Immune checkpoints and their inhibition in cancer and infectious diseases
Abstract
The development of chronic infections and cancer is facilitated by a variety of immune subversion mechanisms, such as the production of anti-inflammatory cytokines, induction of regulatory T (Treg) cells, and expression of immune checkpoint molecules, including CTLA-4 and PD-1. CTLA-4, expressed on T cells, interacts with CD80/CD86, thereby limiting T-cell activation and leading to anergy. PD-1 is predominantly expressed on T cells and its interaction with PD-L1 and PD-L2 expressed on antigen-presenting cells (APCs) and tumors sends a negative signal to T cells, which can lead to T-cell exhaustion. Given their role in suppressing effector T-cell responses, immune checkpoints are being targeted for the treatment of cancer. Indeed, antibodies binding to CTLA-4, PD-1, or PD-L1 have shown remarkable efficacy, especially in combination therapies, for a number of cancers and have been licensed for the treatment of melanoma, nonsmall cell lung cancer, and renal and bladder cancers. Moreover, immune checkpoint inhibitors have been shown to enhance ex vivo effector T-cell responses from patients with chronic viral, bacterial, or parasitic infection, including HIV, tuberculosis, and malaria. Although the data from clinical trials in infectious diseases are still sparse, these inhibitors have great potential for treating chronic infections, especially when combined with therapeutic vaccines.
Introduction
Distinct subtypes of effector T cells, including CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ Th1, Th2, Th17 cells, mediate protective immunity against viruses, bacteria, fungi, parasites, and tumors. However, these effector T cells can also promote acute and chronic inflammation, which can lead to immunopathology or autoimmunity and therefore must be tightly regulated. Immune regulation is mediated by regulatory cells of the innate and adaptive immune system, including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), M2-type macrophages, and regulatory cytokines, including IL-10 and TGF-β, and by immune checkpoints that control T-cell activation. These regulatory cells, molecules, and immune checkpoints are often enhanced during cancer and chronic infections as a mechanism of immune subversion and have therefore become a very important therapeutic target in the treatment of cancer and also have potential to enhance the efficacy of cancer and infectious disease vaccines 1, 2.
T-cell exhaustion or dysfunction is now seen as a key mechanism contributing to impaired T-cell responses against tumors or pathogens. Exhausted T cells were first identified in the context of chronic infections; however, T cells with a similar phenotype were later identified in the tumor microenvironment 3. Exhausted T cells are functionally characterized by a loss of IL-2 production, reduced proliferative capacity, reduced cytotoxic capacity, and impaired production of pro-inflammatory cytokines 1. A major hallmark of T-cell exhaustion is enhanced expression of multiple immune checkpoints, such as PD-1, CTLA-4, lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and ITIM domain (TIGIT), and T-cell immunoglobulin-3 (TIM-3) 4. It has been demonstrated that T-cell function decreases with increased expression of immune checkpoints 4. The interplay of immune checkpoints and their ligands is complex and can occur at different stages of T-cell activation and function. For example, CTLA-4, LAG-3, TIM-3, and TIGIT primarily interact with their ligands during the T-cell priming stage, thereby limiting T-cell activation 5. On the other hand, PD-1 expression is upregulated on activated T cells and ligation of PD-1 with PD-L1 or PD-L2 predominantly occurs in the periphery leading to suppression of activated T cells at the effector phase 6. Importantly, immune checkpoints have distinct mechanisms and nonredundant roles, emphasizing the complexity of the regulation of T-cell responses.
Immune checkpoints control effector T-cell and NK-cell responses by multiple mechanisms
Immune checkpoints have distinct ligands and suppress T-cell function through multiple mechanisms. CTLA-4 was one of the first inhibitory receptors shown to play a role in suppression of T-cell responses 7. Two signals are required for T-cell activation: recognition of peptide antigen presented by MHC (signal 1) and costimulation through CD28 following binding to CD80 or CD86 expressed by APCs (signal 2) (Fig. 1A). CTLA-4 is structurally similar to CD28 and binds to CD80 and CD86 at a higher affinity than CD28 (Fig. 1B) 8. It has been suggested that CTLA-4 expression interferes with T-cell activation by reducing CD28 costimulation (Fig. 1B) 9, 10. An alternatively spliced variant of CTLA-4, which has a decoy function, can be secreted by T cells and exerts its inhibitory function independent of cell–cell interactions 11, 12. CTLA-4 is constitutively expressed on Treg cells, but can also be expressed on effector T cells following their activation 9, 13. However, unlike Treg cells, which express CTLA-4 on their cell surface, activated T cells mainly express CTLA-4 intracellularly 14. This differential expression suggests a dual function of CTLA-4. Expression of CTLA-4 by Treg cells serves as a mechanism of Treg cells to suppress excessive T-cell responses, while intracellular reservoirs of CTLA-4 prevent tissue damage by self-reactive pathogenic T cells 15. Other mechanisms of CTLA-4-mediated suppression of T-cell activation include an increase in T-cell motility, inhibition of TCR and CD28-induced genes, and reduction in CD25 expression 16-18. It has also been shown that high CTLA-4 expression on CD4+ T cells is crucial for the suppressive function of Treg cells 19. Blocking CTLA-4 in vivo has been shown to promote antitumor immunity by inhibiting Treg cells and enhancing effector T-cell function in a mouse model for melanoma 20.
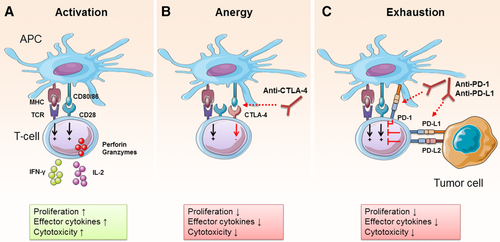
The inhibitory receptor PD-1 and its ligands PD-L1 have gained significance in recent years with the approval of several antibodies that target PD-1 or PD-L1 for the treatment of cancer 21. PD-1 is expressed on T cells, B cells, and certain myeloid cells; however, its role is best characterized in T cells. PD-1 expression on T cells is induced by antigen stimulation 1. Unlike CTLA-4, which limits early T-cell activation, PD-1 mainly exerts its inhibitory effect on T cells in the periphery where T cells encounter PD-1 ligands 6. Two ligands of PD-1 have been identified so far, PD-L1 and PD-L2 22, 23, which are expressed by a large range of cell types, including tumor cells, monocyte-derived myeloid dendritic cells (DCs), epithelial cells, T cells, and B cells. In cancer, tumor cells and myeloid cells are thought to be the main cell types mediating T-cell suppression through PD-1 ligation (Fig. 1C) 24. In addition, PD-L1 expressing γδ T cells from human pancreatic ductal adenocarcinoma were shown to suppress anti-tumor CTL and Th1 cells, which was reversed by blocking PD-1 25. However, it is still unclear whether the effects of PD-L1 and PD-L2 on PD-1 downstream signaling are dependent on the cell type that expresses the ligand. A recent study suggested that PD-L1 expressing murine NK cells can suppress DC maturation and activation, while having no direct effect on T-cell activation 26. To our knowledge, this is the first report to suggest that not all PD-L1 expressing cell types are capable of directly inhibiting T cells through PD-1 ligation. Moreover, there are differences between PD-L1- versus PD-L2-induced effects, which remain to be fully elucidated. A study by Liang et al. demonstrated that PD-L1−/− mice are resistant to Leishmania mexicana infection, while PD-L2−/− mice developed more exacerbated disease 27. Furthermore, a recent study found that PD-L2 expression on immune cells in malaria-infected mice was correlated with improved pathogen clearance by outcompeting PD-L1 for binding to PD-1, thus limiting T-cell exhaustion 28.
Several mechanisms of PD-1-mediated T-cell suppression have been proposed. Chemnitz et al. reported that PD-1 ligation inhibits T-cell activation only upon TCR engagement 29. PD-1 has intracellular immunoreceptor tyrosine-based inhibition motif (ITIM) and immunoreceptor tyrosine-based switch motif 29, and it has been shown that PD-1 ligation leads to the recruitment of the phosphatases src homology 2 domain containing tyrosine phosphatase (SHP)-1 and SHP-2 to the immunoreceptor tyrosine-based switch motif 29. SHP-2 affects T-cell function in a number of ways. Sheppard et al. demonstrated that SHP-2 interferes with TCR downstream signaling by preventing zeta-chain-associated protein kinase 70 and CD3ζ phosphorylation 30. Moreover, PD-1 ligation has been shown to interfere with signaling molecules, such as phosphatidylinositol-4,5-bisphosphate 3-kinase and Ras, which are important for T-cell proliferation, cytokine secretion, and metabolism 1, 31. Analysis of human immunodeficiency virus (HIV) specific T cells also demonstrated PD-1-dependent basic leucine zipper transcription factor upregulation, which inhibited T-cell function 32. Furthermore, Li et al. reported a correlation between SHP-2 and PD-1 expression in tumor-infiltrating lymphocytes from patients with head and neck cancer, which limited Th1-type responses 33. Ligation of PD-1 has also been shown to induce metabolic alterations in T cells. Mitochondrial dysfunction of intratumoral T cells has been correlated with enhanced expression of inhibitory receptors 34. Patsoukis et al. demonstrated metabolic reprogramming of T cells from glycolysis to lipolysis as a consequence of PD-1-mediated impairment of T-cell effector function 35. Bengsch et al. further demonstrated PD-1-induced defects in mitochondrial respiration and glycolysis, which led to impaired T-cell effector function that could be reversed by mammalian target of rapamycin inhibition 36. Since most of the identified mechanisms of PD-1-mediated T-cell suppression are based on in vitro or ex vivo experiments, it remains to be demonstrated that the same mechanisms are responsible for T-cell exhaustion in vivo 3.
In addition to inhibiting effector T-cell responses, the PD-1:PD-L1 axis has been implicated in promoting induction of Treg cells. This hypothesis was supported by in vitro studies showing enhanced Treg conversion in the presence of PD-L1-expressing DCs or PD-L1-coated beads 37, 38. Consistent with these findings, our group has demonstrated that blocking PD-1 suppresses TGF-β and retinoic acid (RA) induced Treg conversion from naïve T cells in vitro 39. Moreover, the treatment with a PD-1-blocking antibody in combination with a tumor vaccine significantly reduced Treg infiltration into CT26 tumors in vivo 39.
While there are many parallels between exhausted T cells in infection and cancer, recent studies have suggested that there are significant differences in the phenotype of T cells between these diseases 40. Schietinger et al. reported that during chronic lymphocytic choriomeningitis virus (LCMV) infections, exhausted T cells become eomesodermin (EOMES)hiPD-1hi and granzyme B (GZMB)hi, while dysfunctional T cells in oncogene-driven inducible liver tumors show a loss of EOMES expression and high levels of GZMB, PD-1, and LAG-3. Moreover, it was suggested that infection-induced exhaustion is reversible, while tumor-induced dysfunction of T cells is only reversible at premalignant and early malignant stages and irreversible at later stages 40.
LAG-3, TIM-3, and TIGIT represent the next generation of immune checkpoints that are being targeted in cancer immunotherapy. LAG-3 binds to MHC class II with higher affinity than CD4+, thereby compromising CD4+ T-cell activation 41. However, LAG-3 expression is also associated with impairment of CD8+ and NK-cell function, suggesting that there may be additional LAG-3 ligands 5. TIM-3 can be expressed by T cells, NK cells, but also myeloid cells, such as DCs and monocytes; binding of galectin-9 to TIM-3 on T cells has been shown to induce cell death 42. Binding of TIM-3 to high mobility group box 1 , a marker of immunogenic cell death that triggers innate cell responses by binding to pattern recognition receptors, prevents innate cell activation 43.
NK cells also express immune checkpoints and this may compromise their protective role in early immune responses against cancer and infectious diseases. Expression of PD-1 is enhanced on NK cells from patients with multiple myeloma 44 or infected with Mycobacterium tuberculosis (MTB) 45 or HIV 46. Blockade of PD-1 enhanced human NK-cell trafficking, effector function, and cytotoxicity against multiple myeloma tumor cells in a killing assay 44. Moreover, NK cells express the transmembrane glycoprotein CD226, which promotes NK-cell function and cytotoxicity upon binding to its ligand CD155 47. The immune checkpoint TIGIT is upregulated on activated NK cells and binds to CD155 at a higher affinity than CD226, thereby inhibiting CD226-mediated activation 48. Da Silva et al. reported that TIM-3 is an exhaustion marker on NK cells; NK cells from melanoma patients overexpressed TIM-3 and were functionally impaired, and this was reversed by treatment with an anti-TIM-3 antibody ex vivo 49.
Immune checkpoints in cancer
Effector and Treg cells in cancer
T cells, especially CTL and IFN-γ secreting Th1 and polyfunctional T cells, play a key role in controlling tumor growth 50. Upon presentation of their cognate antigen and priming by APCs, CD8+ T cells can kill tumor cells by multiple mechanisms. The key killing mechanism involves the release of perforin and granzymes, which disrupt the plasma membrane of tumor cells leading to their apoptosis. FAS ligand expression on T cells has been shown to induce apoptosis on target cells by binding to FAS, which induces a caspase-8 signaling cascade. In addition to direct cytotoxicity, activated CD8+ T cells release IFN-γ, which contributes to tumor cell death by the upregulation of the expression of MHC class I on tumor cells and by direct inhibition of tumor cell proliferation 51. The presence of CD8+ T cells in tumors correlated with a better prognosis in cancer patients, including melanoma, colon cancer, breast cancer, renal cell carcinoma (RCC), and ovarian cancer 52-59. However, a study on primary and metastatic clear cell RCC patients revealed that extensive infiltration of CD8+ T cells that expressed immune checkpoints and absence of functional mature DCs was associated with poor clinical outcome 60. Moreover, patients with preexisting CD8+ T-cell infiltration showed better responses to cancer immunotherapy 61, 62. It has also been reported that the removal of CD8+ T cells exacerbates tumor growth in murine tumor models 63-65.
Tolerance to antitumor immune responses is one of the biggest obstacles to endogenous tumor recognition by T cells and to the induction of antitumor immunity by immunotherapy. Tumor cells acquire mutations that render them resistant to the induction of cell death. Tumor cells downregulate MHC class I expression on their cell surface, which limits immune recognition by CD8+ T cells. Moreover, tumors create an immunosuppressive microenvironment by secretion of anti-inflammatory cytokine and molecules and the recruitment of suppressive immune cells, such as tumor-associated macrophages, MDSCs, and Treg cells.
Treg cell infiltration has been detected in mouse tumor models and in cancer patients. Depletion of Treg cells improved antitumor T-cell responses and reduced tumor growth in murine tumor models for melanoma and colon cancer 64, 66. Moreover, a higher ratio of Treg cells to CD8+ T cells has been associated with poorer disease outcome in cancer, suggesting that Treg-mediated T-cell suppression is an important mechanism by which tumor cells evade immune responses 67-69.
Treg cells’ enrichment in tumors is mediated by their recruitment from the periphery and expansion of tumor-resident Treg cells 70. Treg cells are attracted to the tumor bed by chemokines or vascular endothelial growth factor (VEGF), which are secreted by tumor cells or immune cells in the tumor microenvironment. A range of chemokines have been shown to attract Treg cells into tumors, including CCL17, CCL22, CCL28, and CXCL12 70, 71. CCR4 appears to be the dominant chemokine receptor in the context of cancer and blockade of CCR4-depleted Treg cells and enhanced pro-inflammatory immune responses in a humanized ovarian cancer model and in adult T-cell leukemia-lymphoma patients 72, 73. The immunosuppressive microenvironment of the tumor can promote expansion of Treg cells. Soluble molecules, such as IL-2, TGF-β, RA, and indoleamine 2,3-dioxygenase, contribute to intratumoral Treg expansion 70, 74. Moreover, tolerogenic DCs in the tumor microenvironment have been implicated in the induction of Treg cells 75, 76. A contributing factor for the activation of Treg cells in the tumor is the expression of self-antigens by tumor cells that can induce tolerance. Indeed, Treg cells that recognized tumor-associated antigens have been identified in cancer patients, such as NY-ESO-1 and survivin 77-79. Neoantigens that arise from mutations contribute to tumor cell transformation. Interestingly, tumors with low neoantigen expression have been shown to attract more Treg cells, whereas high neoantigen expression correlated with increased infiltration of CD8+ T cells and Treg depletion 69. The primary effect of Treg cells in tumor is the suppression of effector T cells and NK cells 80. Treg cells can also form a suppressive network by interaction with MDSCs and tumor-associated macrophages, which promote further Treg infiltration 70.
Prognostic value of immune checkpoints in cancer
T cells that show an exhausted or dysfunctional phenotype have been identified in several cancers, including chronic lymphocytic leukemia, ovarian cancer, nonsmall cell lung cancer (NSCLC), and melanoma 81-84. Moreover, the expression of multiple inhibitory ligands has been associated with reduced T-cell effector function in melanoma and ovarian cancer 82, 85.
There is increasing evidence from animal models and clinical data that PD-1 plays a crucial role in limiting anti-tumor T-cell responses. A meta-analysis of 12 different epithelial cancers revealed significantly shorter overall survival for patients with PD-1-positive tumor-infiltrating lymphocytes 86. Analysis of a correlation between PD-L1 expression and disease outcome revealed mixed results. Aberrant PD-L1 expression on tumor cells has been associated with immune escape in multiple cancers, indicating that PD-L1 structural differences contribute to tumor development 87. The expression of PD-L1 correlated with worse prognosis in melanoma, RCC, and hepatocellular carcinoma patients 88-90. No correlation between PD-L1 expression and prognosis has been detected in NSCLC patients 91, 92. In contrast, Dunne et al. found a correlation between PD-L1 expression and immune cell infiltration in colorectal cancer patients and PD-L1 expression correlated with better survival 93. Similarly, patients with PD-L1 positive breast cancers had a better prognosis than patients with PD-L1 negative tumors 94. However, it has been suggested that the expression of PD-L1 is due to enhanced antitumor immune responses, which is associated with improved patient prognosis in some cancers.
There is little evidence to date of a correlation between PD-L2 expression and cancer prognosis. This is in part due to the fact that PD-L1 expression on tumors is more prevalent than PD-L2 expression. No correlation was found in breast cancer patients 94, while PD-L2 expression correlated with a negative prognosis in hepatocellular carcinoma patients 90.
The expression of CTLA-4 has been implicated in limiting anti-tumor T-cell responses. However, the data on CTLA-4 as a prognostic marker are inconclusive. Overexpression of CTLA-4 has been associated with worse prognosis and higher clinical stages in patients with nasopharyngeal carcinoma and breast cancer, respectively 95, 96. In contrast, CTLA-4 expression was associated with a better prognostic impact in NSCLC and B-cell chronic lymphocytic leukemia 97, 98.
Immune checkpoint inhibitors in cancer therapy
The suppression of anti-tumor T-cell responses is largely mediated by Treg cells and immune checkpoints, such as CTLA-4 and PD-1 (Fig. 2A). Immune checkpoint inhibitors have been licensed for use in humans with cancer, notably melanoma, and are predominantly monoclonal antibodies against immune checkpoints on immune cells that block the interaction with the respective checkpoint ligands. The primary aim of immune checkpoint blockade is to reduce suppression of effector T cells, especially CD8+ T cells, and thereby improve tumor-specific immune responses. Moreover, immune checkpoint blockade has the potential to enhance the effectiveness of other immunotherapeutic approaches, such as cancer vaccines, by taking the brake off T-cell suppression and thus enhancing T-cell effector function (Fig. 2B). Antibodies have been developed for most of these inhibitory receptors and tested in preclinical models and clinical trials and some of these have now been licensed for use in humans 99.
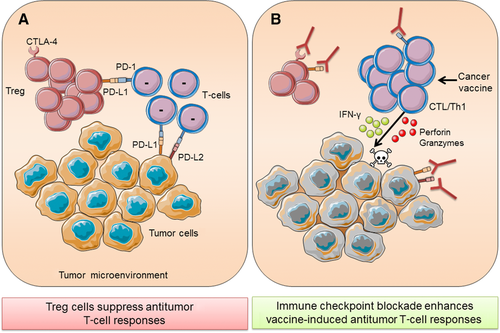
CTLA-4 was the first immune checkpoint that was targeted in the treatment of cancer. Preclinical studies demonstrated that blocking of CTLA-4 reduced tumor growth in mouse models of melanoma, colon carcinoma, and many others 100, 101. The efficacy of CTLA-4 therapy in preclinical models was associated with increased T-cell infiltration into tumors 101. Based on these findings, antibodies targeting CTLA-4 were tested in clinical trials. One of these antibodies, ipilimumab, was the first checkpoint inhibitor to be approved by the U.S. food and drug administration (FDA) in 2011. It was approved for treatment of metastatic melanoma and has shown great success in patients, increasing the median overall survival by 10 months 102. In a parallel study, treatment with ipilimumab and dacarbazine led to superior 1-, 2-, and 3-year survival compared with dacarbazine alone 103. Pooled analysis from 12 clinical trials involving ipilimumab revealed survival of up to 10 years in some melanoma patients 104. Ipilimumab has also been tested in other malignancies, including NSCLC, renal cancer, and prostate cancer. However, these trials did not meet the projected clinical endpoints 21. The efficacy of ipilimumab in a cohort of patients has provided evidence that reactivation of T cells could lead to antitumor responses in patients.
Preclinical studies with PD-1- or PD-L1-blocking antibodies have demonstrated a reduction in tumor growth in several murine tumor models 101, 105-107. Mouse models using PD-1 antibodies showed that protection was associated with increased CD8+ T-cell infiltration and function 101, 107, 108. Moreover, the ratio of CD8+ T cells to Treg cells was elevated when PD-1 was blocked 107. Furthermore, blocking PD-1 enhanced T-cell infiltration into tumors by increasing the secretion of IFN-γ-inducible chemokines 109.
PD-1- and PD-L1-blocking antibodies have been tested in the clinic, and remarkable responses were observed for patients with melanoma and NSCLC. To date, three antibodies have been approved by the FDA that target the PD-1:PD-L1 axis. The first antibody targeting PD-1 pembrolizumab (Merck) was approved in September 2014 for the treatment of advanced or unresectable melanoma in patients failing other treatments and later became the first-line treatment 110. Pembrolizumab is now also approved for NSCLC 111. A second PD-1-blocking antibody nivolumab (Bristol-Myers Squibb) has been approved for the treatment of melanoma, NSCLC, and renal cancer 21. Moreover, atezolizumab (anti-PD-L1; Genentech) has been approved by the FDA in May 2016 for the treatment of bladder cancer 112. Anti-PD-1 therapy has also shown some evidence of efficacy against head and neck, breast, ovarian, and gastric cancers 21.
A study in melanoma patients that directly compared ipilimumab (anti-CTLA-4) with pembrolizumab (anti-PD-1) revealed better responses in the pembrolizumab-treated cohort, with a 12-month survival rate of 68–74% compared with 58% in patients treated with ipilimumab 113. Moreover, the combination of ipilimumab and nivolumab induced significant responses in untreated melanoma patients and was more effective than either treatment alone 114. A recent study reported that patients that showed prolonged benefit from ipilimumab also showed improved outcomes from subsequent treatment with pembrolizumab 115. These findings suggest nonredundant functions for CTLA-4 and PD-1. This is consistent with the evidence that these immune checkpoints exert their effects at different stages of T-cell activation, with CTLA-4 working mainly at the T-cell priming stage in the lymph nodes, whereas PD-1 mainly affects T cells in the periphery 116. This suggests that CTLA-4 blockade increases the pool of activated T cells in the lymph nodes, but does not prevent immune suppression in the tumor microenvironment, whereas PD-1-blocking antibodies prevent T-cell exhaustion at the tumor site.
Although checkpoint inhibitors are proving successful in the treatment of certain cancers, they are associated with significant and frequent side effects, in particular autoimmune syndromes, including colitis, skin disorders, and hepatitis 21. Blockade of CTLA-4 and PD-1 induced similar adverse effects; however, high-grade adverse effects were less frequent in patients treated with pembrolizumab (10.1–13.3 %) when compared with ipilimumab (19.9 %) 113.
The field of immune checkpoint inhibition has progressed to other targets, with recent preclinical studies and clinical trials assessing inhibitors that target TIM-3, LAG-3, and TIGIT. Preclinical studies using mouse models have demonstrated significant reduction in tumor growth using blocking antibodies against the immune checkpoints LAG-3 and TIM-3 106, 108, 117-119. Moreover, TIGIT−/− mice showed a significant reduction in B16 and MC38 tumor growth 120. To our knowledge, no clinical trials have yet assessed TIGIT-targeted therapies. However, two trials with anti-TIM-3 antibodies are currently ongoing in advanced malignancies and metastatic solid tumors (NCT02609268, NCT02817633). Moreover, IMP321 (anti-LAG-3) is being tested in melanoma (NCT02676869), renal cancer (NCT00351949), and breast cancer (NCT02614833). Studies using BMS-986016 (anti-LAG-3) alone or in combination with anti-PD-1 are currently recruiting patients with advanced solid tumors and hematologic malignancies (NCT01968109, NCT02061761). These studies are still in phase 1 and aimed at testing the safety in a small cohort of patients with advanced malignancies. The next years will show whether these novel immune checkpoint inhibitors are able to compete with PD-1-blocking antibodies and whether there will be synergistic effects when combined.
Immune checkpoint inhibitor combination therapies for cancer
Although monotherapies with CTLA-4- or PD-1-blocking antibodies have significantly prolonged survival of some patients with certain cancers, the majority of patients did not respond. The observation that combined treatment with ipilimumab and nivolumab induced better responses than either treatment alone paved the way for the search of effective checkpoint therapy combinations and several combination strategies are now under investigation 114. Experiments in murine tumor models have demonstrated that, due to nonredundant mechanisms, different checkpoint inhibitors can work synergistically. This has been shown for combinations of anti-PD-1 with anti-CTLA-4, anti-TIM-3, or anti-LAG-3 101, 106-108. Moreover, combination of anti-PD-1 with an activating antibody against the costimulatory receptor 4-1BB improved antitumor responses in a mouse model of colon carcinoma 121.
Cancer vaccines typically consist of a source of cancer antigens and adjuvants that activate innate immune cells, especially DCs. Cancer vaccines aim to generate tumor-specific T cells that can kill tumor cells through secretion of IFN-γ or lytic granules (Fig. 2B). Combinations of immune checkpoint blockade with immune activators, such as cancer vaccines, could be particularly favorable for patients with tumors that lack T-cell infiltration. There is some evidence from animal models to support this hypothesis. Curran et al. demonstrated that blocking PD-1 and CTLA-4 significantly improved the efficacy of a vaccine comprising irradiated Flt3 ligand secreting B16 cells 107. The reduction in tumor growth was associated with an elevated CD8/Treg ratio in B16 tumors 107. Similarly, combination of a GM-CSF-CT26 vaccine with anti-PD-L1 and anti-CTLA-4 significantly reduced tumor growth in the CT26 tumor model 101. Moreover, the addition of either anti-CTLA-4 or anti-PD-1 improved the efficacy of a vaccine comprising tumor lysates, GM-CSF, and CpG in the B16 tumor model 122. The inclusion of anti-PD-1 or anti-CTLA-4 significantly elevated the numbers of infiltrating IFN-γ-secreting CD8+ T cells compared with mice that were treated with the vaccine alone 122. Moreover, we have recently shown that PD-1 blockade significantly enhanced the efficacy of a TLR-adjuvanted cancer vaccine in the CT26 model and induced potent anti-tumor memory, protecting mice from tumor growth upon rechallenge 39. The protective effect was associated with enhanced effector T-cell responses and a reduction in Treg infiltration 39.
Immune checkpoint blockade has also been shown to improve the efficacy of therapies that do not directly target the immune system, such as radiotherapy and targeted cancer therapies 123-125. Very promising results were achieved with combinations of immune checkpoint blockade and histone deacetylase inhibitors (HDACi). HDACi induce direct tumor cytotoxicity and improve tumor immunogenicity and are approved for the treatment of cutaneous T-cell lymphoma, peripheral T-cell lymphoma, and malignant myeloma 126. Woods et al. found that HDACi also induce the expression of PD-L1 on murine and human melanoma cells. Consequently, the addition of anti-PD-1 significantly improved HDACi-induced reduction in tumor growth 127. Moreover, Zheng et al. suggested that HDACi enhance therapeutic effects of PD-1 blockade by stimulating the secretion of T-cell attracting chemokines into the tumor microenvironment 128.
Immune checkpoints in infections
Chronic and acute viral infections
Pathogens can exploit immune checkpoints to limit host-protective antigen-specific immune responses. Some of the first descriptions of T-cell exhaustion came from studies of T-cell responses in chronic infections. T-cell exhaustion is mainly a hallmark of persistent infections rather than in acute infections that are usually rapidly cleared by the immune system. Acute infections with chronic LCMV-induced potent activation of pathogen-specific CD8+ T cells 129. However, persistent exposure to viral antigens during chronic LCMV infection caused T-cell reprogramming leading to high PD-1 expression and T-cell exhaustion 129. Although many molecular mechanisms have been implicated in the induction of T-cell exhaustion, persistent antigen exposure seems to be a main driver of T-cell exhaustion during chronic infections 3, 40. Studies of LCMV infection in mice demonstrated a correlation between antigen load and functional impairments in virus-specific CD8+ T cells 130. Moreover, lack of CD4+ T-cell help and inhibitory receptor ligation have been implicated in the induction of T-cell exhaustion 3. Blocking the inhibitory receptor PD-1 restored CD8+ T-cell function in mice with chronic LCMV infection, demonstrating that T-cell exhaustion is reversible 129. Blockade of PD-L1 also enhanced the efficacy of a therapeutic vaccine against chronic LCMV infection, which was effective even in the absence of CD4+ T-cell help 131.
Several studies have suggested that blockade of immune checkpoints, such as PD-1, may lead to a reversion of T-cell dysfunction in the context of chronic infection with HIV, hepatitis B virus (HBV), or hepatitis C virus (HCV). PD-1 is upregulated on HIV-specific CD8+ T cells and PD-1 expression correlated with disease progression 132. Blockade of PD-L1 ex vivo enhanced IFN-γ secretion by HIV-specific CD8+ T cells, suggesting that PD-1 signaling might play a role in limiting T-cell responses against HIV 132-134. In a recent study, Gill et al. identified PD-L1 expressing cells in the lymph nodes of HIV-infected patients 135. Although blockade of PD-L1 in simian immunodeficiency virus (SIV) infected rhesus macaques did not significantly improve antiviral responses, it did result in a minor delay in viral rebound after combined antiretroviral therapy 135. Moreover, a study by Chew et al. suggested a role for the immune checkpoint TIGIT in limiting antiviral T-cell responses in HIV 136. TIGIT binds to its receptor CD155, which sends a negative signal to TIGIT expressing cells 137. TIGIT expression was enhanced on T cells from HIV patients and SIV-infected macaques and was coexpressed with PD-1 136. Dual blockade of TIGIT and PD-L1 ex vivo markedly restored CD8+ T-cell proliferation, but failed to restore effector cytokine secretion 136. These studies suggest that immune checkpoints may limit T-cell responses during HIV infection and that immune checkpoint blockade might be beneficial in HIV-infected patients. Indeed, a recent study has suggested that immune checkpoint inhibitors may be beneficial in enhancing T-cell responses to HIV patients. Eron et al. showed that anti-PD-L1 antibody treatment enhanced HIV-specific CD8+ T-cell responses in two of eight HIV-infected patients on antiretroviral therapy 138. Larger studies will be required to determine the therapeutic benefit of immune checkpoint blockade in HIV-infected individuals on retroviral therapy.
There is circumstantial evidence that blockade of PD-1 or TIM-3 may reverse dysfunctional T-cell responses in HBV infection. Similar to that found during HIV infection, PD-1 is upregulated on CD8+ T cells in HBV patients, and this correlates with viral titers 139. Antiviral therapy in the form of oral direct antiviral agents (telbivudine or lamivudine) reduced expression of PD-1 on T cells, which was associated with enhanced IFN-γ and reduced IL-10 secretion 140. Blockade of PD-1 during ex vivo restimulation of PBMCs from patients with chronic HBV infection increased IFN-γ secretion by CD4+ but not CD8+ T cells 141. Similarly, TIM-3 overexpression was associated with reduced IFN-γ secretion by CD8+ T cells from HBV-infected patients and this was partially enhanced by blocking the TIM-3 pathway 142. Thus, PD-1 overexpression seems to impair antiviral T-cell responses during HBV infection; however, the efficacy of anti-PD-1 or other immune checkpoint blockade strategies remains to be demonstrated in HBV-infected patients.
Consistent with other chronic viral infections, PD-1 has been implicated in the regulation of T-cell responses during HCV infection. Blockade of PD-1 blockade ex vivo successfully reactivated HCV-specific T cells 143. However, it is still unclear whether PD-1 blockade is capable of reversing T-cell exhaustion in vivo in infected patients. In a study with HCV-infected chimpanzees, only one in three infected animals responded to anti-PD-1 treatment with reduced viremia and improved HCV-specific T-cell responses 144. Similarly, single-dose anti-PD-1 treatment induced significant suppression of viral replication in 11% of HCV-infected patients 145. It has been argued that preexisting virus-specific T cells in the liver are essential for the enhancement of T-cell responses by PD-1 blockade 144. It is possible that a combination of immune checkpoint blockade with a therapeutic vaccine, which induces virus-specific T-cell responses, might be more effective.
While the role of immune checkpoints and T-cell exhaustion has been well established in the context of chronic infections, it is still unclear whether immune checkpoints can lead to T-cell exhaustion in acute infections. Immune-competent hosts effectively clear most acute viral infections and generate long-lasting T-cell memory, suggesting that T-cell exhaustion does not occur, or at least does not prevent, effective anti-viral responses during transient infection 146. However, PD-1 expression was shown to be upregulated on CD8+ T cells after infection with a highly pathogenic strain of influenza virus, which was associated with delayed viral clearance and reduced survival in mice 147. Moreover, repeated injections of mice with the pathogenic PR8 strain of influenza virus caused T-cell exhaustion similar to chronic infections, suggesting that chronic exposure to viral antigens is a driver of exhaustion 148. Thus, exhaustion of CD8+ T cells may be contributing to the establishment of chronic and highly pathogenic transient infections.
Parasitic and bacterial infections
Most parasitic infections and many bacterial infections are not cleared by the host and become chronic largely as a result of pathogen-mediated immune subversion strategies evolved to prolong their survival in the host (Fig. 3A). These mechanisms include parasite induction of anti-inflammatory molecules, such as TGF-β, IL-10, and RA 149. Moreover, there is evidence that parasites enhance the expression of immune checkpoints and their ligands and induce Treg cells that suppress effector T-cell responses. Induction of Treg cells has been demonstrated for a range of helminth parasites, including Schistosoma mansoni, Fasciola hepatica, and Heligmosomoides polygyrus 149. T-cell exhaustion and increased PD-1 expression on T cells has been observed in mice infected with Leishmania donovani and humans infected with L. mexicana 150, 151. Moreover, some parasites have been shown to induce the expression of PD ligands on DCs. For example, S. japonicum infection promotes TLR-2-mediated PD-L2 expression on DCs, which can suppress immune responses by binding to PD-1 expressing cells 152. Moreover, Joshi et al. found enhanced PD-1 expression on T cells and increased PD-L1 expression on splenic DCs in L. donovani infected mice 150. Blockade of immune checkpoints thus has the potential to enhance antiparasitic immune responses (Fig. 3B). Blockade of CTLA-4 restored antiparasitic T-cell responses in mice infected with Plasmodium berghei ANKA, L. donovani, and Leishmania chagasi 153-155. Blockade of PD-1:PD-L1 improved antiparasitic responses against Toxoplasma gondii, L. donovani, and Plasmodium falciparum 150, 156, 157. The expression of PD-1 and LAG-3 on T cells has been associated with impaired clearance of malaria infections, which was restored by blocking PD-1, PD-L1, or LAG-3 157. Moreover, blockade of PD-L1 and LAG-3 increased IFN-γ secretion by CD4+ T cells in response to malaria antigens 158. In contrast to previous reports of an immunosuppressive role of PD-L2, a recent study suggested a positive role of PD-L2 in promoting antimalarial T-cell responses 28. The findings suggested that PD-L2 outcompetes PD-L1 for binding to PD-1, thereby preventing T-cell exhaustion.
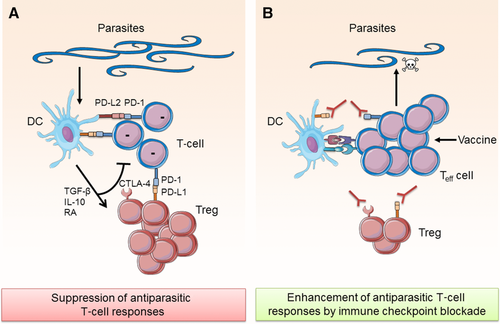
Immune checkpoints have been shown to play a role in the clearance of Helicobacter pylori infection. Expression of PD-L1 is enhanced on gastric epithelial cells during H. pylori infection 159 and PD-L1 expression is associated with an increase in Treg cells 160. However, PD-1−/− mice are more susceptible to T. gondii infection and show increased IL-10 secretion by T cells, demonstrating a potential anti-inflammatory outcome in the absence of PD-1 161.
In MTB infections, the role of the PD-1/PD-L1 pathway is controversial due to conflicting findings from mice and humans. It has been suggested that MTB evades immune responses by promoting PD-L1 expression on DCs, which enhances the induction of Treg cells 162. However, PD-1−/– mice were more susceptible to MTB-induced mortality, and this was associated with excessive inflammation and uncontrolled bacterial proliferation in the lungs 163. Sakai et al. reported that IFN-γ producing CD4+ T cells play a minor role in controlling MTB proliferation in the lungs but are responsible for 80% of bacterial clearance in the spleens 164. They demonstrated that PD-1 ligation limits IFN-γ production by CD4+ T cells that protected mice from exacerbated pulmonary MTB infection and early host mortality 164. These findings suggest a protective role of PD-1 downstream signaling in CD4+ T cells during MTB infections, at least in mice.
In patients with active MTB infections, PD-1 was found to be increased on CD4+ T cells but not CD8+ T cells compared to healthy controls 165, 166. Effective drug treatment in active TB patients was associated with a decrease in PD-1 expression on effector T cells 166. Moreover, PD-L1 and PD-L2 were upregulated on monocytes of active TB patients compared to healthy controls 165. Blockade of the PD-1/PD-L1 pathway on CD4+ T cells and macrophages enhanced phagocytosis and intracellular killing of MTB in vitro, suggesting a negative regulatory role for PD-1 in MTB infections 165. However, to date there are no reports on PD-1/PD-L1 blockade in patients to confirm the potential of immune checkpoint blockade in the treatment of MTB infection in humans.
To date, the findings of recovery of exhausted T cells from patients with chronic infections are mostly limited to ex vivo treatment of T cells. Clinical trials with immune checkpoint inhibitors in combination with antiviral, antibacterial, or antiparasitic therapies or vaccines are needed to fully evaluate the efficacy of such combinations in humans.
Conclusions
The approval of ipilimumab (anti-CTLA-4) for the treatment of melanoma in 2011 marked a paradigm shift in cancer immunology research and placed immunotherapy at the forefront of new treatment approaches for cancer. Immune checkpoints have been identified as major obstacles to the induction of antitumor immune responses. The expression of inhibitory receptors on T cells, such as PD-1 and CTLA-4, contributes to dysfunctional effector T-cell responses. Similarly, immunotherapies that activate antitumor immunity often fail to induce long-lasting responses due the suppressive effects of immune checkpoints. Therefore, therapies that promote the activation of antitumor T-cell responses in combination with immune checkpoint blockade are likely to improve the therapy efficacy in many settings. The outcome of a number of ongoing current clinical trials focused on immune checkpoint inhibitor combinations (https://clinicaltrials.gov/) will give important insight into which combinations are suitable for the different cancer indications and eventually which cohorts of patients within that indication.
In the field of infectious diseases, immune checkpoints constrain protective immunity during many chronic infections, but therapies based on immune checkpoints inhibition have not been tested or developed to the same extent as they have in cancer. It is clear that most if not all successful pathogens have evolved mechanisms to evade and suppress host protective immune responses. There are established roles for Treg cells and the immunosuppressive cytokines IL-10 and TGF-β in constraining antipathogen effector T-cell responses 167, but there is also expanding evidence of a role for immune checkpoints, as well as regulatory cells of the innate immune system, including type 2 macrophages, tolerogenic DCs, and eosinophils. The use of immune checkpoint inhibitors may help to reverse the state of immune suppression that is common in chronic infection, especially with parasite infections, and have significant potential to promote healing immune responses to parasites. However, perhaps the greatest potential lies in combining immune checkpoint blockade with therapeutic vaccination. Indeed, there is proof-of-principle from studies that have demonstrated that vaccine-induced immune responses can be enhanced by depleting Treg cells or by blocking the production or function of immunosuppressive cytokines 168, 169. While blocking Treg cells or immune checkpoints is unlikely to be used for enhancing the efficacy of routine prophylactic vaccination, it does have significant potential to enhance the efficacy of therapeutic vaccines against many chronic infections, such as malaria, tuberculosis, and HIV.
Acknowledgements
This work was supported by a research grants from Science Foundation Ireland (11/PI/1036 and 12/RI/2340) to K.H.G.M.
Conflict of interest
Kingston H.G. Mills is a cofounder and minority shareholder in Opsona Therapeutics Ltd.; he has received honoraria, for giving educational presentations, from Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Cellgene, Sanofi Pasteur, and Novartis. L.D. declares no commercial or financial conflict of interest.
References
Abbreviations
-
- HBV
-
- hepatitis B virus
-
- HDACi
-
- histone deacetylase inhibitors
-
- LAG-3
-
- lymphocyte activation gene-3
-
- LCMV
-
- lymphocytic choriomeningitis virus
-
- MDSCs
-
- myeloid-derived suppressor cells
-
- MTB
-
- Mycobacterium tuberculosis
-
- NSCLC
-
- nonsmall cell lung cancer
-
- RA
-
- retinoic acid
-
- RCC
-
- renal cell carcinoma
-
- TIGIT
-
- T-cell immunoglobulin and ITIM domain
-
- TIM-3
-
- T-cell immunoglobulin-3