The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses
Abstract
IL-1 drives Th responses, particularly Th17, in host defense. Sharing the same co-receptor, the IL-1 family member IL-36 exhibits properties similar to those of IL-1. In the present study, we investigated the role of IL-36 in Aspergillus fumigatus-induced human Th responses. We observed that different morphological forms of A. fumigatus variably increase steady-state mRNA of IL-36 subfamily members. IL-36α is not significantly induced by any morphological form of Aspergillus. Most strikingly, IL-36γ is significantly induced by live A. fumigatus conidia and heat-killed hyphae, whereas IL-36Ra (IL-36 receptor antagonist) is significantly induced by heat-killed conidia, hyphae, and live conidia. We also observed that IL-36γ expression is dependent on the dectin-1/Syk and TLR4 signaling pathway. In contrast, TLR2 and CR3 inhibit IL-36γ expression. The biological relevance of IL-36 induction by Aspergillus is demonstrated by experiments showing that inhibition of the IL-36 receptor by IL-36Ra reduces Aspergillus-induced IL-17 and IFN-γ. These data describe that IL-36-dependent signals are a novel cytokine pathway that regulates Th responses induced by A. fumigatus, and demonstrate a role for TLR4 and dectin-1 in the induction of IL-36γ.
Introduction
The IL-1 family (IL-1F) consists of 11 members 1. The in silico discovery of IL-1 family members IL-1F5, IL-1F6, IL-1F8, and IL-1F9 have recently been renamed IL-36 receptor antagonist (IL-36Ra), IL-36α, IL-36β, and IL-36γ, respectively. Presently, there is a paucity of data on the biological properties and functional roles of the IL-36 subfamily in health and disease. The classic IL-1 family members IL-1α and IL-1β as well as IL-18 and IL-33 polarize Th responses 1. However, little is known about the role of the IL-36 subfamily (IL-36α, IL-36β, and IL-36γ) and the IL-36Ra in antimicrobial host defense. Each member of the IL-36 subfamily binds to IL-1Rrp2 (now renamed IL-36 receptor, IL-36R) and recruits the co-receptor for signal transduction, IL-1RAcP 2, 3. The IL-36R is expressed on dendritic cells (DCs) 4, 5 and T cells 5. Stimulation of T cells by IL-36R ligands results in the induction of IFN-γ, IL-17, and IL-4 5, and DCs primed with IL-36 ligands are inducers of Th1 cells. Although the synthetic TLR3 ligand polyI:C induces IL-36γ secretion 6, nothing is known about the innate pattern recognition pathways that induce IL-36R ligands in the antimicrobial host defense.
The opportunistic fungus Aspergillus fumigatus is ubiquitous; Aspergillus spores are inhaled daily by humans. Inhaled Aspergillus spores (conidia) are efficiently cleared in healthy individuals 7. However, in immunocompromised persons, or in patients with preexisting lung injury, Aspergillus conidia germinate resulting in invasive infections associated with high mortality 8. Th responses are important in the host defense against invasive aspergillosis 9, and the major known cytokine pathways that are involved in the induction of Th1 and Th17 responses are IL-12/IL-18 and IL-1/IL-23, respectively 9, 10. Next to the induction of the specific Th-axis, there is also interplay between these cytokine pathways. IFN-γ-induced IL-18 binding protein was found to downregulate the Th17 response 11, and in several cases the induction of Th17 or IL-17 producing γδT cells was found to be dependent on IL-18 12, 13
In the present study, we have focused on gene expression of the IL-36 subfamily by different morphological phenotypes of A. fumigatus. In addition, we studied the IL-36R pathway in the induction of proinflammatory Th responses induced by A. fumigatus. Furthermore, we aimed to identify which pattern recognition pathways are involved in the induction of IL-36γ during Aspergillus-specific host defense. Here we demonstrate that the IL-36R pathway is involved in the regulation of Aspergillus-induced proinflammatory Th cytokine responses, and that the most important pathways for the induction of IL-36γ in Aspergillus host defense are represented by the dectin-1/Syk and TLR4 pathway.
Results
Aspergillus fumigatus induces the Th cell cytokines IL-17, IL-22, IFN-γ, and IL-13
To study the effect of IL-36 on proinflammatory T-cell responses induced by A. fumigatus, we first investigated the capacity of conidia and hyphae to induce in human PBMCs the production of the Th17 cytokines IL-17 and IL-22, and of the Th1 cytokine IFN-γ, the Th2 cytokines IL-4 and IL-13 and the anti-inflammatory cytokine IL-10. Increasing numbers of heat-inactivated (HI) conidia were added to PBMCs and after 7 days of incubation, IL-17, IL-22, IFN-γ, IL-4, IL-1, and IL-10 levels were determined in the culture supernatants. As shown in Figure 1A, 1 × 107/mL HI conidia or hyphae induced a IL-17, IL-22, and IFN-γ response. IL-13 was induced by 1 × 107/mL conidia, but not by hyphae (Fig. 1A), and production of IL-4 and IL-10 was undetectable after challenge with the fungus (data not shown).

Aspergillus fumigatus-induced Th17 cells and FoxP3 cells
We investigated whether Aspergillus-induced cells with a typical Th17 phenotype (IL-17+RORγt+ CD4+ cells). We gated on CD4+ T cells and subsequently looked at the presence of intracellular IL-17 in PBMCs stimulated with medium or with HI Aspergillus conidia, which was increased after stimulation with Aspergillus (Fig. 1B). Subsequently, we gated on the IL-17+ CD4+ cells and measured expression of RORγt (Fig. 1C). Compared with medium-stimulated PBMCs, Aspergillus-stimulated cells demonstrate a significant increase of RORγt+IL-17+ CD4+ cells (Fig. 1D). Of the Aspergillus-stimulated cells 83.4% (SD ±7.7) IL-17+ CD4+ cells were RORγ+, compared with nonstimulated cells 32.8% (SD ± 13.2). In addition to the RORγ+ Th17 cells, we investigated whether Aspergillus-induced FoxP3+ cells. Compared with medium only, Aspergillus induced a significant expansion of the FoxP3+ population (Fig. 1E).
Aspergillus fumigatus increases steady state levels of IL-36α, IL-36β, IL-36γ, and IL-36Ra mRNA
We next assessed whether A. fumigatus induces the IL-36 receptor ligands and IL-36Ra. The Aspergillus cell wall is highly dynamic and therefore will express different PAMPs at different stages of infection. Due to a hydrophobic (rodlet) protein layer that covers the resting conidia of A. fumigatus, few PAMPs will be exposed 14. When live conidia are incubated at 37°C in RPMI culture medium they initiate swelling after 2–4 h, and form germtubes after 6–8 h (Fig. 2A). During the swelling and germination phase, the hydrophobic rodlet layer is lost exposing PAMPs 14. To investigate the ability of different morphological forms of A. fumigatus to induce IL-36 family members, we compared resting conidia which are generaly considered to be poorly immunogenic, with live conidia that are allowed to germinate and expose numerous PAMPs in the process, and hyphae that are exposing numerous PAMPs. Human PBMCs were stimulated the morphological forms for 4, 8, and 24 h. IL-36α expression was only significantly induced by HI Aspergillus hyphae 4 h after stimulation, conidia (live and HI) did not significantly induce IL-36α expression (Fig. 2B). IL-36β was only significantly induced by heat-killed A. fumigatus conidia (Fig. 2C). Although stimulation with HI conidia did not result in a significant upregulation of IL-36γ expression, PBMCs stimulated with live conidia or with HI A. fumigatus hyphae significantly increased IL-36γ mRNA levels (Fig. 2D). mRNA of IL-36Ra was significantly induced by stimulation with all morphological forms of Aspergillus and ranged from 15 to 120 fold increase; dependent on the time of measurement and morphological form of Aspergillus. Overall, the expression of mRNA for the IL-36 cytokines was maximal at 8 h after stimulation (Fig. 2).
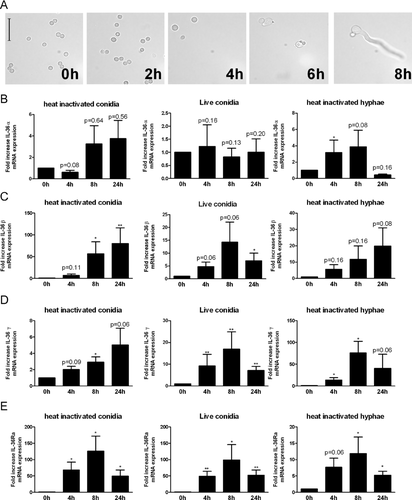
IL-36Ra inhibits Aspergillus-induced IL-17 and IFN-γ responses
To elucidate the role of IL-36R in the induction of the Aspergillus-specific Th1 and Th17 responses, we added IL-36Ra to block the IL-36R pathway. Human PBMCs were stimulated with A. fumigatus conidia and hyphae in the absence or presence of IL-36Ra at increasing concentrations from 0.1 to 100 ng/mL. We observed that IL-36Ra significantly inhibited Aspergillus-induced IL-17 and IFN-γ production, especially in the lower range of the dose response (100 pg/mL to 1 ng/mL) (Fig. 3A and B). The production of IL-22 was only significantly affected at a concentration of 100 pg/mL IL-36Ra (Fig. 3A and B). IL-36Ra did not modulate IL-13 (Fig. 3A) and proliferation of FoxP3+ cells (Fig. 3C) induced by A. fumigatus conidia.

Inhibition of endogenous IL-36Ra increases Aspergillus-induced IL-17 and IL-22
Compared with other fungal pathogens Aspergillus is a relatively poor IL-17 inducer. Since, we observed that A. fumigatus conidia were potent inducers of IL-36Ra mRNA expression, we wondered whether this relatively low IL-17 induction might be due to high induction of endogenous IL-36Ra in the cultures. To investigate this, PBMCs were stimulated with A. fumigatus conidia and endogenous IL-36Ra was inhibited using an anti-human IL-36Ra blocking antibody. Aspergillus was able to induce significantly higher IL-17 and IFN-γ levels in the stimulations where IL-36Ra was inhibited (Fig. 3D). Inhibition of IL-36Ra in medium-stimulated cells did not result in significant IL-17 induction.
IL-36 ligands cannot act as surrogates for IL-1β or IL-18
The IL-36R is expressed on CD4+ T cells and stimulation with IL-36 ligands results in the induction of IL-17, IFN-γ, and IL-4 5. In addition IL-1 family members IL-1β and IL-18 can induce IL-17 or IFN-γ, respectively, in the presence of STAT activators, such as IL-12 and IL-23 15. To investigate whether IL-36 ligands can directly induce Th cytokines we stimulated isolated CD4+ T cells with increasing concentrations of IL-36γ or IL-36β. We did not observe any detectable cytokine concentrations of IL-17 or IFN-γ in the supernatants (Supporting Information Fig. 1). When IL-36γ or IL-36β was combined with IL-12 or IL-23, we could not detect IL-17 or IFN-γ production, in contrast to the combinations of IL-1/IL-23 and IL-18/IL-12 (Fig. 4A).
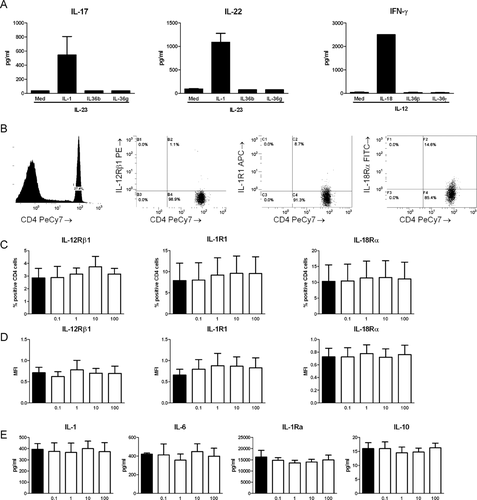
Furthermore, we investigated whether the observed differences in IL-17 and IFN-γ production were due to downregulation of the receptors which sense IL-17 and IFN-γ inducing cytokines. We stimulated PBMCs with A. fumigatus and subsequently stained the cells for the T-cell marker CD4 and the receptors IL-1R1, IL-12Rβ1, and IL-18Rα. To determine the presence of the receptors we gated for the CD4+ cells and determined the percentage of positive cells (Fig. 4B). There were no significant differences in percentage of CD4+ cells expressing the receptors (Fig. 4C) or the mean fluorescence intensity of receptor expression (Fig. 4D) between Aspergillus-stimulated cells and cells stimulated with Aspergillus in the presence of addition of IL-36Ra in the range of 0.1 to 100 ng/mL. There were no differences in the total expression of these receptors in CD4− cells, and PBMCs stimulated with medium did not show differences after the addition of IL-36Ra (Supporting Information Fig. 2).
IL-36Ra does not affect Aspergillus-induced IL-1β, IL-23, IL-18, IL-6, IL-1Ra, and IL-10
We next investigated whether IL-36Ra had an effect on the cytokines that are important for the induction of human Th1 and Th17 responses, such as IL-1β, IL-6, IL-1Ra, IL-23, IL-18, and IL-12p70 production 9. As a anti-inflammatory cytokine we measured IL-10. Addition of IL-36Ra did not result reduce IL-1β, IL-6, IL-1Ra, or IL-10 production induced by A. fumigatus. (Fig. 4E) IL-12p70 and IL-23 concentrations in the supernatants of PBMCs stimulated with Aspergillus were undetectable (data not shown), and no differences were found in mRNA levels of IL-12p35 and IL-18 (Supporting Information Fig. 3).
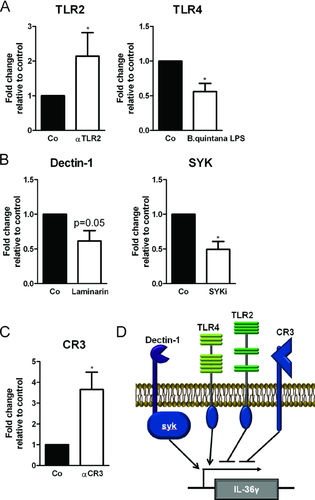
Induction of IL-36γ by A. fumigatus is dependent on dectin-1 Syk signaling and TLR4
The TLRs, TLR2 and TLR4, were previously reported to recognize A. fumigatus 16-20. In addition, the β-glucan receptor dectin-1 and its downstream signaling kinase Syk are also involved in the recognition of A. fumigatus 21-24. To identify whether TLR2, TLR4, CR3, dectin-1, or Syk are required for the induction of IL-36γ by A. fumigatus, we stimulated PBMCs in the absence or presence of specific blockers of pattern recognition receptors that have been associated with the recognition of A. fumigatus. PBMCs were stimulated with live A. fumigatus for 8 h, since at this time point we observed the highest level of mRNA of IL-36γ (Fig. 2B). Inhibition of dectin-1 or Syk resulted in significant reduction of Aspergillus-induced IL-36γ transcription (Fig. 5A). Blockade of TLR4 also resulted in decreased IL-36γ transcription (Fig. 5B). In contrast, when complement receptor 3 (CR3) or TLR2 were blocked, we observed that IL-36γ transcription increased (Fig. 5B and C). Taken together these data demonstrate that the dectin-1/ Syk and TLR4 pathway are involved in the induction of IL-36γ, whereas TLR2 and CR3 induce signaling that negatively regulates the induction of IL-36γ by Aspergillus (Fig. 5D).
Discussion
In the present study, we investigated the role of the IL-36R pathway in the induction of Aspergillus-specific proinflammatory Th responses. In addition, we identified the pattern recognition pathways responsible for the induction of IL-36γ by A. fumigatus. We demonstrate that A. fumigatus induces Th1 and Th17 responses in human PBMCs and that these responses are dependent on the IL-36R pathway. The induction of IL-36γ by A. fumigatus is mediated through the dectin-1/Syk and TLR4 pathway, while for unknown reasons, triggering TLR2 and CR3 suppresses the
induction of IL-36γ. These data describe a novel cytokine pathway that regulates Th responses induced by A. fumigatus and describes for the first time the recognition pathways that regulate IL-36 responses in host defense.
Invasive aspergillosis is a devastating disease in the immunocompromised patient and is associated with a high mortality 8. Adjunctive therapeutic strategies are needed to overcome this significant disease burden in immunocompromised patients. Furthermore, invasive pulmonary aspergillosis is increasingly recognized in nonneutropenic intensive care unit patients that are treated with corticosteroids 25, 26. Knowledge of the host defense against A. fumigatus is essential to understand the susceptibility to invasive aspergillosis and to develop new treatment strategies. Th responses play an important role in the host defense against A. fumigatus 9. IL-1 family members drive the induction and polarization of Th responses 1, and the IL-36 subfamily (IL-36α, β, and γ) induce IFN-γ and IL-17 in mouse splenocytes during mitogenic stimulation 5.
Here, we show that the IL-36 receptor pathway plays a role in the A. fumigatus-induced Th1 and Th17 responses in human cells, since blockade of the IL-36R with the addition of exogenous IL-36Ra inhibits IL-17 and IFN-γ production induced by A. fumigatus. Moreover, blocking the endogenous IL-36Ra results in a significant increase in IL-17 and IFN-γ, providing additional evidence that the IL-36R pathway is involved. It is tempting to speculate that the relatively low IL-17 production induced by Aspergillus compared with other pathogens is due to the capacity of Aspergillus to induce IL-36Ra. We observed that low concentrations of IL-36Ra were more efficient in inhibiting Th cytokine production than higher concentrations, this effect is still unexplained and needs further investigation. Moreover, we also demonstrate that the different IL-36 family members are induced by different morphological forms of A. fumigatus. Interestingly, we observed that heat-inactivated resting conidia, which are generally thought to have a low stimulatory capacity due to their hydrophobic protein layer, are potent inducers of both IL-36 ligands (IL-36β and IL-36γ) and the antagonist IL-36Ra, while hyphae, which are associated with invasive fungal growth, are potent inducers of IL-36γ expression, but induce the antagonist IL-36Ra to a lesser extent. This could indicate that when hyphae are formed during invasive fungal growth, the balance between receptor agonist and antagonist is shifted; however, it remains to be determined whether this is detrimental or beneficial for the host.
An important issue that remains to be resolved is whether the effect of IL-36 cytokines on Aspergillus-induced Th responses is due to an effect of IL-36 cytokines on T cells or DCs or both. IL-36 cytokines act directly on murine DCs and murine CD4+ T cells 5. In humans, dendritic cells express high levels of IL-36R and DCs primed with ligands for this receptor are very potent inducers of Th1-cell proliferation 4. We observed that isolated CD4+ T cells stimulated with IL-36 cytokines do not produce IL-17 or IFN-γ production. Furthermore, we found that IL-36 cytokines cannot serve as a surrogate for the IL-1 family members IL-1β or IL-18 in inducing Th polarization. Collectively, these data suggest that the primary effects of IL-36 cytokines on Th responses is probably through antigen presenting cells, and that IL-36 cytokines might be able to modulate Th responses, but only in the setting of activated Th cells.
The IL-36R agonist IL-36γ is expressed in the lung and is responsible for neutrophil influx in a model of house dust mite-induced inflammation in the lung 27. In human bronchial epithelial cells, IL-36γ induces the chemokines IL-8 and CCL20, which is an important Th17 chemokine 28. This suggests that IL-36γ can directly recruit neutrophils through the induction of IL-8 and that it is important for CCL20-dependent recruitment of Th17 cells, which in turn will result in additional neutrophil recruitment to the site of infection. Although the TLR3 ligand poly I:C induces secretion of IL-36γ 6, 28, other cell surface receptors that induce IL-36R ligands by pathogenic microorganisms have not yet been assessed. We demonstrate that the induction of IL-36γ by A. fumigatus is dependent on the TLR4 and dectin-1 pathways, whereas the TLR2 and CR3 pathway reduce the induction of IL-36γ. These pathways are each important for anti-Aspergillus host defense 29. Both TLR4 and dectin-1 polymorphisms have been linked to susceptibility to invasive aspergillosis 30-32, and TLR4 and dectin-1 play a role in the protective anti-Aspergillus host defense during a murine model of A. fumigatus keratitis 33. Furthermore, dectin-1 deficiency results in defective IL-17 and IL-22 responses in the lungs of mice challenged intratracheally with A. fumigatus 22. In this model of invasive pulmonary aspergillosis, IL-22 deficient and IL-17 deficient mice show an impaired recruitment of neutrophils in the lungs and increased fungal burden 22. It was found that monocyte derived macrophages from healthy individuals express IL-1β and IL-1α and later make a switch to IL-36β expression after stimulation with A. fumigatus. While cells of patients with chronic cavitary pulmonary aspergillosis did not show such a switch 34.
Posttranslational processing by the enzyme caspase-1 facilitates the secretion of IL-1 family members IL-1β and IL-18, which play important roles for the induction of protective Th1 and Th17 responses in antifungal host defense 35, 36. Interestingly, IL-36 receptor ligands also require posttranslational processing for full agonist or antagonist activity 3. Although it has been suggested that the release of active IL-36γ from the cell is dependent on caspase-1 6, the amino-acid sequences surrounding the truncation sites of IL-36γ do not resemble a caspase-1 site 3. It is therefore possible that other proteases are responsible for posttranslational processing of IL-36γ, and it remains to be elucidated whether the pathways that we identified in the regulation of IL-36γ transcription are involved in the release and activation of IL-36 and IL-36Ra.
The IL-36 receptor pathway has recently gained a lot of interest, since it plays a crucial role in the pathogenesis of psoriasis. Overexpression of IL-36γ in mice results in psoriatic skin lesions and these lesions are exacerbated when IL-36γ was overexpressed in mice that are knock-out for IL-36Ra 37. Moreover, IL-36 and IL-36Ra are expressed in human psoriatic skin lesions and IL-36Ra polymorphisms lead to familial pustular psoriasis 37-39. Therefore, the IL-36R pathway represents an attractive therapeutic target for treating psoriasis. Considering this, it is relevant to know the effects of blocking the IL-36R pathway on the host defense against pathogenic microorganisms.
Although further research is required to investigate the role of IL-36 in the immune responses to other pathogens, we have observed that IL-36Ra can also inhibit Candida albicans-induced IL-17 and IL-22 production 40. Furthermore, the IL-36 cytokines do not only regulate the expression and enhance the function of Th17 cytokines, but are also regulated by IL-17 and IL-22 41. This suggests a feedback loop between the IL-36 and Th17 cytokines, which makes the IL-36R pathway a central player in Th17-related mucosal host defense, and the role of this feedback in host defense against fungal pathogens is currently under investigation.
In conclusion, we demonstrate that IL-36R signaling modulates the production of A. fumigatus-induced Th cytokines IL-17 and IFN-γ, and that A. fumigatus induces IL-36γ in a TLR4 and dectin-1/Syk-dependent manner. These findings describe for the first time the innate recognition pathways involved in the induction of IL-36R ligands by a microorganism, and describe a novel pathway involved in the induction of Th responses by A. fumigatus. The future development of therapeutic strategies that will target the IL-36R pathway, and the high mortality associated with invasive aspergillosis, warrants further exploration on the importance of the IL-36R pathway in the host defense against Aspergillus.
Materials and methods
Aspergillus fumigatus
A clinical isolate of A. fumigatus V05-27, which has been previously characterized 42, was used for all stimulations. Conidia and hyphae were prepared as described previously 43, the hyphae were quantified as the number of conidia used to grow the hyphae, and all hyphae were prepared in one batch to account for batch to batch variations. Aliquots from both the conidia and the hyphae were HI at 56°C for 1 h and were checked for viability in Sabouraud glucose broth. HI conidia, hyphae, and live conidia were stored at −80°C until use. A concentration of 1 × 107/mL was used in the experiments, unless otherwise indicated. To study germination of live A. fumigatus conidia, 1 × 107 conidia/mL were plated in 12 wells plates. At 0, 2, 4, and 8 h, a 10 μL sample was taken and observed and photographed using a brightfield microscope at 1000× magnification (Leica DM 3000).
Cytokines
Recombinant, IL-1β, IL-18, IL-12, and IL-23, were purchased from R&D Systems (Minneapolis, MN). IL-36 cytokines were produced in Escherichia coli by R&D Systems. After extraction, refolding and purification of IL-36 cytokines, the level of LPS was determined to be less that 1.0 EU/microgram by the Limulus Amebocyte Lysate method. The molecular weight of recombinant IL-36Ra is 17 000 with an N-terminus at valine 2. On silver stained SDS PAGE under reducing conditions, there is a single band and the purity is >95%.
PBMC isolation and stimulation
Venous blood from healthy subjects was drawn into 10 mL EDTA tubes after written informed consent was obtained. Blood sampling from healthy volunteers was approved by the ethical board of the Radboud University Nijmegen Medical Centre. Subsequently, PBMCs were isolated as described previously 44. Blood was diluted in PBS (1:1) and fractions were separated by Ficoll (Ficoll-Paque Plus, GE Healthcare, Zeist, The Netherlands) density gradient centrifugation. Cells were washed twice with PBS and resuspended in RPMI-1640 culture medium supplemented with 10 μg/mL gentamicin, 10 mM l-glutamine, and 10 mM pyruvate (Gibco, Invitrogen, Breda, The Netherlands). The cells were counted using a particle counter (Beckmann Coulter, Woerden, The Netherlands) and the cell number was adjusted to 5 × 106/mL. PBMCs were plated in 96-well round bottom plates (Corning, NY, USA) at a final concentration of 2.5 × 106/mL and were stimulated with medium, 1×107/mL HI A. fumigatus conidia, or live A. fumigatus conidia in a total volume of 200 μL. Cells were stimulated for 0, 4, 8, 24 h and 7 days at 37°C and 5% CO2. After stimulation culture supernatants were collected and stored at −20°C and the cells were lysed in TRIzol reagent (Invitrogen) and stored at −20°C until RNA isolation was performed. Pattern recognition pathways were inhibited in PBMCs by preincubation for 1 h with a specific inhibitor. LPS derived from Bartonella quitana was used to block TLR4 45. Bartonella quitana LPS was extracted and purified as described previously 46. Mouse anti-human TLR-2 (eBioscience, Halle-Zoersel, Belgium) and control mouse IgG1 (eBioscience) were used at a final concentration of 10 μg/mL. Anti-human integrin β2 (αCR3) and control goat IgG were purchased from R&D systems (Minneapolis, MN) and used in a final concentration of 10 μg/mL. Laminarin (Sigma, St Louis, MO) was used in a final concentration of 50 ng/mL to inhibit dectin-1. Syk kinase inhibitor (3-(1-methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide) was purchased from Calbiochem (Merck, Darmstadt, Germany) and was used in a concentration of 50 nM to inhibit Syk signaling. This inhibitor is a cell-permeable oxindole compound that acts as a potent, reversible, and ATP-competitive Syk inhibitor.
IL-36 and IL-36Ra expression
RNA was isolated according to the protocol supplied with the TRIzol reagent. Isolated mRNA (1 μg) was reverse transcribed into cDNA using the iScript cDNA synthesis kit (BIORAD, Hercules, CA). Quantitative real-time PCR (qPCR) was performed using power SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA) and primers hIL-1F6-F 5′-TTG-CCT-TAA-TCT-CAT-GCC-GAC-3′ and hIL-1F6-R 5′-CCG-ACT-TTA-GCA-CAC-ATC-AGG-3′ for amplification of IL-36α, hIL-1F8-F 5′-AGA-AAT-TCA-GGG-CAA-GCC-TAC-3′ and hIL-1F8-R 5′-CAG-CCA-GGG-TAA-GAG-ACT-GAC-3′ for amplification of IL-36β, hIL-1F9-F 5′-GAA-ACC-CTT-CCT-TTT-CTA-CCG-TG-3′ and hIL-1F9-R 5′-GCT-GGT-CTC-TCT-TGG-AGG-AG-3′ for amplification of IL-36γ, hIL1F5-FW 5′-ACT-CGG-CAT-TGA-AGG-TGC-TTT-3′, hIL1F5-REV 5′-GGG-ACC-ACG-CTG-ATC-TCT-T-3′ for amplification of IL-36Ra, humIL-18 F 5′-TGT-CGC-AGG-AAT-AAA-GAT-GGC-T-3′ and humIL-18 R 5′-CCT-TGG-TCA-ATG-AAG-AGA-ACT-TGG-T-3′ for amplification of IL-18, IL-12p35 hum F 5′-CCT-TGC-ACT-TCT-GAA-GAG-ATT-GA-3′ and IL-12p35 hum R 5′-ACA-GGG-CCA-TCA-TAA-AAG-AGG-T-3′ for amplification of human IL-12p35 and Beta2M hum F 5′-ATG-AGT-ATG-CCT-GCC-GTG-TG-3′ and Beta2M hum R 5′-CCA-AAT-GCG-GCA-TCT-TCA-AAC-3′ for amplification of β2M. The PCR was performed using an Applied Biosystems 7300 real-time PCR system using PCR conditions 2 min 50°C, 10 min 95°C followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The RNA levels of IL-36γ and IL-36Ra were corrected for differences in loading concentration using the signal of house-keeping protein β2M and RNA isolates from freshly isolated PBMCs (T = 0) were set at 1.
IL-36 receptor blockade
PBMCs were plated in 96-well round bottom plates (Corning) at a final concentration of 2.5×106/mL and were preincubated for 1 h with medium or IL-36Ra at increasing concentrations. After prestimulation the PBMCs were stimulated for 24 h or 7 days with medium, 1×107/mL HI A. fumigatus conidia or hyphae (7 days) or live A. fumigatus conidia (24 h) at 37°C and 5% CO2. After stimulation, culture supernatants were collected and stored at −20°C until analysis.
IL-36Ra blockade
PBMCs were plated in 96-well round-bottom plates (Corning) at a final concentration of 2.5 × 106/mL and stimulated for 7 days with medium, 1 × 107/mL HI A. fumigatus conidia at in the presence of Goat IgG isotype control or Goat anti-human IL-36Ra (R&D Systems) at a concentration of 10 μg/mL. After stimulation, culture supernatants were collected and stored at −20°C until analysis.
RORγ and FoxP3 expression
Supernatant was removed from PBMCs that were stimulated for 7 days with medium or 1 × 107/mL HI A. fumigatus conidia. The cells were restimulated for 4–6 h with PMA (50 ng/mL) (Sigma-Aldrich), ionomycin (1 μg/mL) (Sigma-Aldrich) and Golgiplug (BD Biosciences, Breda, The Netherlands) according to the protocol supplied by the manufacturer. Cells were first stained extracellularly using PeCy7-conjugated anti-CD4 (BD Biosciences). Subsequently the cells were fixed and permeabilized with Cytofix/Cytoperm solution (eBioscience) according to the protocol supplied by the manufacturer. Following permeabilization the cells were intracellularly stained with FITC-conjugated anti-IL-17 (eBioscience) and allophycocyanin-conjugated anti-RORγ (eBioscience), or allophycocyanin-conjugated anti-FoxP3. The RORγ+ IL-17+ CD4+ cells and FoxP3+ cells were detected on a FC500 flowcytometer (Beckman Coulter) and the data were analyzed using CXP analysis software v2.2 (Beckman Coulter).
Cytokine measurement
IL-1β, IL-1Ra, IL-4, IL-6, IL-10, IL-12P70, IL-13, IL-17, IL-22, IL-23, and IFN-γ were measured using commercially available ELISAs (R&D Systems or Sanquin, Amsterdam, The Netherlands) according to the protocols supplied by the manufacturer. Detection limits of the assays are 39, 780, 78, 15.6, 11.7, 39, 39, 78, 15.6, and 7.8 pg/ml, respectively.
Receptor expression analysis
Supernatant was removed from PBMCs that were stimulated for 7 days with medium or 1 × 107/mL HI A. fumigatus conidia. The cells were stained with PeCy7-conjugated anti-CD4, PE-conjugated anti-IL-12Rβ1, allophycocyanin-conjugated anti- IL-1R1, and FITC-conjugated anti-IL-18Rα. Cells were detected on a FC500 flowcytometer (Beckman Coulter) and the data were analyzed using CXP analysis software v2.2 (Beckman Coulter).
Statistical analysis
The differences between stimulations with control stimulation versus inhibitors or IL-36Ra were analyzed with the Wilcoxon signed rank test. A p-value of <0.05 was considered statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001). All experiments were performed at least twice and data represent cumulative results of all experiments performed. Data was analyzed using GraphPad Prism v 5.0.
Acknowledgements
The authors thank R&D Systems and specifically Vassili Kalabokis and Ruyi Hao for their help in providing the IL-36 cytokines for these studies. F.L.vdV. was supported by a Veni grant of the Netherlands Organization for Scientific Research, a stipend from the Niels Stensen foundation and a NCMLS grand. M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research. C.A.D. was supported by a NIH grant AI-15614 and AR-45584.
Conflict of interest
The authors declare no financial or commercial conflict of interest
References
Abbreviations
-
- CR3
-
- complement receptor 3
-
- HI
-
- heat inactivated
-
- IL-1F
-
- IL-1 family




