Active dissemination of cellular antigens by DCs facilitates CD8+ T-cell priming in lymph nodes
See accompanying commentary by Mueller. https://doi.org/10.1002/eji.201747248
Abstract
Antigen (Ag) specific activation of naïve T cells by migrating dendritic cells (DCs) is a highly efficient process, although the chances for their colocalization in lymph nodes (LNs) appear low. Ag presentation may be delegated from Ag-donor DCs to the abundant resident DCs, but the routes of Ag transfer and how it facilitates T-cell activation remain unclear. We visualized CD8+ T cell-DC interactions to study the sites, routes, and cells mediating Ag transfer in mice. In vitro, Ag transfer from isolated ovalbumin (OVA)+ bone marrow (BM) DCs triggered widespread arrest, Ca2+ flux, and CD69 upregulation in OT-I T cells contacting recipient DCs. Intravital two-photon imaging revealed that survival of Ag-donor DCs in LNs was required for Ag dissemination among resident CD11c+ DCs. Upon interaction with recipient DCs, CD8+ T cells clustered, upregulated CD69, proliferated and differentiated into effectors. Few DCs sufficed for activation, and for efficient Ag dissemination lymphocyte function associated antigen 1 (LFA-1) expression on recipient DCs was essential. Similar findings characterized DCs infected with a replication-deficient OVA-expressing Vaccinia virus known to downregulate MHC-I. Overall, active Ag dissemination from live incoming DCs helped activate CD8+ T cells by increasing the number of effective presenting cells and salvaged T-cell priming when Ag-donor DCs could not present Ag.
Introduction
To initiate an adaptive immune response, rare antigen (Ag) specific naïve T cells must efficiently locate their cognate Ag-presenting dendritic cells (DCs) in lymphoid organs 1. The frequency of clonal T cells specific to a given MHC-I restricted Ag, within the total repertoire of naïve T cells, is extremely low (∼1/106–107) 2, 3. This may not pose a problem when the lymph node (LN) is awash with soluble Ag presented on resident DC, but when ingested Ag is carried to the LN by small numbers of migratory DCs 4, the prospects of the two cell types finding each other seem low.
Despite the low numerical odds for a T-cell DC encounter, T-cell activation is a very efficient process: it was shown that very few DCs are enough to effectively initiate an immune response 4 and that the entire clonal population of naïve Ag-specific T cells is activated even at low Ag doses 3.
-
We have previously visualized DCs introduced into the skin as they actively crawl through lymphatics 5 join sessile networks of resident DCs in LNs, and survive in the LN for several days 6.
-
It was shown that T cells do not stop and form immunological synapses immediately upon encountering low concentration of peptide-MHC (pMHC) presented in the LN, but continue to migrate for several hours 7. This would allow them to select the DCs that present the highest concentrations of antigenic peptides 8.
-
Finally, it has been shown that Ag originating from incoming DCs can be presented on resident DCs in the LN, presumably because fragments of dying incoming DCs were processed by the resident ones 9. It was later shown that such Ag transfer participates in activation of both CD4 10 and CD8 11-13 T cells. These studies suggested several cellular routes through which Ag might be transferred, but no consensus emerged.
These latter works applied an indirect approach to the question of Ag transfer, relying mostly on T-cell activation as readout. Thus it remained unclear where Ag was transferred, in what form and through what routes, as well as how it benefitted T-cell activation.
Here, we focused on the spatiotemporal aspects of Ag transfer by using in vitro live cell imaging and two-photon microscopy in LNs of living mice to follow the behavior of Ag-donor DCs, Ag-recipient DCs, and Ag-specific T cells. Specifically, we imaged ovalbumin (OVA) specific OT-I CD8 T cells as they physically interacted with OVA-bearing DCs and recipient DCs whose Ag-presentation capacities had been genetically compromised by knocking out the H-2Kb MHC-I gene 14. Limited numbers of donor DCs were diluted in large numbers of recipient DCs to form mixed DC cultures in which Ag could be transferred. In addition, donor DCs were injected into the footpad and observed in the draining LNs. In both cases, the extent of Ag transfer was deduced from the behavior of the T cells exposed to these mixed DC networks and correlated with short- and long-term immunological indicators of T-cell activation.
We observed OT-I T cells arresting and interacting with the recipient DCs while fluxing calcium, clearly indicating that Ag was transferred. The presence of H-2Kb+ (presentation-competent) recipient DCs boosted T-cell activation and proliferation. When H-2Kb- (presentation-deficient) incoming donor DCs were used, T cells clustered on nearby recipient CD11c+ DCs, and were efficiently activated. Ag dissemination required the migration of viable Ag-donor DCs to draining LNs and proximity between incoming and resident DCs. These effects were evident even at low numbers of Ag-donor DCs and recipient DC deficient in lymphocyte function associated antigen 1 (LFA-1) suboptimally activated T cells. Finally, we demonstrated that Ag transfer between DCs is crucial in salvaging immune response to viral infection of an immune-evasive virus.
Overall, our study suggests that rare incoming DCs facilitate T-cell activation by actively disseminating some of their Ag to a widespread network of resident CD11c+ DCs. We propose that disseminated Ag serves as a retention signal for T cells alerting them that cognate incoming DCs are present in the LN.
Results
Ag-specific interactions of CD8+ T cells with recipient DCs indicate that Ag spreads among DCs in vitro
To detect if Ag can spread among DCs in culture, we followed the cellular interactions between naïve T cells and Ag-presenting DCs, using epifluorescence and differential interference contrast (DIC) on a wide-field microscope. To that aim, we first established an in vitro system that mimics T cell and DC patterns of motility in the T zone of LNs, by plating bone marrow-derived DCs (BM-DCs) on ECM matrix decorated with CCL21, the major T-cell zone chemokine 15. Under these conditions, BM-DCs spread and formed reticular monolayers of sessile cells with motile dendrites (Supporting Information Video 1). Naïve T cells, on the other hand, became polarized and migrated vigorously (Supporting Information Video 2). These behaviors resemble the motility of DCs and T cells in vivo 6.
We isolated CD8+ T cells from OT-I mice and produced BM-DCs from Act-mOVA mice (OVA+ mice) 16, H-2Kb+ or H-2Kb−/− mice. OVA+ DCs, unlike OVA-pulsed DCs, continuously shuttle the Ag through the MHC-I pathway for stable presentation 12. We found that OVA+ DCs presented the Ag to T cells more efficiently (Supporting Information Fig. 1A) and with less variability than OVA-pulsed DCs.
To monitor the spread of Ag, we mixed OVA+ donor DCs and OVA− recipient DCs at a ratio of 1:200; both DC types could be either H-2Kb+ or H-2Kb−. After overnight coculture, OT-I T cells were applied to the DC preparations. T-DC Ag-specific interactions were identified based on established parameters: formation of T-DC conjugates, T-cell deceleration, and calcium flux 17.
To evaluate the formation of T-DC conjugates, OT-I T cells were applied to preparations containing only recipient OVA−H-2Kb+ DCs, only donor OVA+H-2Kb+ DCs, or a mixture of both DC types (Fig. 1A). We monitored T-cell clustering around DCs and calculated the mean duration of T-DC contacts. In OVA− cultures, T cells interacted minimally with DCs and migrated randomly. In OVA+ cultures, T cells interacted with most DCs and formed multicellular clusters. Importantly, in mixed cultured, T cells interacted not only with Ag-donor DCs (blue) but also with recipient DCs which were scattered throughout the imaging field (Supporting Information Video 3). Clustering was reflected in prolonged DC T-cell interaction in OVA+ cultures and intermediate contact durations in mixed cultures (Fig. 1A).
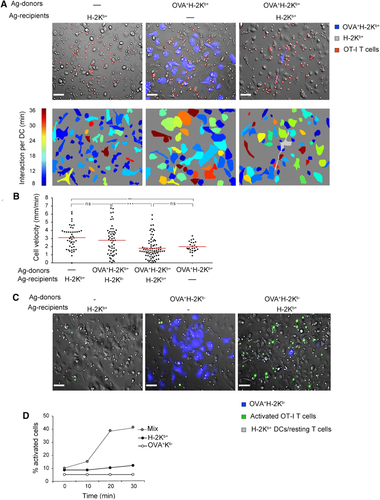
In vitro microscopy of T-cell DCs interactions indicates that Ag spreads among DCs Wide-field microscopy of OT-I T-cell behavior was used to assess the dissemination of Ag among plated DCs. Unmixed preparations of OVA-bearing (OVA+, blue) or OVA-free (OVA−, no color) DCs were prepared as controls. Mixed preparations of Ag-donor and Ag-recipient DCs at a ratio of 1:200 were prepared to examine Ag transfer. DCs were applied overnight to slides precoated with CCL21 to form a monolayer of sessile DCs. OT-I CD8 T cells were then applied and imaged for 30–60 min. Ag-donor DCs were cultured from OVA+CFP+ mice, T cells were labeled with CMTMR or with the Ca2+ indicator Fluo-4 AM. (A) T-DC interactions: CMTMR-stained OT-I CD8 T cells (red) were applied to the three indicated DC preparations. Upper panels: snapshots taken after 30 min. Lower panels: contact duration between T cells and DCs—warmer colors indicate longer mean interaction times per DC. The CFP OVA+ H-2Kb+ cell in the mixed culture (marked gray in the lower right panel) was not included in calculations. In the mixed culture, contact durations were long, resembling interactions with the unmixed preparation of Ag-donor DCs. The images are representative snapshots from 25 to 40 min movies taken at DIC mode with a 20× objective. The scale bar represents 50 μm. The experiment is representative of nine fields of view captured in three different experiments. (B) Velocities: T-cell migration was tracked on the four indicated DC preparations and cell velocities were compared. In mixed DC preparations, T-cell velocities resembled unmixed Ag-free DC preparations when H-2Kb- recipients were used and unmixed OVA-bearing DC preparations when H-2Kb+ recipients were used. The two mixed preparation were significantly different. Data were pooled from three experiments with three fields of view captured in each experiment. Each dot represents one tracked T cell, red bars represent mean velocities. Two and three asterisks indicate p < 0.01 and p < 0.001, respectively, as indicated by ANOVA. (C) Calcium flux: OT-I CD8 T cells were applied to the indicated three types of DC preparations. Snapshots showing Fluo-4 AM signals (green) at 30 min are presented. The images are representative snapshots from 25 to 30 min movies taken in DIC and epifluorescence modes with a 20× objective. The scale bar represents 50 μm. The experiment is representative of nine fields of view captured in three different experiments. (D) The percentage of calcium-fluxing T cells was followed. Only when H-2Kb+ recipient DCs were present did calcium flux indicate TCR engagement. Data are representative of three experiments.
To evaluate T-cell deceleration, OT-I T cells were applied to the DC preparations containing OVA−H-2Kb+, OVA+H-2Kb+ (as negative and positive controls) or OVA+ DCs mixed with either H-2Kb+ or H-2Kb- recipient DCs. We tracked the T cells and calculated their average velocity (Fig. 1B). As expected, T cells crawled fastest (3.1 μm/min) in the OVA−H-2Kb+ culture where there was no Ag, and slowest (2 μm/min) in the OVA+H-2Kb+ culture, where all DCs presented the Ag. Importantly, the speeds of T cells in mixed cultures were significantly affected by the identity of the recipient DCs (Supporting Information Video 4), When recipient DCs were H-2Kb−, the average velocity was similar to that observed in the OVA− culture (∼2.8 μm/min); when recipient DCs were H-2Kb+, the average velocity was similar to that seen by the OVA+ culture (∼1.9 μm/min).
To verify directly that T-cell deceleration was related to Ag presentation, we checked if the interaction of T cells with recipient DCs triggered Ca2+ flux. To this aim, OT-I T cells stained with the calcium indicator Fluo-4 AM were plated on mixed cultures composed of H-2Kb+ recipient DCs and OVA+H-2Kb− donor DCs. In this preparation, only recipient cells could present the SIINFEKL peptide to T cells. Cultures composed exclusively of OVA- DCs or OVA+H-2Kb−DC were used as negative controls. As expected, when no Ag was present, very few T cells, probably dying ones, became green (Fig. 1C and D, Supporting Information Video 5). Similarly, when Ag was present but could not be presented by any DCs (OVA+H-2Kb−), there was negligible green signal. In contrast, in the mixed DC culture, T cells fluxing Ca2+ accumulated gradually as they came into contact with recipient DCs (Fig. 1D). Here again, T-cell activation occurred regardless of how distant recipient DC were from Ag-donor DCs.
Our in vitro live cell imaging experiments demonstrate that when rare Ag-donor DCs are mixed with presentation-capable recipient DCs, T cells decelerate, stop to interact with recipient DCs and form clusters. These interactions were TCR dependent as reflected by Ca2+ flux in Ag-specific T cells. Taken together, these results indicate that Ag was widely disseminated from the original rare Ag-donor DC to be presented on recipient DCs.
Ag-recipient DCs greatly enhance T-cell activation and proliferation in vitro
After we detected thorough imaging that rare Ag-donor DCs spread Ag to recipient DCs, we asked if this dissemination leads to efficient T-cell activation and priming. To address this question we again used mixtures of Ag-donor and recipient DCs, as described in the Supporting Information.
At 5 h, T cells activated by such mixed cultures upregulated CD69 (Fig. 2A). The percentage of activated T cells increased with more Ag-donor DCs in the culture. When recipient DCs were H-2Kb−, maximal activation was only reached when one in five DCs carried the Ag. In contrast, when H-2Kb+ recipient DCs were used, one in 25 DCs was enough to fully activate the T cells. Mature recipient DCs enhanced T-cell activity more than immature DCs, but only if they were H-2Kb+ (Supporting Information Fig. 1B and C). Fixation of H-2Kb+ recipient DCs abolished their contribution completely (Supporting Information Fig. 1D) indicating that they acquire Ag through an active cellular process.

Ag transfer between DCs in culture induces CD69 upregulation and leads to T-cell proliferation OVA+H-2Kb+ Ag-donor DCs were cocultured for 16 h with recipient DCs at various ratios to form mixed cultures. Two types of recipient DCs were used: H-2Kb+ or H-2Kb−. OT-I T cells were then cocultured with the mixed DCs. (A) CD69 upregulation: T cells were stained for CD8 and CD69 at 5 h. At left, flow cytometry dot plots show upregulation of CD69 on pregated CD8+ T cells after coculture with a 1:125 mixture of Ag donor and the indicated recipient DCs. At right, CD69 upregulation is plotted as a function of the ratio between Ag donor and recipient cells. T-cell activation increased gradually with more Ag donor DCs present, and this activation was greatly enhanced when recipient DCs were H-2Kb+. Data are represented as mean + SEM of three replicates. Results are representative of five experiments. The arrow indicates the DC ratio depicted at the top. (B) T-cell proliferation: OT-I T cells proliferation was estimated by CFSE dilution at 72 h. At left, sample histograms show division of OT-I T cells after coculture with a 1:125 mixture of Ag donor and the indicated recipient DCs. At right, T-cell proliferation is depicted at different ratios between Ag donor and recipient cells. T-cell proliferation increases gradually as more Ag donor DCs were present; proliferation was dramatically enhanced when recipient DCs were H-2Kb+. The arrows point to data depicted on the right. Data are represented as mean + SEM of three replicates. Results are representative of three experiments. The arrow indicates the DC ratio depicted at the top.
Even more striking results were obtained when T-cell proliferation was measured (Fig. 2B). Here, when H-2Kb+ recipient DCs were used, one Ag-donor DC in 625 was enough to induce maximal T-cell proliferation.
Together, these results indicate that T-cell priming does not depend only on the original Ag-donor DCs but is assisted by recipient DCs presenting the Ag that they had acquired. Similar phenomena might occur in vivo among Ag-donor DCs that migrate into a LN and interact with resident DCs. Sensing Ag disseminated from incoming to recipient CD11c+ DCs, CD8+ T cells decelerate and cluster.
To study Ag transfer in vivo, we used two-photon imaging in anesthetized mice and monitored DC-T cell interactions within the T zones of popliteal LNs following injection of Ag-donor DCs. We subcutaneously injected H-2Kb+ mice with OVA+ H-2Kb-/− DCs in the footpad. These DCs had reached the T-cell zones (Fig. 3A). They showed no sign of early rejection, extended and retracted their dendrites but moved little laterally (Supporting Information Video 6). We examined the time-dependent rejection of H2Kb−/− DCs in WT hosts using in vivo imaging (Supporting Information Fig. 2B and C) and flow cytometry (Supporting Information Fig. 2D and E) and found that the DCs survived in the LN for at least 48 h, regardless of their Kb status.
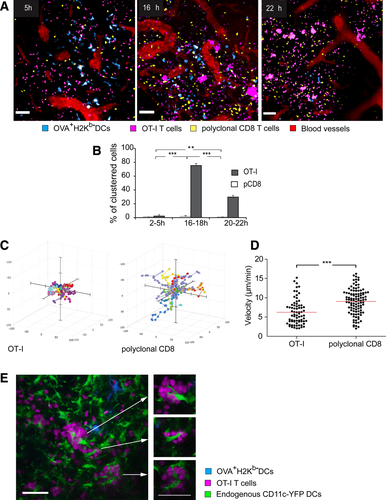
To examine the dynamics of Ag dissemination, we i.v. injected the above mice with fluorescently-tagged Ag-specific OT-I T cells and, as a control, naïve polyclonal CD8+ T cells stained with a different dye. LNs were imaged 5 to 22 h later. At 5 h, both polyclonal CD8 T cells and OT-I T cells distributed evenly in the T-cell zone and migrated vigorously. At 16 and 22 h, as indicated in the video, OT-I cells crawled more slowly than polyclonal CD8+ T cells and formed numerous clusters (Fig. 3A, Supporting Information Video 7). Quantification demonstrated that the peak of T-cell clustering occurred around 16 h after transfer (Fig. 3B). Focusing on this time-point we compared the migration patterns of OT-I and polyclonal T cells. Ag-specific cells traveled shorter distances than polyclonal ones (Fig. 3C) and crawled more slowly (Fig. 3D, Supporting Information Video 8).
We next determined around which APCs the T cells cluster. For that purpose, we used CD11c-YFP host mice whose LN DCs selectively express YFP 6. These mice allowed us to visualize the resident YFP+ recipient DCs alongside injected CFP+ Ag-donor DCs. Following injection of the OVA+H-2Kb−CFP+ DCs, T cells clustered in the vicinity of the transferred DCs, but not necessarily in direct contact with them (Supporting Information Video 9). These clusters typically contained CD11c+YFP cells at their core (Fig. 3E), strongly suggesting that these are the APCs that present disseminated Ag. Notably, T cells clustered around widespread resident DCs, not necessarily adjacent to Ag-donor DCs. This observation favors a contact-independent mechanism of Ag transfer.
These observations mirror our in vitro findings and suggest that Ag is disseminated within the LNs from migrating DCs to nearby CD11c+ recipient DCs for presentation to T cells. The widespread pattern and gradual kinetics of T-cell arrest suggest that small quantities of Ag are broadly dispersed through the network of LN DCs 18.
Ag dissemination in LNs facilitates CD8+ T-cell priming in vivo
Our in vivo imaging experiments indicate that Ag is disseminated from incoming DCs to resident DCs in LNs and presented to CD8 T cells. To check if such transfer can functionally affect immunity, we studied both early and late indicators of T-cell activation. For that purpose, we s.c. injected host mice, which were either H-2Kb+ or H-2Kb−,with H-2Kb+ or H-2Kb- OVA+ DCs. OVA− DCs were used as a negative control.
In mesenteric LNs, used as internal negative controls, no T-cell activation occurred, as CD69 was not upregulated (Fig. 4A). As expected, in popliteal LNs of mice injected with OVA– DCs, T cells were not activated. The highest activation was observed when both injected Ag donor and host-recipient DCs could present the Ag (70% activation). In H-2Kb+ mice injected with OVA+ H-2Kb− DCs or in the converse situation, when H-2Kb− mice injected with OVA+ H-2Kb+ DCs, T-cell activation was moderately reduced and reached similar levels (around 40% activation). Importantly, CD69 upregulation preceded T-cell clustering, which at 5 h was not yet evident (Fig. 4A).
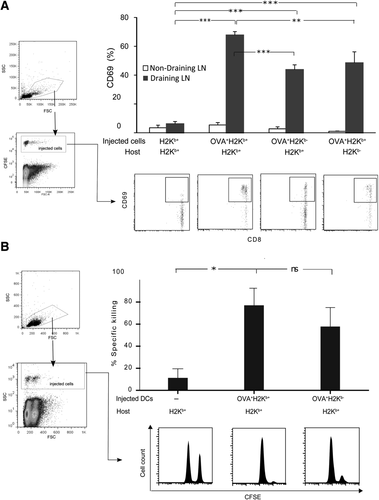
Ag transfer from incoming to resident DCs in LNs facilitates T-cell priming Host mice of the indicated strains were s.c. injected with the indicated types of Ag-donor DCs, and i.v. injected with OT-I CD8 T cells. (A) CD69 upregulation. A total of 5 × 106 CFSE-tagged OT-I CD8 T cells were transferred 16 h after injection of 2 × 105 DCs. The popliteal (draining) and mesenteric (nondraining) LNs were harvested 5 h after T-cell injection. CD69 expression was estimated using flow cytometry for CFSE+CD8+ T cells. T-cell activation was maximal when both transferred and endogenous DCs were H-2Kb+ and could present the Ag. When either Ag donor or recipient cells were H-2Kb−, activation was significantly reduced. Data were pooled from five experiments. Each bar represents an average of 7 to 20 mice. Data are represented as mean + SEM. Representative FACS plots from each condition are shown. **p < 0.01 and ***p < 0.001, respectively, as indicated by ANOVA. (B) In vivo CTL activity. H-2Kb+ hosts were subcutaneously injected with 5 × 103 Ag-donor DCs which were either H-2Kb− or H-2Kb+. A total of 5 × 103 OT-I CD8 T cells were injected i.v. on the same day. The specific killing of Ag-pulsed target splenocytes was evaluated using flow cytometry 1 day later. Transfer of H-2Kb+ DCs resulted in robust CTL activity compared to unimmunized control (p < 0.05), CTL activity was somewhat lower when H-2Kb−OVA+ DCs were transferred but not significantly so (p > 0.05). Data were pooled from four experiments. Each bar represents an average of five to nine mice. Data are represented as mean + SEM. Representative FACS plots showing surviving pulsed and nonpulsed targets (high and low CFSE) from each condition are shown.
We next wanted to investigate whether this initial activation can prime T cells and generate functional CTLs. A standard in vivo cytotoxicity assay 19 was performed. When 2 × 105 OVA+ DCs were used for immunization, regardless of their H-2Kb expression, target cells were specifically eliminated (Fig. 4B). Thus, although T-cell activation was somewhat diminished, enough CTLs developed to fully eliminate all target cells.
We typically conducted our experiments with relatively high numbers (2 × 105) of DCs coinjected with LPS; these conditions were required to visualize the cells in LNs. To estimate the contribution of Ag dissemination in more physiological conditions, we sterilely injected mice with 40-fold lower numbers of DCs (5 × 103 per footpad). Even at this low number of injected DCs, T-cell activation and proliferation were significantly reduced in H-2Kb−/− hosts, whose resident DCs could not present the Ag, (Supporting Information Fig. 3).
Taken together, these data indicate that Ag dissemination from Ag-donor DCs to the endogenous DC network can facilitate T-cell priming and proliferation at low Ag doses and result in full maturation of T cells and development of Ag-specific cytotoxicity.
Ag dissemination is an active process occurring within LNs
It has been postulated that LN DCs cross-present Ag captured from dead or dying DCs 9, 10, 20; this capture can occur either in the periphery or within LNs. In our experiments, presentation-deficient DCs elicited T-cell activation as soon as they reached the LN and survived there for several days (data not shown) much like wild-type (WT) DCs 6. We therefore hypothesized that Ag-donor DCs actively disseminated Ag only as they reached the LN.
We first ruled out the possibility that DCs dying in the periphery could be a substantial source of Ag presented by DCs in the LN. We injected the footpads of WT mice with equal numbers of either live or UV-treated OVA+ DCs, and compared the consequent clustering and activation of OT-I T cells in the draining LN. UV irradiation resulted in apoptosis of 80% of DCs based on Annexin-V and PI staining (Supporting Information Fig. 4). Two-photon microscopy indicated that no surviving UV-irradiated DCs had reached the LN (Fig. 5A). As a result, OT-I T cells did not cluster and showed a similar distribution to polyclonal CD8 T cells (Fig. 5A and B, Supporting Information Video 10). Correspondingly, OT-I T-cell activation was significantly reduced (Fig. 5C). Thus, the uptake of dead DCs in the periphery seems to contribute little to Ag transfer between DCs.
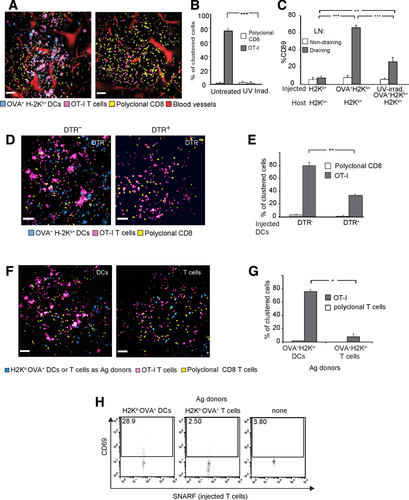
Ag dissemination in LNs requires migration and survival of Ag-donor DCs (A–C) Mice were injected with untreated or UV-irradiated Ag-carrying DCs to evaluate the relative contribution of Ag dissemination in the skin and within LNs. (A) T-cell clustering. A live two-photon imaging snapshot showing the T-cell clustering patterns in the LNs of mice injected with the different DCs. OT-I CD8+ T cells (magenta) and polyclonal CD8 (yellow) were transferred into H-2Kb+ mice 16 h before imaging. Scale bar = 50 μm. The images depict representative snapshots from in vivo movies. Representative data from three independent experiments involving three mice are shown. (B) Decreased clustering of T cells after UV-irradiated DCs were injected into H-2Kb+ hosts (p < 0.001) point to inefficient delivery of Ag to the LN. (C) CD69 upregulation. The indicated mice strains were intradermally injected with the indicated types of Ag-donor DCs and intravenously injected with OT-I CD8 T cells. OT-I CD8 T cells were injected 16 h after DC injection. Draining and nondraining LNs were harvested 5 h after T-cell injection. CD69 expression was estimated for CD8+Vα2+ T cells. Each bar represents an average of 6 to 22 LNs, from 3 to 11 mice. **p < 0.05, ***p < 0.01, and ***p < 0.001, respectively, as indicated by ANOVA. Data are represented as mean + SEM. (D and E) H-2Kb− mice were subcutaneously injected with OVA+H-2Kb− DCs (blue) or DTR+ OVA+H-2Kb− DCs to right and left footpad respectively. OT-I T cells (magenta) and polyclonal CD8 T cells (yellow) were injected i.v. 3 h later, followed 3 h later by DTx at dose of 16 ng/g body weight. (D) T-cell clustering. Twelve hours after DTx treatment, the two contralateral LNs were imaged by two-photon microscopy. Only DTR− incoming DCs could be detected (blue). Representative images from three independent experiments involving three mice are shown. The images are representative snapshots from in vivo movies. The scale bar represents 50 μm. (E) Analysis of clustering: The significantly different percentage of clustered T cells in the contralateral LNs points to a significant reduction in dissemination of Ag when DCs undergo local apoptosis (0.001< p <0.01). *p < 0.05, **p < 0.01, and ***p < 0.001, respectively, as indicated by ANOVA. (F–H) C57BL/6 Mice were injected with either DCs or CD4+ T cells isolated from OVA+ H-2Kb− mice. (F) T-cell clustering. Live two-photon imaging shows the pattern of OT-I CD8 T-cell clustering in the LNs of mice injected with the Ag donors. Injection of 2 × 105 DCs s.c. or 4 × 106 CD4 T cells i.v. yielded comparable densities of Ag-donor cells in the T zones of the LN. OT-I CD8+ T cells (magenta) and polyclonal CD8 (yellow) were transferred into H-2Kb+ mice 16 h before imaging. The images are representative snapshots from in vivo movies. The scale bar represents 50 μm. (G) Analysis of OT-I T-cell clustering indicated that Ag was inefficiently disseminated by CD4 T cells compared to DCs (p < 0.001). (H) CD69 upregulation. Draining LNs were harvested 16 h after the injection of Ag-donor DCs and T cells. CD69 expression was estimated for SNARF+ T cells. Data are representative of two independent experiments.
We next tested whether apoptosis of incoming DCs within the LN would enhance or interfere with Ag dissemination. To induce apoptosis of Ag-donor DCs after they had reached the T zone, we used DCs from CD11c-DTRtg mice (where DTR is diphtheria toxin receptor). Intraperitoneal injection of diphtheria toxin (DTx) results in specific depletion of such CD11c+ DCs 21. WT mice were injected with OVA+H-2Kb− DCs from DTR+ mice into the right hind paw and from DTR– mice into the left one. Host mice were systemically treated with DTx 6–8 h after DCs transfer. This delay was sufficient for DCs to reach the LNs (Supporting Information Fig. 2A). Twelve hours later, two-photon imaging detected live DTR– DCs the in left popliteal LN, but no DTR+ DCs in the right one (Fig. 5D, Supporting Information Video 11). As seen before, OT-I T cells clustered in the vicinity of incoming OVA+H-2Kb− DCs; in contrast, in the contralateral LN, OT-I T-cell clustering was significantly reduced (Fig. 5D and E). This observation illustrates that efficient Ag transfer requires active participation of Ag-donor DCs, although cross-presentation of Ag from dead cells may play a minor role.
The Ag-donor DCs we have used express OVA as a membrane-bound protein. Such proteins can be proteolytically cleaved or shed from the cell surface. It was therefore possible that the resident DCs cross-presented this shed form of OVA. To examine this scenario we injected C57BL/6 mice with high numbers of CD4 T cells isolated from OVA+ H-2Kb− mice. These cells migrate to the same T-cell zones occupied by incoming DCs and can serve as potential donors of shed OVA (Fig. 5F–H). This transfer yielded comparable densities of Ag-donor cells in the T zones to that seen with s.c. injection of DCs (Fig. 5F). As before, Ag-specific OT-I CD8 T cells were injected to assess Ag dissemination. Although the CD4 T cells could in principle shed membranal OVA in the LN, no clustering of OT-I T cells was observed (Fig. 5F and G). Correspondingly, OT-I T cells did not upregulate CD69 (Fig. 5H). Together, these results indicate that Ag dissemination is a capacity reserved to incoming DCs and is unlikely the result of passive Ag-shedding.
In conclusion, for efficient Ag dissemination, Ag-donor DCs, and not other cells, must migrate into the draining LN and survive there, strongly suggesting an active route of Ag transfer.
Ag dissemination involves an LFA-1-dependent mechanism
DCs express both the integrin LFA-1 22 and its ligand ICAM1 23; the interaction between these molecules may promote communication between DCs. We therefore examined the involvement of LFA-1 in Ag dissemination using H-2Kb+ LFA-1− mice 24, which we used as a source of recipient DCs in culture and as hosts in in vivo experiments. We first verified that BM-DCs from WT mice indeed express LFA-1 and their counterparts from LFA-1− mice do not (Supporting Information Fig. 5A) .We then validated that LFA-1 deficiency in DCs does not directly interfere with their Ag presentation capability. LFA-1− DCs were as efficient as wild-type DCs in presenting soluble OVA protein and SIINFEKL peptide, across a wide range of doses (Supporting Information Fig. 5B; SIINFEKL-pulsed LFA-1− DCs migrated into the LNs of WT mice and triggered the clustering of OT-I cells (Supporting Information Video 12). Finally, LFA-1− mice supported normal proliferation of adoptively transferred OT-I T cells in response to soluble OVA immunization in the LN (Supporting Information Fig. 5C) and spleen.
Unlike their normal function in direct Ag presentation, LFA-1- DCs showed defects as recipient DCs: First, we tested how LFA-1 deficiency affects Ag dissemination in vitro as reflected by early T-cell activation (Fig. 6A). Ag-donor OVA+H-2Kb+ DCs were mixed with three types of recipient DC, as indicated. In agreement with our previous results, T-cell activation was highest when LFA-1+H-2Kb+ recipients were used and lowest when LFA-1+ H-2Kb- recipients were used. LFA-1-H-2Kb+ recipients induced an intermediate level of T-cell activation.
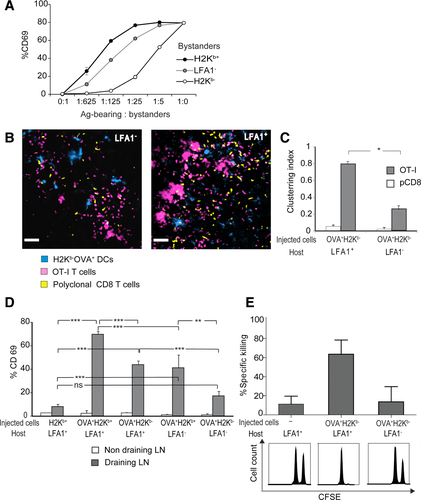
We proceeded to investigate if LFA-1 deficiency in recipient DCs interferes with Ag transfer and T-cell activation in vivo. To examine the clustering of OT-I T cells in LNs, we injected OVA+H-2Kb− DCs into the footpads of H-2Kb+ LFA-1− mice. Fluorescently labeled Ag-specific T cells and control polyclonal T cells were injected 24 h after DC injection. In LFA-1− hosts, OT-I T cells failed to cluster (Fig. 6B and C, Supporting Information Video 13) and resembled polyclonal CD8 T cells in their distribution.
Correspondingly, LFA-1 deficiency in host DCs markedly reduced T cells activation induced by Ag dissemination (Fig. 6D). Specifically, when OVA+H-2Kb+ DCs were injected into LFA-1− hosts, T-cell activation was significantly reduced. To directly test whether LFA-1 is involved in the uptake of Ag, OVA+ H-2Kb¯ DCs were injected into H-2Kb+ hosts which were either LFA-1+ or LFA-1−, such that only recipient DCs could present the Ag. As seen before, OVA from these donor DCs could be presented by LFA-1+ host DCs fairly efficiently; in contrast, LFA-1 deficiency almost abolished T-cell activation, suggesting poor uptake of disseminated Ag.
To examine the long-term implications of reduced Ag dissemination in LFA− mice, we investigated if it would diminish CTL formation. Performing an in vivo killing assay we found that injection of H-2Kb¯ OVA+ DCs induced SIINFEKL-specific CTL development in LFA-1+ hosts but was significantly reduced in LFA-1− mice (Fig. 6E).
Taken together, these findings suggest that LFA-1− recipient DCs acquire Ag from neighboring DCs poorly, and thus fail to assist T-cell activation.
Ag dissemination salvages immune response to virally infected Ag donor
In previous experiments, we used adjuvant-stimulated OVA-expressing BM-DCs as a convenient model for in vitro and in vivo experiments. To validate the importance of Ag dissemination to more physiologically relevant situations we used viral infection of BM-DCs. We used genetically modified vaccinia virus Ankara (MVA), a replication-deficient virus encoding GFP and OVA proteins in tandem 25. We have previously shown that MVA infection of BM-DCs suppresses expression of CD86 and MHC-I, impairing their ability to stimulate naïve T cells 26. We took advantage of this phenotype to test if Ag dissemination from infected DCs can salvage T-cell activation.
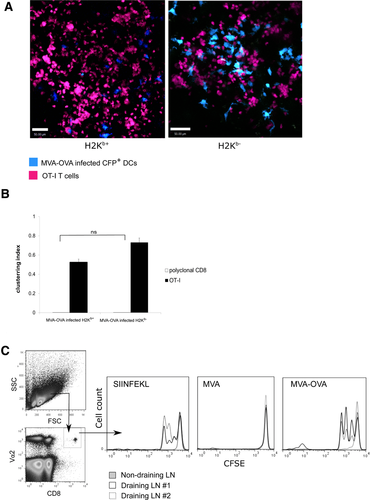
We infected BM-DCs with either MVA-GFP or MVA-OVA-GFP viruses. We could detect GFP and OVA expression in BM-DCs in vitro (Supporting Information Fig. 6A). MVA-infected H2Kb+ and H2Kb−/− DCs were intradermally injected into mice when their OVA expression was maximal and cell death was minimal (Supporting Information Fig. 6B) and the interaction of OT-I T cells with recipient DCs in the draining LNs was observed. In both cases, OT-I T cells formed numerous clusters around neighboring DCs throughout the T-cell zone indicative of dissemination and presentation of OVA (Fig. 7A, Supporting Information Video 14). In both cases the degree of clustering was significant and similar (Fig. 7B). Our results suggest that Ag presentation is impaired in MVA-infected Ag-donor cells and is delegated to LN-resident DCs. The imaging experiment was followed up with functional assay of T-cell activation, which revealed efficient T-cell proliferation in case of H2Kb+ and H2Kb−/− DCs infected with MVA-OVA-GFP (Fig. 7C). These data suggest that Ag dissemination from incoming DCs that are incapable of presenting Ag to resident DCs can salvage immune responses.
Discussion
The field of Ag presentation has been struggling with evidence that DCs immigrating into the LNs are not necessarily the ones that present the Ag to T cells 10, 11. The mechanisms allowing the cellular transfer of Ag, and the advantages it holds for T-cell activation are debated, as it is unclear why Ag presentation is not limited to the DCs that originally encountered the Ag.
Our research demonstrates, by observing the intact LNs of mice in real time, that incoming DCs that express Ag, disseminate it to resident DCs for presentation. Specifically, we showed how this Ag dissemination affects the behavior and activation of CD8 T cells and pointed to possible mechanisms employed.
Working initially with in vitro preparations, our study demonstrated that scarce Ag-donor DCs can efficiently transfer Ag to abundant recipient DCs. By examining CD8 T-cell activation and behavior we showed that the antigenic peptide is presented by the recipient DCs, increasing the total number of DCs participating in Ag presentation. Ag dissemination affected the migration of T cells within DC cultures, promoted their clustering and greatly assisted their priming, allowing them to proliferate even when Ag-recipient cells outnumbered Ag-donor cells several hundreds to one. Using Ca2+ flux imaging we demonstrated that the interaction of CD8 T cells with Ag-recipient DCs was TCR-dependent, and that rather than being transferred between discrete adjacent DCs, Ag was widely disseminated to distant cells.
For greater physiological relevance, we studied Ag dissemination by incoming Ag-expressing DCs in the LNs of living mice while manipulating the DCs’ capabilities to transfer and present the Ag. Central to this set of experiments was the transfer of limited numbers of OVA+H-2Kb− donor DCs into WT hosts, producing a situation in which the recipient DCs were the only cells capable of presenting the Ag. In accordance with previous findings using CD4 10 and CD8 T cells 11, 12, T-cell activation in such LNs demonstrated that Ag had been transferred from donor to recipient DCs. We could also show, using long-term assays, that CD8 T-cell activation by recipient DCs can lead to productive development of Ag-specific CTLs, even with low numbers of injected DCs. Correspondingly, the presence of presentation-competent recipient DCs in the LN, doubled the efficiency of T-cell activation, especially when numbers of incoming DCs were limiting. The physiological relevance of these findings was further demonstrated using low numbers of injected DCs as Ag donors.
We utilized the unique advantages of intravital two-photon microscopy to examine the distribution and integrity of incoming cells, and follow the Ag-dependent changes in the behavior of T cells interacting with recipients DCs. Following footpad injection, presentation-deficient Ag-donor DCs reached the draining LN, integrated within resident CD11c+ DCs, and exhibited intact morphology and dendrite motility. Ag-specific CD8 T cells slowed down in T zones permeated by donor DCs, confirming that Ag was disseminated to resident DCs. T cells then clustered around endogenous CD11c-recipient DCs. Interestingly, T cells congregated around a limited number of DCs, which were not necessarily adjacent to donor DCs. This likely indicates that some resident DCs outperform others at collecting or presenting the Ag 8.
In the presence of disseminated Ag, OT-I T cells followed the three-phase migration pattern described before 7. T cells did not stop immediately in the presence of Ag, even though CD69 expression at the same time point indicated that they have sensed its presence. Rather, they continued to migrate for several hours before slowing down and clustering. This pattern is characteristic of Ag presentation at low avidity 27, which does not trigger the full arrest of CD8 T cells and allows them to migrate further. It suggests that migrant DCs disseminate low amounts of Ag to a large number of resident DCs allowing the T cells to continue searching for the optimal DCs to interact with.
We examined several potential scenarios for the transfer of Ag among DCs: (a) Peripheral uptake of dying DCs – in which DCs would cannibalize apoptotic DCs in the periphery and migrate to the LN to cross-present the Ag they acquired. This scenario seems unlikely, as T-cell clustering and activation diminished, rather than increased when UV-irradiated DCs, known to be excellent substrates for cross-presentation 9, 28 were injected into the footpad. (b) Central uptake of dying DCs—in which donor DCs would migrate from the periphery, die in the LN, and resident DCs take up their Ag and cross-present it. This scenario was rejected as deliberate killing of DTR+ DCs using DTx in the LNs reduced T-cell clustering. (c) Central transfer of Ag between live DCs—in which donor DCs would migrate to the LN and transfer the Ag via functional cell-to-cell communication. This scenario was supported by our in vivo T-cell motility and activation data.
Several mechanisms have been proposed for transfer of antigens between DCs. These include phagocytosis of DC fragments 9, 10, trogocytosis, and cross-dressing 13, 29, 30. Other routes, such as gap junctions 31 or tunneling nanotubes 32, may also be suggested based on in vitro observations. Although, in the in vivo setting, it was generally agreed that migrating DCs need to reach the LNs alive 10, 11, it is not clear if they die before their Ag in disseminated to resident DCs, and whether they transfer antigens as pMHC complexes or unbound Ags.
Although the molecular mechanisms of Ag transfer remains incompletely understood, our study provides insights into it. We made sure that Ag-dissemination was unique to DCs by demonstrating that high numbers of Ag-expressing T cells reaching the same areas in the LN cannot accomplish it. This experiment indicated that Ag was not passively shed from the cell membranes in the LN. Based on the patterns of OT-I T-cell migration within LNs, we conclude that Ag was disseminated from live donor DCs to recipient CD11c+ DCs without evidence of direct contact. MHC-cross-dressing, the swapping of surface pMHC molecules between cells, has been implicated in Ag transfer in several studies 13, 29. In our experiments this phenomenon seems less relevant because when H-2Kb− DCs, which do not have intact peptide-MHC complexes to transfer, were used as donor DCs, Ag was efficiently transferred.
Since DCs express both the integrin LFA-1 and its ligand ICAM-1, making highly adhesive DC to DC interactions possible, we studied the efficiency of Ag-transfer using LFA-1−/− Ag-recipient DCs. Even though LFA-1−/− DCs displayed normal migration and Ag presentation, they were inefficient Ag presenters when exposed to OVA+H-2Kb− donor DCs (both in vitro and in vivo).
Ligation of LFA-1 and ICAM-1 might mediate direct interactions between Ag-donor and Ag-recipient DCs. Nevertheless, in our experiments, T cells could be imaged clustering (in vivo) and fluxing calcium (in vitro) while contacting DCs located away from the Ag-donor DCs, suggesting that Ag had been dispersed independently of cell contacts.
A route of Ag transfer that is consistent with such findings involves exosomes. LFA-1 acts as a scavenging receptor mediating cellular uptake of DC-derived exosomes 33-35. Indeed, uptake of purified exosomes by DCs has been demonstrated in vitro 36, and administration of DC-derived exosomes can result in immunization 37 and is pursued clinically 38. However, physiological exchange of exosome between DCs has never been demonstrated in vivo, and can be suggested based on our findings.
Our findings suggest that in pathological situations, in which migrating DCs cannot present the Ag (as emulated in our experiments using H-2Kb- DCs), recipient DCs can take over and salvage the immune response. This situation arises when migrating DCs are infected not only by vaccinia but also by other immune-evasive viruses (e.g., HSV, EBV, CMV) that interfere with Ag processing and MHC-I presentation to avoid detection by CD8 T cells 39. The mechanisms we propose could act as a countermeasure by allowing the noninfected resident DCs to activate CD8 T cells 40.
Taken together, our findings suggest that live incoming DCs actively disseminate some of their Ag to nearby CD11c+ DCs which collect the Ag in an LFA-dependent manner. The disseminated Ag is presented on the resident DCs and may then serve as a retention signal for T cells in the LN, increasing the chances of their priming.
Materials and methods
Mice
Mice were maintained in a specific pathogen-free facility under conditions approved by the institutional animal care and use committee of the Weizmann Institute of Science (IACUC approval #02020313-2 ). Male and female mice were 8 to 12 weeks old when used. Adoptive cell transfers were sex matched. The mouse strains used in this work are described in the Supporting Information. The following mouse strains were used: Act-mOVA (OVA+), JAX Stock #005145 16; Actin-CFP mice (CFP+), JAX stock #004218, 41; LFA-1-deficient mice (LFA-1−/−) 24, a gift from Ronen Alon, WIS; H2-Kb deficient mice (H2Kb−/−) 14, a gift from Lea Eisenbach, WIS; CD11c-DTR (JAX stock #004509) 21; ubiquitin-EGFP mice (where EGFP is enhanced GFP; JAX stock #004353) 42; CD11c-EYFPhi (where EYFP is enhanced yellow fluorescent protein; CD11c-YFP) mice (JAX stock #008829) (Lindquist et al., 2004); OT-I (JAX stock #003831) 43; C57BL/6 mice, either CD45.2 or CD45.1, were provided by Harlan Laboratories, Israel. The above strains were all on the genetic background of C57BL/6 and were crossed as indicated in the section Results.
Generation and manipulation of BM-DCs
BM-DCs were generated by modifying an established protocol 41. Analysis of harvested cells revealed that 85–95% expressed CD11c, with 40–60% positive for MHC-II. BM-DCs were further activated with 20 μg/mL poly (I:C) (Sigma, Israel) for 16 h. For imaging purposes, CFP+ cells were sometimes used. To fix DCs, the cells were treated with 0.001% glutardehyde for 30 s, followed by deactivation with 0.2 M glycine as detailed elsewhere (45). To obtain apoptotic Ag-donor DCs, the culture plates were irradiated with 50 J/m2 of ultraviolet light. In the experiments requiring DTR-mediated depletion of DCs, DTx was injected i.p at 16 ng/g body weight 12 h before imaging 21.
To estimate the efficiency of Ag transfer in popliteal LNs, we s.c. injected 2 × 105 BM-DCs together with 50 ng LPS (Sigma) to the hind footpad.
To infect BM-DCs with MVA, we followed a protocol described previously 25. Briefly, BM-DCs were harvested at day 10–12 and 2 × 106 DCs were mixed with 2 × 106 viral particles in 100 μL medium. The cells were incubated for 1 h at 37 °C and mixed every 10 min.
T-cell purification and staining
Polyclonal or OT-I CD8+ T cells were negatively selected from spleens and LNs using EasySep™ Mouse CD8+ T-Cell Enrichment Kit. The kit contains a cocktail of biotinylated antibodies directed against several leukocyte lineages (CD4, CD11b, CD11c, CD19, CD45R/B220, CD49b, TER119). The purity of enriched cells exceeded 90% based on CD8 and Vα2 TCR staining.
For imaging purposes, T cells were either harvested from GFP+ mice or labeled with vital fluorescent dyes. Cells were incubated in Roswell Park Memorial Institute medium (RPMI) for 20 min with SNARF (seminaphtharhodafluor, Invitrogen) or with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) (CellTracker Orange, Life Technologies) at 37°, access dye was then reacted with 10% fetal calf serum.
Flow cytometry
Flow cytometry was performed according to standard procedures on an LSRII flow cytometer (Becton Dickinson) and analyzed with FlowJo software (Tree Star). Antibodies used for analysis were anti-CD11c-APC (Biolegend, clone N418), anti-CD45.1-biotin (Biolegend, A-20), anti-MHC-II (I-Ab)-PE (where PE is phycoerythtin; Biolegend, AF6-120.1), anti-CD80-PE (eBioscience, 16-10A1), anti-CD40-PE (eBioscience, 1C10), anti-CD8-PE (Biolegend, 53–6.7), anti-Vα2 TCR-APC (Biolegend, B20.1), and anti-CD69-APC (Biolegend, H1.2F3).
In vitro wide-field microscopy of DC T-cell interactions and calcium flux
BM-derived DCs were plated for 16 h on chamber microslides (μ-Slide VI; ibidi) precoated as described before 15 with 2 μg/mL CCL21 chemokine (R&D) followed by 5 μg/mL fibronectin (Sigma) and 15 μg/mL collagen-I (Chemicon). Glass imaging chambers were coated with adhesive matrix decorated with 100 sites/μm2 of CCL21–
To analyze DC T-cell interactions, CFP+ and CFP− DCs were used and OT-I T cells were stained with CMTMR (Life Technologies) according to standard protocol. For calcium flux analysis, T cells were preloaded with 2 μM Fluo-4-AM (Life technologies) as described before 42. T cells were added and imaged by DIC and epifluorescence microscopy using DeltaVisionRT® system at stable temperature (37°C) and humidity. Time-lapse images were collected at 10-s intervals for 30 min.
In vitro Ag transfer assay
BM-DCs were plated in a 96-well conic plate at 1.5 × 105 cells/well. OVA+ and OVA− DCs were mixed at different ratios and cocultured for 16 h. Purified naïve OT-I T cells were applied to the mixed DC cultures for 5 h. T-cell activation was assessed by flow cytometry of CD69 expression. T-cell proliferation was assessed at 72 h using a standard CFSE-dilution assay 43.
In vivo killing assay
To evaluate CTL activity in live mice, we performed a standard CFSE-based assay 44.
Intravital two-photon microscopy in the LN
Before imaging, mice were anesthetized by i.p injection of 100 mg/kg ketamine + 15 mg/kg xylazine + 2.5 mg/kg acepromazine. Anesthesia was supplemented hourly with half this dose. Mice were placed on a warmed plate and kept at a core temperature of 37°C. The popliteal LN was surgically exposed while preserving normal blood flow in it. The foot was placed on a silicone stage and the LN covered with a glass-bottom imaging chamber.
We used a two-photon microscope (Ultima; Prairie Technologies) incorporating a pulsed laser (Deep See Mai Tai, Newport Corp.). The laser was tuned to 890 nm to simultaneously excite all used fluorophores: ECFP, EYFP, EGFP, SNARF, CMTMR, and 647 Q-dots (Life technologies) for blood flow visualization. Water-immersed 20× (NA 0.95, where NA is numerical aperture) or 40× (NA 0.8) objectives (Olympus) were used. To create a typical time-lapse sequence, a 50- to 80-μm-thick section of LN was scanned at 4- to 6-μm Z-steps every 40–50 s.
Image analysis
Image analysis was performed with Volocity 4.3.2 from Perkin Elmer. To calculate the 2D or 3D velocity of T cells, the coordinates of their centroid were tracked for 30–60 min. The duration of T-DC interactions was calculated per DC as the average time it overlapped with all the T cells it contacted. The percentage of Ca2+ T cells was calculated as the number of Fluo-4-AM-bright cells out of all T cells in field. The percentage of clustered T cells was calculated as the percentage of T cells which were in contact with five or more T cells.
Statistical analysis
Data were statistically analyzed using GraphPad Prism software. For simple comparisons, a two-tailed Student's t test was used. Multiple comparisons were performed by one-way ANOVA followed by Bonferroni correction for planned contrasts. Significance was set to p < 0.05. *p < 0.05, **p < 0.01, and ***p < 0.001. Data in figures are shown as mean ± SEM.
Acknowledgments
We thank Professors Ronen Alon and Steffen Jung for critical discussion. This work was supported by a grant from the Minerva Foundation.
Conflict of interest
The authors declare no commercial or financial conflict of interest
References
Abbreviations
-
- CMTMR
-
- 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine
-
- DIC
-
- differential interference contrast
-
- DTR
-
- diphtheria toxin receptor
-
- DTx
-
- diphtheria toxin
-
- ECFP
-
- enhanced cyan fluorescent protein
-
- EGFP
-
- enhanced green fluorescent protein
-
- EYFP
-
- enhanced yellow fluorescent protein
-
- LFA-1
-
- lymphocyte function associated antigen 1
-
- LN
-
- lymph node
-
- MVA
-
- modified vaccinia Ankara
-
- NA
-
- numerical aperture
-
- PE
-
- phycoerythtin
-
- pMHC
-
- peptide MHC
-
- SNARF
-
- seminaphtharhodafluor
-
- WIS
-
- Weizmann Institute of Science




