manuscript. J.A.C. and T.J.K. contributed to the writing of the manuscript. S.Y.S. was supported by a grant from the Innovative Research Institute for Cell Therapy (A062260), a grant from the Korean Health Technology R&D Project (HI11C-0990-010013), Ministry of Health & Welfare, Republic of Korea, and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2011–0030739).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
Abbreviations
-
- AD
-
- autoimmune diseases
-
- BET
-
- bromodomain and extra-terminal motif protein
-
- CHO
-
- Chinese hamster ovary
-
- CL
-
- cardiolipin
-
- DAMP
-
- damage-associated molecular pattern
-
- DOPC
-
- dioleoylphosphatidylcholine
-
- LUV
-
- large unilamellar vesicle
-
- NFP
-
- N-formyl peptide
-
- O.D.
-
- optical density
-
- PL
-
- phospholipid
-
- PRR
-
- pattern recognition receptor
-
- PAMP
-
- pathogen-associated molecular pattern
-
- PK
-
- protein kinases
-
- PIM1
-
- proto-oncogene serine/threonine-protein kinase
-
- STS
-
- staurosporin
-
- SUV
-
- small unilamellar vesicle