IL-21 promotes the production of anti-DNA IgG but is dispensable for kidney damage in lyn−/− mice
Abstract
The autoimmune disease systemic lupus erythematosus is characterized by loss of tolerance to nuclear Ags and a heightened inflammatory environment, which together result in end organ damage. Lyn-deficient mice, a model of systemic lupus erythematosus, lack an inhibitor of B-cell and myeloid cell activation. This results in B-cell hyper-responsiveness, plasma cell accumulation, autoantibodies, and glomerulonephritis (GN). IL-21 is associated with autoimmunity in mice and humans and promotes B-cell differentiation and class switching. Here, we explore the role of IL-21 in the autoimmune phenotypes of lyn–/– mice. We find that IL-21 mRNA is reduced in the spleens of lyn–/–IL-6–/– and lyn–/–Btklo mice, neither of which produce pathogenic autoantibodies or develop significant GN. While IL-21 is dispensable for plasma cell accumulation and IgM autoantibodies in lyn–/– mice, it is required for anti-DNA IgG antibodies and some aspects of T-cell activation. Surprisingly, GN still develops in lyn–/–IL-21–/– mice. This likely results from the presence of IgG autoantibodies against a limited set of non-DNA Ags. These studies identify a specific role for IL-21 in the class switching of anti-DNA B cells and demonstrate that neither IL-21 nor anti-DNA IgG is required for kidney damage in lyn–/– mice.
Introduction
The autoimmune disease systemic lupus erythematosus (SLE) is driven by the production of autoantibodies and exacerbated by innate immune system hyperactivation. This leads to inflammation and damage to multiple organs, including the kidneys. Genetic studies in humans and mice have identified multiple pathways that contribute to the autoimmune phenotypes associated with lupus 1, 2. Despite these advances, the majority of current treatments for SLE involve nonspecific immunosuppression. A more thorough understanding of the mechanism(s) responsible for the initial loss of tolerance and the subsequent end organ damage might facilitate the development of more targeted therapies.
Lyn-deficient mice lack a critical negative regulator of B-cell and myeloid cell activation 3. These mice exhibit hyper-active B cells, plasma cell (PC) accumulation, autoantibodies, and glomerulonephritis (GN) 4-6, all features of SLE. Reduced Lyn expression has been observed in B cells from SLE patients 7, 8, and polymorphisms in the lyn gene have been associated with SLE 9, 10. By defining the requirements for autoantibody production and kidney damage in lyn–/– mice, we hope to better understand the events that disrupt normal B-cell tolerance checkpoints and the consequences of these for disease pathology.
We previously identified two stages in the development of humoral autoimmunity in this model 11. The first involves the accumulation of PCs and IgM autoantibodies, while the second controls the class switching of autoreactive B cells specific for lupus-associated autoantigens such as dsDNA. The latter step requires IL-6, a proinflammatory cytokine associated with autoimmunity in mice and humans 11, 12. Understanding how IL-6 promotes autoantibody production in lyn–/– mice may have important clinical applications, as anti-IL6R antibodies are currently in trials as a therapy for SLE 13.
While IL-6 has pleiotropic effects 14, it likely promotes autoantibody production via the cytokine IL-21. IL-6 induces IL-21 expression by multiple subsets of CD4+ T cells 15-17. IL-21 is a potent stimulator of B-cell differentiation 15, 18-24 and class switching 18, 19, 25-27 and promotes GC maintenance 21, 28. IL-21 and/or IL-21-producing cells such as T follicular helper (Tfh) cells or extrafollicular T helper cells are elevated in several murine lupus models 18, 29-32. In BXSB.Yaa 31 and MRL.lpr mice 33, 34, blocking IL-21 signaling can prevent autoimmune pheno-types. Furthermore, polymorphisms in IL-21 and its receptor are associated with SLE 35, 36. IL-21 is thus a candidate to mediate pathogenic autoantibody production in Lyn-deficient mice.
Consistent with this hypothesis, we found significantly reduced IL-21 mRNA levels in the spleens of lyn–/–IL-6–/– mice. We therefore generated lyn–/–IL-21–/– mice to address the role of IL-21 in the autoimmune phenotypes of lyn–/– mice. Loss of IL-21 did not affect total Ig levels, nor did it prevent the accumulation of PCs or IgM autoantibodies. However, IL-21 was required for IgG against DNA and several other, but not all, autoantigens. Despite this, lyn–/–IL-21–/– mice developed GN to a similar extent as lyn–/– mice. Thus, IL-21-dependent class switching of anti-DNA B cells to IgG is not required for kidney pathology. These studies also suggest that IL-6 contributes to kidney damage via mechanisms in addition to promoting IL-21 expression.
Results
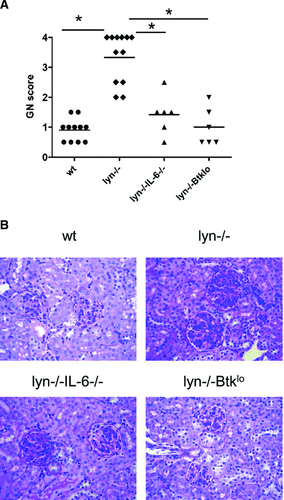
Deficiency of IL-6 ameliorates kidney damage in lyn–/– mice
We previously demonstrated that IL-6 is required for the production of IgG against lupus-associated autoantigens, including nucleic acids, in lyn–/– mice 11. IgG autoantibodies with these specificities are known to be pathogenic 37, 38. Indeed, IL-6-deficiency ameliorated the severity of GN in lyn–/– mice (Fig. 1). This confirms a recent report which also demonstrates that lyn–/–IL-6–/– mice lack IgG deposits in their kidneys 12.
IL-6 promotes expression of IL-21 by T cells in lyn–/– mice
IL-6 can induce class switching in B cells indirectly via IL-21 15. We asked whether IL-21 levels were altered in lyn–/– and/or lyn–/–IL-6–/– mice. We examined 3- to 5-month-old mice because IL-6-driven autoantibody production occurs by this time in lyn–/– animals 11, 12. Somewhat surprisingly, IL-21 mRNA expression was not significantly elevated in lyn–/– spleens (Fig. 2). The majority of IL-21 mRNA in both WT and lyn–/– spleens was expressed by CD4+ T cells (Supporting Information Fig. 1), similar to results obtained with WT mice expressing an IL-21 reporter 39. Consistent with the ability of IL-6 to induce IL-21 expression by T cells 15-17, splenic IL-21 mRNA was reduced in the absence of IL-6 in both lyn+/+ and lyn–/– mice (Fig. 2).
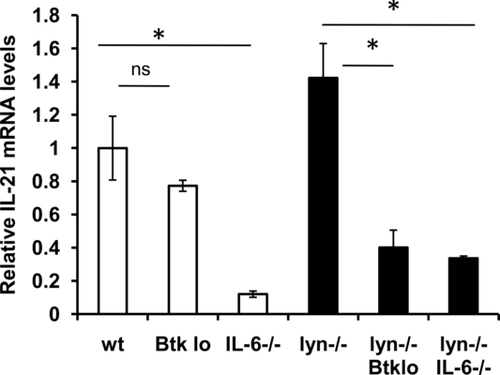
Btk promotes kidney damage and IL-21 expression in lyn–/– mice
Autoantibody production 40 and GN (Fig. 1) are also impaired in lyn–/– mice expressing low levels of Btk, a target of Lyn-dependent inhibitory pathways. Splenic IL-21 mRNA was decreased in these lyn–/–Btklo mice (Fig. 2). Thus, although IL-21 levels are not dramatically upregulated in the absence of Lyn, two manipulations that prevent IgG autoantibodies and GN in lyn–/– mice also limit IL-21 expression. This suggests a role for IL-21 in the differentiation or class switching of autoreactive B cells in lyn–/– mice. To test this hypothesis, we generated and characterized lyn–/–IL-21–/– mice.
B-cell developmental defects in lyn–/– mice are independent of IL-21
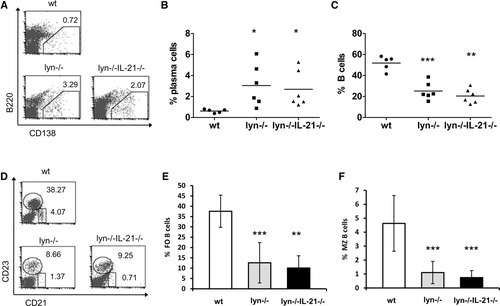
lyn–/– mice have several B-cell defects, including increased PCs and fewer marginal zone B cells 11, 41. IL-21 can induce PC differentiation 15, 18-24 and promote apoptosis of marginal zone B cells during chronic inflammation 42. However, as in lyn−/− mice, PCs (B220loCD138hi) were elevated and follicular (CD23+CD21+) and marginal zone (CD23−CD21+) B cells were reduced in lyn−/−IL-21−/− spleens compared with those in WT (Fig. 3). Thus, B-cell developmental defects in lyn−/− mice are independent of IL-21.
IL-21 is required for anti-DNA IgG in lyn–/– mice
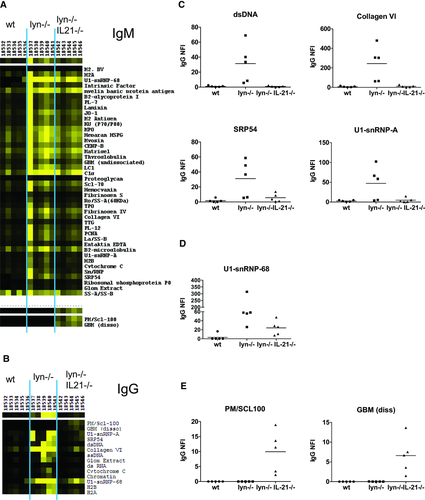
We next evaluated sera from 4- to 5-month-old mice for autoantibodies by ELISA. At this age, IL-6-dependent IgG autoantibodies are consistently observed in lyn–/– mice 11, 12. While lyn–/–IL-21–/– mice had similar levels of anti-dsDNA and anti-ssDNA IgM as lyn–/– mice (Fig. 4A and B), they did not produce anti-dsDNA and anti-ssDNA IgG (Fig. 4A and B). This was not due to a general class switching defect since total IgM and IgG levels were unaffected by IL-21-deficiency (Supporting Information Fig. 2). Nor was this a kinetic effect, as anti-DNA IgG was not detected in lyn–/–IL-21–/– mice as old as 12 months of age (Fig. 4C and D). Aged lyn–/–IL-21–/– mice also did not produce IgG autoantibodies against dsDNA plus histones (Fig. 4E). IL-21 is therefore required for class switching of anti-DNA B cells.

lyn–/–IL-21–/– mice have some IgG autoantibodies
To determine whether IL-21 affects autoantibody specificity in lyn–/– mice, sera were hybridized to an autoantigen array containing approximately 70 Ags commonly targeted in lupus and other autoimmune diseases 43. lyn–/– mice produce IgM against a wide range of autoantigens even in the absence of IL-6 11. In contrast, their IgG autoantibodies depend on IL-6 11, 12 and are focused toward nucleic acid-containing and glomerular Ags 11. Similar results were obtained in a comparison of lyn–/– and lyn–/–IL-21–/– mice. Both strains produced IgM against multiple autoantigens (Fig. 5A), while the majority of IgG autoantibodies observed in lyn–/– mice were absent in lyn–/–IL-21–/– mice (Fig. 5B and C). However, some autoreactive IgG was evident against a limited number of Ags (Fig. 5B, D, E), two of which were unique to lyn–/–IL-21–/– mice (Fig. 5E).
ICOS+ T cells in lyn–/– spleens are reduced in the absence of IL-21
In addition to acting directly on B cells to promote class switching, IL-21 supports differentiation of ICOS+ CD4+ T cells that are efficient B-cell helpers 17, 31, 34, 44, 45. We asked whether IL-21-deficiency altered the frequency of cells with the phenotype of Tfh (ICOS+CXCR5+PD1+) or extrafollicular T helper cells (ICOS+CXCR5−PD1+) in lyn–/– mice. Both populations have been implicated in lupus 31, 32, 46. There was no change in ICOS+CXCR5+ cells in lyn–/– mice, consistent with the lack of GC formation in these animals, either basally or in response to immunization 4, 47, 48. However, we did observe an increase in ICOS+CXCR5−PD1+ cells among lyn–/– CD4+ T cells, which was normalized in the absence of IL-21 (Fig. 6A, B and Supporting Information Fig. 3). IL-21-deficiency also reduced the frequency of ICOS+CXCR5+ cells. Low levels of PSGL1 expression mark an IL-21-producing T-cell population that is expanded in other lupus models and promotes class switching 30, 34. These cells were reduced in frequency in lyn–/–IL-21–/– mice relative to lyn–/– animals (Supporting Information Fig. 3).
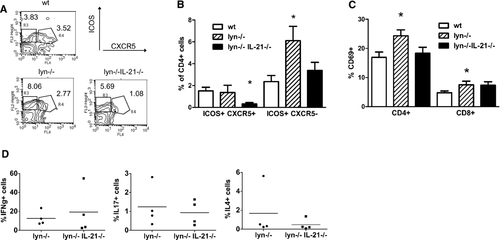
Myeloid cells, but not CD69+ T cells, are elevated in spleens from aged lyn–/–IL-21–/– mice
As lyn–/– mice age, their T cells become activated and produce IFN-γ and/or IL-4 49, 50. This contributes to disease pathology, in part via positive feedback loops between T and myeloid cells 49, 50. The percentage of CD4+ cells expressing the activation marker CD69 was elevated compared with that in WT in lyn–/–, but not lyn–/–IL-21–/– mice (Fig. 6C and Supporting Information Fig. 4). However, the frequency of IFN-γ, IL-4, and IL-17-producing cells among CD4+ T cells was similar in aged lyn–/– and lyn–/–IL-21–/– mice (Fig. 8D, Supporting Information Fig. 4). In the myeloid compartment, we observed an elevated frequency of CD11b+ cells in both lyn–/– and lyn–/–IL-21–/– spleens (Fig. 7). This increase was primarily in the CD11b+Gr1+CD11c− subset (Fig. 7). Because of variability in the total number of splenocytes in aged lyn–/– and lyn–/–IL-21–/– mice (Supporting Information Fig. 5), it was difficult to detect significant changes in the total number of T and myeloid cell subpopulations. However, since the relative frequency of myeloid cells is increased significantly in both lyn–/– and lyn–/–IL-21–/– mice, other cell types will have greater exposure to them and the factors they produce than in WT mice.

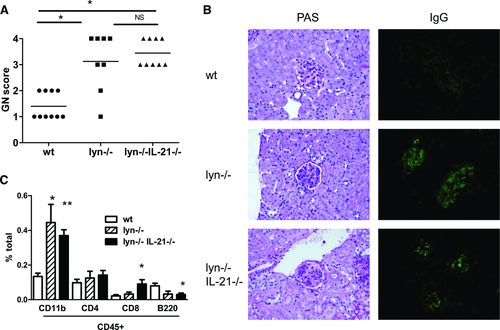
lyn–/–IL-21–/– kidneys demonstrate GN, IgG deposits, and increased myeloid cells
Finally, we asked whether IL-21 mediates kidney damage in lyn–/– mice. Despite the lack of anti-DNA IgG, aged lyn–/–IL-21–/– mice experienced severe GN (Fig. 8A and B). They also demonstrated an increased frequency of CD11b+ (both CD11c−/lo and CD11c+ subsets) and CD8+ cells in the kidneys (Fig. 8C and Supporting Information Fig. 6). Each of these populations has been shown to
be elevated in the nephritic kidneys of other lupus models 51, 52. IgG deposits were observed in four of four lyn–/–IL-21–/– kidneys examined (Fig. 8B and Supporting Information Fig. 6), likely due to residual autoreactive IgG against non-DNA Ags (Fig. 5). Tubular interstitial nephritis was minimal, although mildly elevated (Supporting Information Fig. 6). These results are consistent with a predominant role for immune complex-mediated kidney damage.
Discussion
IL-21 is associated with lupus in both humans and mice 18, 29-36. While IL-21 mRNA is not significantly elevated in Lyn-deficient mice, several manipulations that reduce autoantibodies also dampen IL-21 expression. This suggested a role for IL-21 in the autoimmune phenotype of lyn–/– mice. Indeed, we show that IL-21 is required for IgG against DNA and some other, but not all, self-Ags in lyn–/– mice. However, IL-21 is dispensable for kidney damage in these animals.
IL-21 could promote autoreactive B-cell class switching in two ways; by directly acting on B cells 18, 19, 21, 25-28, and/or by maintaining ICOS+CXCR5− and ICOS+CXCR5+ CD4+ T cells. These subsets are efficient B-cell helpers in extrafollicular and GC responses, respectively 29, 30. Autoreactive B cells are likely activated in an extrafollicular response in lyn–/– mice. These animals fail to form GCs, either spontaneously or in response to immunization 4, 47, 48. Furthermore, secondary peripheral lymphoid organs from lyn–/– mice have a disorganized structure, without defined follicles or B/T zones 4, 53-55.
Although lyn–/–IL-21–/– mice lacked anti-DNA IgG, they still developed GN. The remaining IgG antibodies specific for non-DNA self-Ags have pathogenic potential since they recognize dissociated glomerular basement membrane and RNA-containing Ags. Indeed, IgG deposits were present in four of four lyn–/–IL-21–/– kidneys examined. Inflammation initiated by these non-DNA IgG autoantibodies could then be amplified by positive feedback loops between cytokine-producing T cells and CD11b+Gr1+CD11c− myeloid cells in the periphery 49, 50 and by elevated CD11b+ and CD8+ cells in the kidney, none of which are significantly altered by IL-21-deficiency.
We find that the majority of splenic IL-21 mRNA is produced by CD4+ T cells in an IL-6-dependent manner in both WT and lyn–/– mice, consistent with previous reports 15-17, 39, IL-6 is required for expansion of Tfh cells and/or their expression of IL-21 upon chronic, but not acute, lymphocytic choriomeningitis virus infection 56, 57. These observations suggest that IL-6 maintains steady-state levels of IL-21 expression by T cells basally and during chronic infection or autoimmunity, while IL-6-independent events can induce IL-21 during acute responses to certain pathogens or Ags.
Kidney damage in lyn–/– mice is abrogated by deficiency of IL-6, but not IL-21 11, 12. Thus, IL-6 has both IL-21-dependent and -independent functions in the autoimmune phenotype of lyn–/– animals. There are several mechanisms by which IL-6 could drive the latter events. IL-6 promotes Th17-cell development and inhibits Treg-cell activity 58. We observed a slight increase in Th17 cells among CD4+ T cells in lyn–/– mice (WT 0.34 ± 0.04%, n = 5 versus lyn–/– 1.25 ± 1.09%, n = 4), although this was not significant. Treg cells are present in lyn–/– mice but fail to suppress disease 53. IL-6-deficiency also promotes myelopoiesis 59 and likely contributes to the increase in myeloid cells and their role in proinflammatory feedback loops in lyn–/– mice 12, 49, 50. Finally, IL-6 acts on endothelial cells to alter homing of leukocytes to sites of inflammation 60. This may contribute to kidney damage in lyn–/– mice.
Disruption of IL-21 signaling also prevents IgG autoantibody production and reduces ICOS+CXCR5− T cells in BXSB.Yaa 31 and MRL.lpr mice 33, 34. However, a more profound effect on other aspects of the autoimmune phenotype was observed in BXSB.Yaa and MRL.lpr mice lacking the IL-21R than was seen in lyn–/–IL-21–/– mice 31, 34 In contrast, IgG autoantibody production is independent of IL-21 in Roquinsan/san mice 46, despite increased Tfh cells and IL-21 overexpression. This varying dependence of autoimmune phenotypes on IL-21 signaling may be explained by different disease mechanisms in each model. There is also a formal possibility that there is a more severe effect of deletion of the IL-21R 31, 34 than of IL-21 (the current study and 46). Although IL-21R- and IL-21-deficiency each prevent mortality in BXSB.Yaa mice 31, a detailed description of BXSB.Yaa.IL21–/– mice has not been reported. Together, these studies indicate that neither IL-21 overexpression nor expansion of Tfh or extrafollicular T helper cells can predict a requirement for IL-21 in autoimmune pathology. This suggests that only certain subsets of patients would benefit from therapeutic inhibition of IL-21. In contrast, IL-6, which acts upstream of and more broadly than IL-21, may be a more widely effective target 11-13.
Materials and methods
Mice
lyn–/– 6, lyn–/–Btklo 61, 40, and lyn–/–IL-6–/– 11 mice were described previously. All mice used in lyn–/–Btklo and lyn–/–IL-6–/– studies were backcrossed onto the C57BL/6 background. IL-21–/– (B6.129S-Il21tm1Lex/Mmcd) mice were obtained from the Mutant Mouse Regional Resource Center and crossed with lyn–/– mice to generate lyn–/–IL-21–/– mice. Mice used in the lyn–/–IL-21–/– studies were of mixed C57BL/6 × 129 background; WT and lyn–/– littermates were used as controls. All animals were housed in a specific pathogen free barrier facility, and all procedures were approved by the UT Southwestern Institutional Animal Care and Use Committee.
Flow cytometry
Single-cell suspensions of spleens or collagenase-digested (30 min at 37°C) kidneys were Fc-blocked with anti-mouse CD16/CD32 prior to incubation with some combination of the following monoclonal antibodies: FITC-conjugated anti-CD21, anti-PD1, anti-CD11b, or anti-CD45; PE-conjugated anti-CD23, anti-ICOS, anti-PSGL-1, anti-CD8, or anti-CD11b; PerCP-conjugated anti-B220 or anti-CD4; and biotin-conjugated anti-CD138, anti-CXCR5, anti-CD11c, or anti-CD69. Biotinylated antibodies were detected with strepavidin-allophycocyanin.
Intracellular cytokine staining as described in 62 was adapted for murine cells. Briefly, splenocytes were resuspended at 106/mL and stimulated for 5 h with PMA and ionomycin. Cytokine secretion was blocked by incubating with brefeldin A. Cells were stained extracellularly with PacBlue-conjugated anti-CD4. Permeabilization and fixation were performed using a Foxp3 Staining Kit (Miltenyi Biotec) per the manufacturer's instructions. Cells were then washed and incubated with anti-mouse cytokine antibodies: PE-Cy7-conjugated anti-IFN-γ, PE-conjugated anti-IL4, and allophycocyanin-conjugated anti-IL17.
All antibodies were from BD Biosciences. Samples were acquired on a FACSCalibur or LSRII cytometer and analyzed using CellQuest (all BD Biosciences) or FlowJo (Tree Star) software.
ELISAs
Total Ig and autoantibody ELISAs were performed as in 11 and 40 with the following modifications. ssDNA was prepared by boiling dsDNA and promptly chilling on ice. For experiments with dsDNA plus histones, dsDNA-coated plates were subsequently incubated with total histones (Roche) in 0.06 M HCO3−.
Autoantigen arrays
Autoantibodies were measured on an autoantigen proteomic array as in 43. The array includes 70 autoantigens and eight control proteins. One microliter of serum samples were pretreated with DNAse I for 30 min and diluted 1:100 in PBS + Tween 20 before being added to the arrays in duplicates. Arrays were incubated with samples at room temperature for 1 h with agitation. Detection was with Cy3-labeled anti-mouse IgM and Cy5-labeled anti-mouse IgG (Jackson ImmunoResearch). A Genepix 4000B scanner with laser wavelengths 532 (for Cy3) and 635 (for Cy5) was used to generate images for analysis. Images were analyzed using Genepix Pro 6.0 software to generate a Gene Pix results file. Background subtracted fluorescence intensities of duplicated spots were averaged and then normalized using mouse IgG or IgM which were spotted onto each array as internal controls. Hierarchical clustering analysis of autoantibodies was performed using Cluster and Treeview software (http://rana.lbl.gov/EisenSoftware.htm).
Kidney histology
Kidneys from 8- to 12-month-old mice were fixed in 10% buffered formalin (Fisher Scientific). Sagittal sections were stained with H&E and with periodic acid Schiff and examined by pathologists who were blind to the identity of the samples. GN and tubular interstitial nephritis severity were graded on a scale of 0–4 as described in 63, 64.
For IgG staining, a representative piece of fresh kidney cortex was embedded with Tissue-Tek O.C.T. Compound (Sakura Finetek) and frozen in a Leica CM1850 cryostat (Leica Biosystems). A frozen section was cut at 3–5 μm thickness, placed on a positively charged slide and air dried at room temperature for 30 min. The slide was then rinsed with PBS, fixed in 95% ethanol, hydrated with PBS, and placed in a darkened humidity chamber. One hundred microliters of diluted (1:250), FITC-conjugated, goat polyclonal Ab to mouse IgG (ab97022, Abcam) was added and the slide incubated at room temperature for 30 min, followed by rinsing with PBS. The stained slide was mounted with a coverslip using Aquamount (Thermo Fisher Scientific) and viewed with Olympus BX51 fluorescence microscope (Olympus). The intensity of staining was graded on a scale of 0–3 by a pathologist blind to the identity of the samples.
Quantitative real-time PCR
Splenocytes were lysed in Trizol® (Invitrogen). Total RNA was prepared using a Qiagen RNeasy Kit (Qiagen), and cDNA was generated with a cDNA Archive Kit (Applied Biosystems) according to the manufacturers’ instructions. Quantitative PCR was performed in a Bio-Rad CFX96 machine using Taqman reagents specific for IL-21 and GAPDH (Applied Biosystems). Data were normalized to GAPDH using the delta comparative threshold cycle method 65.
Acknowledgments
We thank Arturo Menchaca, Lyndsay Joson, and Veronica Gaffney for excellent technical assistance and Veronica Gaffney for critical reading of the manuscript. This work was funded by NIH grants P01 AI039824 (A.B.S.) and 1 F31 GM076982 (T.G.). A.B.S. is a Southwestern Medical Foundation Scholar in Biomedical Research. Autoantigen microarray assays were performed in UT Southwestern Microarray Core Facility and funded by NIH P50 AR055503. Intracellular T-cell studies were funded by NIH grant K24AI079272 (N.J.K.).
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- GN
-
- glomerulonephritis
-
- PC
-
- plasma cell
-
- SLE
-
- systemic lupus erythematosus
-
- Tfh
-
- T follicular helper




