Flt3 ligand expands CD4+FoxP3+ regulatory T cells in human subjects
Abstract
CD4+CD25+FoxP3+ naturally occurring regulatory T (Treg) cells play a crucial role in the maintenance of immune tolerance and in preventing autoimmune pathology. Interventions that expand Treg cells are highly desirable, as they may offer novel treatment options in a variety of autoimmune and transplantation settings. Paralleling previous preclinical studies, we demonstrate here that administration of the hematopoietic growth factor Flt3L to human subjects increases the frequency and absolute number of Treg cells, and reduces the ratio of CD8+ T cells to Treg cells in the peripheral blood. The increase in Treg cells was due to enhanced Treg-cell proliferation rather than release of Treg cells from the thymus. Further studies revealed that Flt3L-induced proliferation of Treg cells was an indirect effect that occurred via the interaction of Treg cells with the Flt3L-expanded pool of CD1c+ myeloid dendritic cells. On the basis of these findings, Flt3L may represent a promising agent for promoting immune tolerance in a variety of clinical settings.
Introduction
FoxP3-expressing Treg cells play a major role in maintaining peripheral tolerance and limiting self-harming effects of immune responses 1. There is significant interest in exploiting the suppressive activity of Treg cells clinically for the treatment of conditions such as autoimmune disease, graft-versus-host disease, and allograft rejection. Current attempts are mainly focused on isolating Treg cells from blood, expanding them ex vivo, and re-infusing them into patients 2. However, this approach is limited by insufficient expansion and loss of suppressive capacity in some of the expanded cells 3. It also requires the necessary logistics to produce cell products and will be very costly. Strategies to manipulate Treg cells in vivo could overcome these hurdles. Although various approaches in mice have produced increases in Treg-cell frequencies 4, 5, initial attempts in humans using a CD28 superagonist antibody led to a disastrous outcome 6.
Flt3L is a hematopoietic growth factor important for the development of myeloid and plasmacytoid DCs. Accordingly, injection of Flt3L into mice leads to dramatic increases in these DC subsets in secondary lymphoid organs and blood 7. Similarly, daily subcutaneous injections of Flt3L in humans result in several-fold increase in the frequencies of myeloid and plasmacytoid DCs, and monocytes in the peripheral blood 8, 9.
It was recently demonstrated that peripheral Treg-cell homeostasis in mice is highly dependent on interactions with DCs, such that increases in DC number lead to increases in Treg-cell number 10. Moreover, administration of Flt3L to mice induces significant expansion of Treg cells, as a secondary effect of DC expansion 11, 12. Importantly, this increase in Treg cells was associated with amelioration of autoimmunity and graft-versus-host disease. We demonstrate here that administration of Flt3L to humans expands Treg cells via a similar mechanism to that discovered in mice, suggesting that Flt3L may represent a novel treatment option in autoimmune and transplantation settings.
Results and discussion
Flt3L increases the frequency and absolute number of human Treg cells
Daily subcutaneous injection of human subjects with Flt3L for 14 days led to large increases in monocytes, myeloid DCs, and plasmacytoid DCs in the blood (Fig. 1A), similar to previous studies 8, 9. In addition, we observed for the first time a significant increase in the percentage of Treg cells, as a proportion of CD4+ T cells, following Flt3L administration (mean 68% increase; p < 0.0001; n = 22; Fig. 1B-C). As shown in Figure 1D, the increase in Treg-cell frequency translated to an increase in Treg-cell absolute number, with a greater than twofold increase in the mean number of Treg cells per mL of blood at day 15 (p < 0.05; n = 22). Several patients had samples available from additional time-points; time-course studies in these patients revealed that Treg-cell frequency was maintained or increased even further out to day 22, after Flt3L administration had ceased (Fig. 1E). We also assessed the ratio of CD8+ T cells to Treg cells, and detected a significant decrease after Flt3L treatment (Fig. 1F), which may favor the development of an immune-tolerant environment.
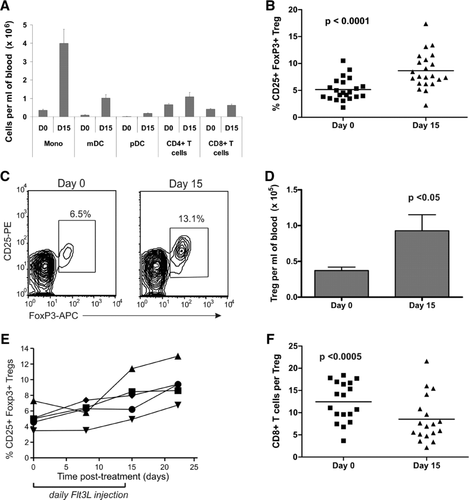
Flt3L-expanded human Treg cells are suppressive
We next aimed to determine the suppressive activity of Treg cells after Flt3L treatment. FoxP3 and CD25 can both be transiently induced on activated effector T (Teff) cells, and it was therefore important to confirm that the increased number of CD4+CD25+ FoxP3+ T cells after Flt3L treatment was due to an increase in genuine Treg cells. To this end, Treg cells were sorted from PBMCs collected before and 15 days after treatment, according to a CD4+ CD25+CD127− phenotype, previously shown to be identical to CD4+FoxP3+ Treg cells 13, 14. Analysis of cells prior to sorting confirmed that, as expected, Treg cells identified using this phenotype were increased at day 15 compared with that on day 0 for all patients tested (Fig. 2A). Furthermore, analysis of Treg cells after sorting confirmed that the majority of sorted cells expressed FoxP3 (mean 87.1%; range 79–94% FoxP3+; Fig. 2B). When tested for their ability to suppress the proliferation of healthy donor CD8+ T cells, the Treg cells from both time-points were equally suppressive on a per-cell basis (Fig. 2C), thereby confirming that Flt3L-induced Treg cells are functional.

Expansion is due to increased proliferation of pre-existing Treg cells
To explore the mechanism behind Treg-cell expansion, we first investigated whether Flt3L had any direct effect on Treg cells. Although initial reports found no evidence for expression of Flt3 at the mRNA or protein level in mature lymphoid cells 15, low level Flt3 expression in T cells has been recently demonstrated 16. We could also detect marginal expression of FLT3-mRNA in Treg cells in some donors using RT-PCR (Fig. 3A). However, culturing CFSE-labeled Treg cells with various concentrations of Flt3L failed to induce proliferation, excluding any cell-intrinsic effect of Flt3L on Treg cells (Fig. 3B).

Indirect mechanisms to explain the Treg-cell increase after Flt3L administration include increased thymic output of Treg cells, the induction of a regulatory phenotype in conventional T cells, or increased proliferation of pre-existing Treg cells. Previous studies in mice have demonstrated that Flt3L can, under certain circumstances, lead to increased thymopoiesis through expansion of lymphoid progenitor cells 17. To address this possibility, we assessed Treg cells for expression of the memory markers CD45RA and CD45RO, together with CD31, a marker of human CD4+ T cells that have recently emigrated from the thymus 18 (Fig. 3C). The percentage of CD31+ naïve (CD45RA+) cells in the Treg population was low, consistent with the low thymic output in adults, and there was no significant increase between day 0 and 15, ruling out increased thymopoiesis as a major mechanism for the Flt3L-induced increase in Treg-cell numbers. This was supported by an unchanged ratio of CD45RA+ naïve Treg cells to CD45RO+ memory Treg cells.
To address the role of peripheral conversion of conventional T cells to Treg cells, we performed staining for the Helios transcription factor that has been reported to be expressed only by thymus-derived Treg cells 19, although this association is somewhat controversial 20. As shown in Figure 3D, the proportion of Treg cells expressing Helios was unchanged after Flt3L administration, suggesting that peripheral conversion may not be a major mechanism by which Flt3L increases the pool of circulating Treg cells, although a more reliable marker of thymic-derived Treg cells is required to confirm this result.
Finally, to determine whether the Flt3L-induced increase in Treg-cell frequency was due to enhanced proliferation, we performed intracellular staining for Ki67 (Fig. 3E). Prior to treatment, proliferating (Ki67+) cells were already more frequent within the Treg-cell population compared with the Teff-cell population, in keeping with the previously reported higher basal proliferation rate of Treg cells 21. After 15 days of Flt3L treatment, there was a nearly twofold increase in the proportion of proliferating (Ki67+) Treg cells compared with the pretreatment measurement, without any increased proliferation in the CD4+ Teff cell compartment. Thus, the altered balance between Treg cells and Teff cells following Flt3L treatment appears to be primarily due to increased proliferation of pre-existing Treg cells.
Myeloid DCs induce Treg-cell proliferation in vitro
As Flt3L treatment led to large increases in myeloid DCs, plasmacytoid DCs, and monocytes in peripheral blood, we cocultured these antigen-presenting cell subsets with Treg cells to directly investigate whether any of these populations could induce Treg-cell proliferation. In mice, CD4+CD8‑ lymphoid DCs are the only DC subset capable of inducing Treg-cell proliferation in the absence of antigen 22, 23. Human CD1c+ myeloid DCs are seen as the human counterpart of this population 24, and accordingly, were the only cell type able to promote significant Treg-cell proliferation in our studies (Fig. 3F). Furthermore, flow cytometric analysis of CD1c+ DCs in patient samples revealed a significant increase in HLA-DR expression after Flt3L administration without any change in expression of the costimulatory molecules CD80 and CD86 (Fig. 3G). These data suggest that increased availability of MHC class II molecules on the expanded DC population contributed to the increased Treg-cell proliferation. This is in keeping with findings in an animal model where the TCR/MHC class II interaction was crucial for the Flt3L-mediated Treg-cell expansion 10.
Concluding remarks
Our results demonstrate that human Treg cells can be expanded in vivo by injection of Flt3L, in keeping with previous studies in mice 11, 12. This increase in Treg-cell frequency occurs indirectly, via expansion of myeloid DCs, which in turn induce Treg-cell proliferation. Our findings are supported by a recently described human DC deficiency syndrome that is associated with a significant reduction in circulating Treg-cell frequency 25, and by studies of normal human skin demonstrating that autologous Langerhans cells promote the proliferation of skin-resident Treg cells via an MHC class II-dependent mechanism 26.
The patients analyzed in this study were participants in a clinical trial designed to investigate potential immune-enhancing effects of Flt3L on endogenous and vaccine-induced anti-tumor immune responses in melanoma. We were unable to detect any significant induction or boosting of melanoma antigen-specific immune responses in our patients, which may be due to the Flt3L-mediated Treg-cell expansion described here 27. On the other hand, agents that can shift the balance between Teff cells and Treg cells to promote an immune-tolerant environment may offer new treatment options for autoimmune conditions and the prevention of alloimmune responses in transplantation medicine 28, 29. Preclinical studies investigating Flt3L administration to mice 11, 12 demonstrated an increase in peripheral Treg-cell number of a very similar magnitude to that observed here (∼twofold). Importantly, this increase was sufficient to ameliorate colitis and prevent death from graft-versus-host disease. Our new data in humans suggest that Flt3L, which already has a well-established safety profile 8, 9, may have similar efficacy in the treatment of humanw disease.
Materials and methods
Clinical trial
PBMCs were obtained from patients with resected stage II/III/IV melanoma participating in a clinical trial involving Flt3L administration 27. Patient PBMCs were cryopreserved in 10% DMSO, and stored in liquid nitrogen. The trial was approved by the Austin Health Human Research Ethics Committee.
Flow cytometry
Leukocyte populations were characterized using fluorochrome-coupled antibodies to surface markers from BD Biosciences (Franklin Lakes, NJ), allophycocyanin-labeled anti-human FoxP3 Staining Kit (eBioscience, San Diego, CA), and Pacific Blue-coupled anti-human Helios antibody (Biolegend, Cambridge, UK). Anti-Ki67 from BD Biosciences was used to assess proliferation, as previously described 30.
Suppression assay
Frozen PBMC samples were thawed and rested in culture for 48 h. Live cells were collected using Lymphoprep, stained and sorted as follows: responder (CD8+CD127+CD25−), Treg cells (CD4+CD25+CD127−), and non-Treg cells (CD4+CD25−CD127−). CD8+ T cells were labeled with 5 μM CFSE and 2000 cells cultured with 1000 Treg cells in 96-well plates containing 1 μg/mL anti-CD3 (OKT3) antibody. The percentage of divided CD8+ T cells (CFSE-low) was determined after 4 days’ culture and used to calculate percent suppression as follows: 1 – (% divided test /% divided max) × 100, where ‘test’ was the suppressor population being tested, and ‘max’ was the non-Treg-cell control.
Detection of Flt3 by RT-PCR
Treg cells were isolated from bulk PBMCs using the Treg isolation kit (Milteny Biotech). The Treg-cell-depleted fraction of CD4+ T cells was used as a source of non-Treg cells. RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA synthesized as previously described 31. The FLT3 primers were designed and PCR performed as described 15.
Treg-cell proliferation
Treg cells, monocytes, CD1c+ myeloid DCs, and plasmacytoid DCs were isolated from PBMCs of healthy donors using Treg-cell, myeloid, and plasmacytoid DC isolation kits and CD14 microbeads (Miltenyi Biotec, Auburn, CA). The Treg cells were stained with 1 μM CFSE, and 2 × 105 cells were incubated with the autologous DC populations in a 5:1 ratio for 5 days. Alternatively, CFSE-labeled Treg cells were cultured with human Flt3L (R&D Systems, Minneapolis, MN), or anti-CD3/CD28 beads (Invitrogen) for 5 days.
Statistical analysis
Pre- and posttreatment data were compared using a paired, two-tailed t-test, calculated using Prism software (GraphPad Software, La Jolla, CA). Other analyses were performed using an unpaired t-test.
Acknowledgments
This work was supported by a Grant-in-Aid to LME and JC from the Cancer Council of Victoria and Operational Infrastructure Support from the Victorian State Government. EM is an employee of CSL Limited and an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research. JF was supported by a grant from the Ministry of Education, Czech Republic. IDD was supported by a Victorian Cancer Agency Clinician Researcher Fellowship and is an honorary NHMRC Practitioner Fellow. JC is an NHMRC Practitioner Fellow.
Conflict of interest
E.M. is employed by CSL Limited, but no reagent or potential product of CSL was used in this work. All other authors declare no financial or commercial conflict of interest.
References
Abbreviation
-
- Teff cell
-
- effector T cell




