Effective T-cell recall responses require the taurine transporter Taut
Abstract
T-cell activation and the subsequent transformation of activated T cells into T-cell blasts require profound changes in cell volume. However, the impact of cell volume regulation for T-cell immunology has not been characterized. Here we studied the role of the cell-volume regulating osmolyte transporter Taut for T-cell activation in Taut-deficient mice. T-cell mediated recall responses were severely impaired in taut−/− mice as shown with B16 melanoma rejection and hapten-induced contact hypersensitivity. CD4+ and CD8+ T cells were unequivocally located within peripheral lymph nodes of unprimed taut−/− mice but significantly decreased in taut−/− compared with taut+/+ mice following in vivo activation. Further analysis revealed that Taut is critical for rescuing T cells from activation-induced cell death in vitro and in vivo as shown with TCR, superantigen, and antigen-specific activation. Consequently, reduction of CD4+ and CD8+ T cells in taut−/− mice upon antigen challenge resulted in impaired in vivo generation of T-cell memory. These findings disclose for the first time that volume regulation in T cells is an element in the regulation of adaptive immune responses and that the osmolyte transporter Taut is crucial for T-cell survival and T-cell mediated immune reactions.
Introduction
The specific activation of T cells immediately involves mechanisms of cell volume regulation allowing T cells to become T-cell blasts. Subsequent to activation, most T lymphocytes undergo apop-tosis mediated by proapoptotic molecules such as Fas and Bim to avoid harmful and autoaggressive immune disorders [1-5]. On the other hand, survival of activated T lymphocytes is a prerequisite for the generation of T-cell memory able to mount effective recall responses [6]. Thus, regulation of T lymphocyte survival is crucial for rapid effector immune functions and establishment of T-cell memory. Still the exact mechanisms underlying this selective process of acquiring resistance to apoptosis, consecutive survival, and generation of T-cell memory remain enigmatic [5, 7, 8].
Survival of cells critically depends on the maintenance of cell volume [9]. As cell membranes are usually highly permeable to water, the constancy of cell volume requires an osmotic equilibrium between intracellular and extracellular fluid. Cellular mechanisms employed to adjust intracellular osmolarity include the cellular accumulation of organic osmolytes. Cellular increase of osmolytes can be achieved by metabolic generation [10] or by Na+ coupled transport, as in the case of taurine [11]. Beyond their impact on cellular osmolarity and thus cell volume, osmolytes, such as taurine, stabilize proteins [10] and protect cells against injury such as oxidative stress [12]. Even though regulation of cell volume and osmolyte transport should also be critical for the generation of T-cell memory, it is unknown whether and how cell volume regulation contributes to T-cell survival and consecutively to T-cell memory responses.
The semi-essential amino acid taurine was found to accumulate in T lymphocytes where it accounts for approximately 44% of the total free amino acid pool [13]. Hence, taurine is considered to be important for cell volume regulation, survival, and function of those cells [12, 14-18]. Cellular taurine accumulation is accomplished by Taut, a Na+, Cl−, taurine co-transporter. Recently, gene-targeted mice lacking Taut (taut−/−) have been generated, and shown to have markedly decreased taurine concentrations in plasma, kidney, liver, eyes, and skin [18, 19]. Consequently, these animals suffer from severe retinal degeneration [19] and chronic liver diseases [20]. Concerning lymphocytes, Taut was shown to be expressed in human cord blood-derived T lymphocytes and an efficient taurine uptake into these cells was demonstrated [21]. Moreover, administration of a taurine-free diet resulted in lymphopenia and regression of spleen and lymph nodes in cats [22]. Conversely, supplementation of taurine increased T-cell proliferation after PMA/ionomycin stimulation [23, 24]. Despite those observations, functional consequences of Taut for T-lymphocyte function have not been analyzed.
Here we show that the absence of Taut profoundly enhanced apoptosis during T-cell activation in vitro and in vivo. Consequently, taut−/− mice have severe defects in establishing T-cell memory and delayed-type hypersensitivity reactions such as tumor rejection and contact hypersensitivity responses (CHS). These data demonstrate that regulation of T-cell volume by Taut is crucial for T-cell survival, T-cell mediated immune reactions, and T-cell memory development thus disclosing a novel element in the regulation of immune responses.
Results
Impaired tumor rejection in taut−/− mice
To investigate the potential role of Taut for T lymphocyte function we immunized taut+/+ and taut−/− mice against B16 melanoma cells and subsequently challenged these mice with melanoma cells into the back skin (Fig. 1A). Melanomas increased in size over 6 days in nonsensitized animals while in sensitized mice tumor growth could be controlled (Fig. 1B). However, and in contrast to sensitized taut+/+ mice, which all rejected the melanomas within 6 days, sensitized taut−/− mice failed to reject the melanomas leading to (i) persistence of tumors in all mice on day 6 and (ii) significantly larger tumors when comparing the groups (Fig. 1 B and C). There was also a tendency for larger melanomas in nonsensitized taut−/− mice, but this difference was not significant (Fig. 1B and C). To extend our analysis, we challenged sensitized mice with an increased tumor load and analyzed these mice for 15 days. While taut+/+ animals again were able to control melanoma, in taut−/− mice tumor size further increased over time (Supporting Information Fig. 1). Since T cells are critical for tumor rejection in this model, our data indicate impaired T-cell memory responses in taut−/− mice.
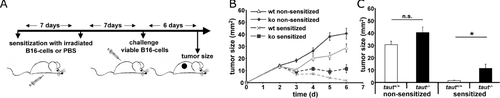

Impaired CHS responses in taut−/− mice
To investigate the validity of this finding, we tested for a role of Taut in a second T-cell mediated immune response, the model of CHS against trinitrochlorobenzene (TNCB) [25]. Taut+/+ and taut−/− mice were sensitized with 5% TNCB at the shaved abdomen and 7 days later challenged with TNCB at the ears to elicit the TNCB-specific T-cell mediated contact dermatitis. While taut+/+ mice responded with a typical delayed-type hypersens-itivity recall reaction as determined by the increase of ear thickness, CHS was reduced in taut−/− mice with a significant difference already at 12 h (Fig. 2A). Histological analysis confirmed that infil-trating leukocytes were significantly decreased in taut−/− mice compared with wild-type (WT) mice (Fig. 2B and C).
Unaltered lymphocyte populations in unprimed taut−/− mice
One possible explanation for reduced T-cell mediated immune responses in taut−/− mice would be a general decrease or shift of leukocyte numbers. However, total white blood cell counts from blood of unprimed mice did not yield significant differences between taut−/− and their WT littermates (taut+/+ 5 ± 1 × 103/μL; taut−/− 6 ± 1 × 103/μL). Likewise, lymph node FACS analysis of unprimed mice showed that the percentages of lymph node CD3+ CD45+ T cells were almost identical in taut−/− compared with those in taut+/+ mice (Fig. 3A and C) with no significant differences observable between taut−/− and taut+/+ mice. Moreover, no differences were found in lymph node CD4+ or CD8+ T cells from these unprimed taut−/− and taut+/+mice (Fig. 3D). Additionally, we could not detect any obvious differences in the architecture of lymphoid organs by histological analysis (Supporting Information Fig. 2).

Significantly reduced T lymphocytes in taut−/− mice after in vivo T-cell activation
As we found no alterations of lymphocyte populations in untreated taut−/− mice, we analyzed lymphocytes from skin draining lymph nodes after elicitation of CHS. CD3+ T cells from taut−/− CHS mice were significantly reduced (Fig. 3B right panel, 3E), affecting both CD4+ (29% of gated cells corresponding to 1431 ± 88 gated cells for WT and 16% of gated cells corresponding to 779 ± 282 gated cells for taut−/−) and CD8+ T lymphocytes (21% of gated cells corresponding to 1049 ± 100 gated cells for WT and 12% of gated cells corresponding to 579 ± 42 gated cells for taut−/−) (Fig. 3F). This suggested that in the absence of Taut in vivo generation or maintenance of activated T cells is impaired. Thus, deficiency of Taut may either lead to aberrant homing or to diminished T-cell numbers and consequently to reduced T-cell mediated immune responses.
Increased cell death and reduced survival in taut−/– T lymphocytes after activation
The observed decrease of T lymphocytes in taut−/− mice after challenging the immune system could result from modified survival of activated T cells or could be a consequence of other in vivo dysfunctions. Therefore, lymphocyte 3H-thymidine uptake, survival, and apoptosis were investigated in vitro after specifically activating T lymphocytes with mAbs to CD3 and CD28. After activation, 3H-thymidine uptake was strongly diminished in taut−/− T cells compared with that of WT T cells (Fig. 4A). Moreover, survival of T cells following activation in the absence of Taut was found to be markedly reduced for both, whole lymph node cells (Fig. 4B) and purified CD4+ and CD8+ T cells (data not shown), indicating that Taut-mediated cell volume regulation might be crucial to rescue T cells from cell death by apoptosis. Indeed, AnnexinV staining detecting phosphatidylserine exposure as sign of early apoptosis was two- to threefold increased in both CD4+ and CD8+ taut−/− T cells as compared with WT T cells (Fig. 4C and D, respectively). Increased apoptosis in taut−/− CD4+ and CD8+ T cells was not restricted to activation of CD3/CD28, but was seen with other modes of T-cell activation tested, such as stimulation with PMA/Ionomycin (Supporting Information Fig. 3). These experiments demonstrate that Taut is crucial for in vitro T-cell survival after activation and that in the absence of Taut, both CD4+ and CD8+ T cells rapidly undergo activation-induced apoptosis.
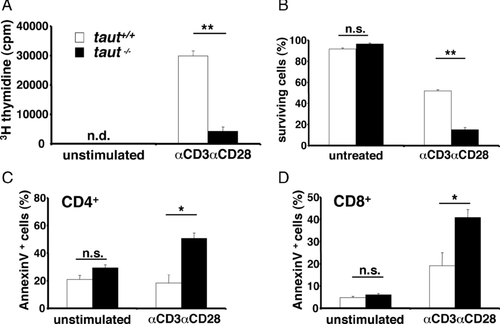
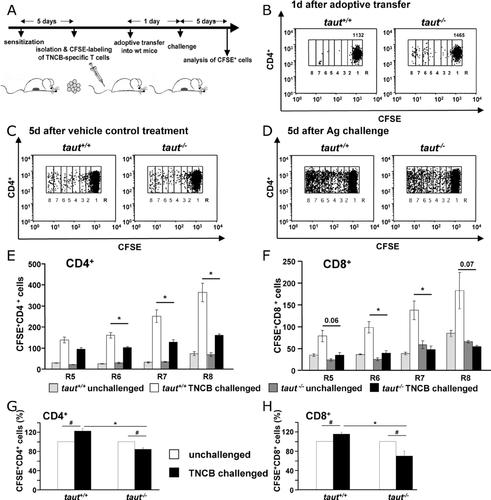
Reduced survival of proliferating taut−/− T lymphocytes after antigen-specific activation in vivo
Activated taut−/− T lymphocytes showed increased cell death in vitro and T-cell mediated recall immune responses were impaired in taut−/− mice. Therefore, we investigated antigen-specific activation of T lymphocytes in vivo. We adoptively transferred T lymphocytes to specifically monitor proliferation and survival after antigen-specific activation in recipient mice. WT and taut−/− mice were epicutaneously sensitized with TNCB, and T cells were isolated from skin draining lymph nodes 5 days later and loaded with carboxyfluorescein diacetate succinimidyl ester (CFSE). Equal amounts of CFSE+ taut−/− or CFSE+ taut+/+ T cells were adoptively transferred into individual unprimed WT mice. This experimental setup excludes additional constraints due to the loss of Taut in other than T cells and allows direct conclusions for the role of Taut in T-cell response in vivo, as the antigen-specific T cells had been transferred to an intact WT environment. T-cell analysis revealed unequivocal homing of taut+/+ and taut−/− CFSE+ CD4+ (Fig. 5B) as well as CFSE+ CD8+ T cells (Supporting Information Fig. 4A) to the host skin draining lymph nodes and spleen (not shown). One day after transfer, recipient mice received either TNCB or vehicle control challenge and 5 days later, mice were sacrificed and skin-draining lymph nodes were analyzed for the presence of dividing donor T cells as determined by CFSE brightness. In the absence of antigen we found only little proliferation and no difference between taut+/+ and taut−/− T-cell subpopulations (Fig. 5C, Supporting Information Fig. 4B). Challenging the recipient mice with TNCB led to an increase of CFSElow dividing CD4+ and CD8+ T cells in both the taut+/+ and taut−/− T-cell population. However, the number of proliferating taut−/− CFSElow T cells was significantly reduced as compared with taut+/+ CFSElow T cells (Fig. 5D, Supporting Information Fig. 4C). Separate quantification of CD4+ and CD8+ T lymphocytes showed a significantly lower number of dividing cells (Fig. 5E and F, with R5 to R8 corresponding to cells that have undergone four to seven cell divisions respectively) indicating that taut−/− T cells are more susceptible to apoptosis after antigen-specific activation also in vivo.
Increased cell death during activation of taut−/− T cells could consequently lead to decreased overall survival of T lymphocytes after antigen-specific activation. CFSE+ WT T cells from mice with or without antigen challenge exhibited a ∼25% increase in total CD4+ and a ∼20% increase in total CD8+ T cells following antigen challenge (Fig. 5G, H left panel). In contrast, total taut−/− CFSE+ CD4+ and CFSE+ CD8+ T cells were significantly reduced by about 20% and 35%, respectively, in challenged compared with those in unchallenged recipients (Fig. 5G, H right panel).
Increased apoptosis and reduced memory generation in taut−/− T lymphocytes in vivo
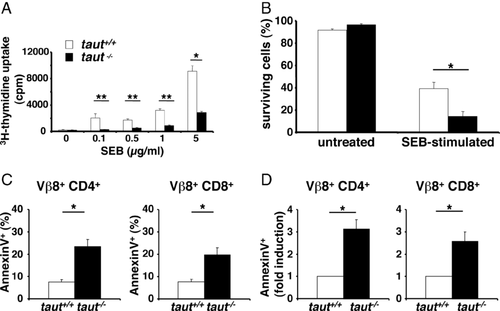
Selective monitoring of activated T cells in vivo can be achieved by examining T-cell responses to the bacterial superantigen staphylococcal enterotoxin B (SEB). SEB specifically activates T cells carrying the T-cell receptor Vβ8 chain and the expression of the Vβ8 chain can be monitored by FACS analysis using the F23.1 mAb. We first specifically stimulated Vβ8+ T lymphocytes with SEB in vitro. Similar to TCR activation with anti-CD3 mAbs (Fig. 4), T-cell activation with SEB-induced strong proliferation of WT T cells (Fig. 6A). In contrast, taut−/− T cells taking up 3H-thymidine were significantly reduced at all SEB concentrations tested (from 0.1 to 5.0 μg/mL SEB, Fig. 6A). It is well known that activation of T cells with SEB leads to activation-induced cell death in Vβ8+ SEB responsive T cells [26]. Indeed, following stimulation with SEB survival of taut−/− T cells was significantly more reduced as compared with survival of taut+/+ T cells, an effect already apparent at a dose of 0.1 μg/mL SEB (Fig. 6B). As in the experiments with anti-CD3 mAbs, T-cell activation by SEB resulted in significantly higher rates of apoptotic CD4+ and CD8+ Vβ8+ T cells in the absence of Taut (Fig. 6C) and induction of phosphatidylserine exposure was two- to threefold higher in taut−/− compared with WT T cells (Fig. 6D, determined by AnnexinV staining). This difference of phosphatidylserine exposure was SEB-specific, as analysis of Vβ6+ T cells, which are nonresponsive to SEB, revealed comparable percentages of AnnexinV+ T cells from taut+/+ and taut−/− T cells (Supporting Information Fig. 5). Next, induction of apoptosis by SEB activation was analyzed in vivo. One day after SEB injection, T cells from both WT and taut−/− mice were analyzed. Following SEB injection, early apoptosis was detectable in Vβ8+ CD4+ and Vβ8+ CD8+ T cells of both genotypes (Fig. 7A and B, respectively). However, the increase of AnnexinV staining was significantly higher in T cells from taut−/− animals. This increase was four- to fivefold higher in SEB-treated taut−/− mice compared with SEB-treated taut+/+ mice (Fig. 7C). Consequently, the reduction of Vβ8+ CD4+ and Vβ8+ CD8+ T cells following SEB treatment in vivo was also more than doubled in T cells lacking Taut (Fig. 7D). Thus, activation-induced apoptosis was selectively increased in SEB responsive taut−/− T cells in vivo.
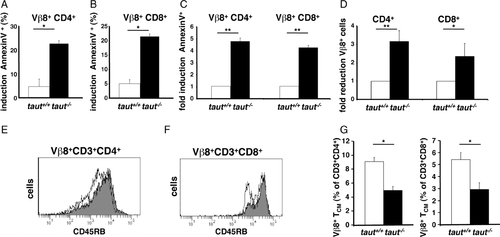
Reduced T-cell memory generation in taut−/− mice
Following activation, T cells downregulate CD45RB, an isoform of the glycoprotein CD45 highly expressed on naïve T cells [27]. Investigating CD45RB expression in T cells from sensitized mice allows us to specifically monitor T cells, which were previously activated (antigen-experienced). In line with the analysis of T-cell numbers in unprimed mice (Fig. 3A, C, and E), no differences in regard to CD45RBlow T-cell numbers were observed comparing unprimed taut−/− and WT mice (Supporting Information Fig. 6A). Similarly, no significant differences were observed in TNCB-sensitized taut−/− and WT mice investigating the T-cell population that was not affected by activation and remained naïve (CD45RBhigh naïve T cells, Supporting Information Fig. 6B). In contrast, we selectively found reduced numbers of CD45RBlow taut−/− T cells corresponding to activated and memory T cells (Supporting Information Fig. 6B, CD45RBlow) confirming the selective increase of apoptosis after activation in taut−/− T cells (Fig. 3B, D, and F; Fig. 5). Thus, these data indicate that increased apoptosis of taut−/− T cells selectively kills T cells following activation in vivo as shown for taut−/− T cells in vitro (Fig. 4 and Supporting Information Fig. 3).
Increased apoptosis in activated T cells is expected to reduce the formation of memory T cells. Central memory T (TCM) cells represent the long-lived T-cell memory population that rapidly responds to its specific antigen and upon antigen re-exposure gives rise to effector T cells migrating into peripheral tissues. TCM cells were shown to establish persistent T-cell memory also in primates [28], to preferentially migrate to lymph nodes, and TCM cells are CD45RBlow, CD62Lhigh, and CCR7+, whereas naïve cells are CD45RBhigh, CD62Lhigh, and CCR7+. Taut−/−mice showed decreased T-cell immune responses and impaired T-cell survival upon activation in vivo, suggesting that taut−/− mice generate reduced numbers of memory T cells. To address this question, we investigated long-lived T-cell memory in vivo. To this end, SEB responding Vβ8+ T cells were analyzed 5 weeks after repeated SEB injections. Analyzing antigen-experienced effector and memory Vβ8+ T cells we found reduced CD45RBlow populations in taut−/− compared with taut+/+ mice for CD4+ and CD8+ T cells (Fig. 7E and F). Moreover, focusing exclusively on SEB-specific long-lived T-cell memory by quantifying the TCM cells as CD45RBlow, CD62Lhigh, and CCR7+ T cells we found that 5 weeks after the last SEB activation Vβ8+ CD4+ TCM and Vβ8+ CD8+ TCM cells from Taut-deficient mice were significantly reduced compared with WT mice (Fig. 7G), while there was no difference between taut−/− and taut+/+ Vβ6+ CD4+ TCM and Vβ6+ CD8+ TCM cells (data not shown).
Collectively these data demonstrate that the taurine transporter Taut determines T-cell responses by regulating T-cell survival following activation. Consequently, in the absence of Taut, apoptosis is increased leading to impaired T-cell mediated recall responses and reduced memory generation in vivo. Therefore, mechanisms of cell volume regulation in T cells that follow T-cell activation (i) critically involve Taut, (ii) regulate the pool of effector and memory T cells, and (iii) determine the outcome of T-cell mediated immune responses.
Discussion
Cell proliferation and apoptotic cell death are tightly connected to regulation of cell volume [9]. We found that T cells from mice deficient for the cell volume regulating transporter Taut are more susceptible to apoptosis and consequently to cell death, especially when exposed to activation stimuli such as mitogens or specific antigen. Apparently, T-cell activation depends on cell volume regulation for survival and the absence of Taut leads to reduced T-cell memory and T-cell mediated recall responses. In this context, it is especially interesting that lymph node resident T cells were unequivocally present in unprimed taut−/− and taut+/+ mice. This indicates that naïve T cells, which have not experienced antigen exposure and activation, apparently do not depend on cell volume regulation by Taut. Another T-cell subset that resides within lymph nodes are the resting memory T cells called TCM cells [29]. Generation of T-cell memory is still incompletely understood, but TCM cells were shown to develop from activated T cells and to go through two major phases of development: expansion and contraction [5, 8, 30]. Subsequent to antigen exposure, relative resistance to apoptosis allows activated T cells to undergo massive expansion leading to up to 10,000-fold increase of antigen-specific T cells [7, 8]. Consecutively, susceptibility to apoptosis reduces activated CD4+ and CD8+ T-cell numbers by approximately 90% and only activated T cells experiencing sufficient antiapoptotic signals can survive and become memory T cells [5, 7, 8, 30]. Here, we demonstrate that Taut is not a matter of life and death for lymph node resident naïve T lymphocytes but that Taut confers resistance against apoptosis of activated T cells. Consequently, deficiency in cellular taurine uptake in T lymphocytes significantly decreases lymph node resident memory T cells and T-cell mediated recall responses.
One hallmark of apoptotic cell death is shrinkage, which is accomplished by release of ions [31, 32] and organic osmolytes [9, 33, 34]. Lack of the osmolyte transporter Taut impairs cell volume regulation and leads to cell shrinkage and apoptosis and it was shown that CD95-induced DNA fragmentation and phosphatidylserine exposure of Jurkat T cells is immediately preceded by taurine release [33-35]. The present study indeed demonstrates enhanced rates of apoptosis in taut−/− T lymphocytes and it is tempting to speculate that the inability of T cells to accumulate taurine favors cell shrinkage and apoptosis [33-36]. However, lack of taurine does apparently not foster apoptosis in all cell types and under all circumstances. Taut−/− erythrocytes even proofed to be more resistant against several triggers of apoptosis including osmotic cell shrinkage [37]. On the other hand, taurine has been demonstrated to protect endothelial cells and cardiomyocytes against apoptosis [38]. The survival and function of retinal cells has similarly been shown to critically depend on the presence of taurine and taurine uptake [19, 39]. Interestingly, and confirming the finding of this study, addition of taurine reduced the rate of activation-induced apoptosis in human T cells and supplementing taurine in vivo enhanced T-cell mediated tumor rejection thus increasing survival of the animals in a model of metastatic melanoma [23, 40]. To the best of our knowledge, patients presenting with immune deficiencies or not responding to (tumor) vaccination have not been investigated for a role of Taut and cell volume regulation nor were loss of function mutations of Taut analyzed so far. But analyzing the role of Taut may allow us to identify individuals with a Taut-dependent T-cell memory weakness within these groups of patients and taurine supplementation may help to improve immunity [41].
Our data demonstrate that susceptibility to apoptosis in Taut-deficient memory CD4+ and CD8+ T cells is responsible for reduced T-cell survival leading to decreased T-cell numbers. Apoptosis in Taut-deficient T cells decreases the pool of remaining memory T cells and consequently, T-cell mediated immune responses are impaired highlighting the importance of cellular taurine accumulation for lymphocyte survival.
In summary, these findings show that cell volume regulation by Taut is crucial for T-cell survival and T-cell mediated immune reactions and unravel a novel element in the regulation of immune responses.
Methods
Animals
Generation of Taut-deficient mice has been described previously [19]. Eight- to twelve-week-old Taut knockout mice (taut−/−) and their age-matched littermates (taut+/+) were used for contact hypersensitivity and melanoma experiments. C57BL/6N mice for transfer experiments were purchased from Charles Rivers (Sulzfield, Germany). All experimental procedures were made according to European guidelines and German law and have been approved by the local authorities (Regierungspräsidium Tübingen, permission number Py6/07).
White blood cell isolation and lymphocyte immunophenotyping
White blood cell analysis was performed from peripheral whole blood obtained by retro-orbital plexuspunctation or from cell suspension of auxiliary and inguinal lymph nodes. Cells were stained with labeled antibodies for CD45, CD19, CD3, CD4, CD8, CD25, and CD69, CD45RB, CCR7, CD62L (BD Pharmingen, Heidelberg, Germany) and analyzed by flow cytometry using a FACS Calibur and Cellquest software (Becton Dickinson, Heidelberg, Germany). Cells present in the mononuclear living cell fraction as determined by propidium iodide exclusion (Sigma, Munich, Germany) were analyzed. Whole cell counts of 20 μL whole blood were performed using an electronic hematology particle counter (Medical Diagnostics Marx, Butzbach, Germany).
T-cell culture
T cells were cultured as previously described [42, 43]. To expand TNCB-specific T cells in vitro, 2.5 × 105 T cells mixed with 5 × 105 of trinitrobenzenesulfonic acid solution (TNBS)-treated antigen presenting cells (APCs) were cultured in DMEM, supplemented with 10 mM HEPES, 1 mM Na-pyruvate, MEM-aminoacids, 50 U/ml Penicillin, 50 μg/ml Streptomycin, 25 μM β-mercaptoethanol, 10% fetal calf serum (FCS), Biochrom, Berlin, Germany). T cells were stimulated every other week with freshly isolated APCs loaded with TNBS.
Activation-induced apoptosis of T lymphocytes and Annexin binding assay
CD4+ and CD8+ T cells were isolated from lymph nodes by MACS selection kit (MiltenyiBiotec, BergischGladbach, Germany), seeded separately in 96-well plates (4 × 105/200 μL), and treated with 0.5 μg/mL phorbol 12-myristate 13-acetate (PMA; Sigma, Taufkirchen, Germany) and 1 μM ionomycin (Sigma) or with carrier control for 8 h. For αCD3/αCD28 stimulation plates were coated with 5 μg αCD3/mL (BiolegendEurope, Uithoorn, Netherlands) o.n. and washed with PBS. 2 × 106 whole lymph node cells were seeded with 20 μg αCD28/mL (Biolegend) and cultured for 3 days. To evaluate activation-induced apoptosis phosphatidylserine surface exposure on CD4+ and CD8+ T cells lymphocytes were quantified utilizing AnnexinV (BD Pharmingen) and subsequent flow cytometry analysis. Survival of activated T cells was determined by Trypan blue dye exclusion.
Proliferation assay
For proliferation assays cells were seeded in 96-well plates and stimulated with SEB or αCD3/αCD28. 0.25 μCi 3H-thymidine (Amersham, GE Healthcare, Freiburg, Germany) was added per well and 10 h later cells were harvested. Incorporated 3H-thymidine was measured with a micro beta counter (Perkin Elmer, Wiesbaden, Germany).
Mouse melanoma model
Taut−/− and taut+/+ mice were sensitized as described by Van Loveren [44] by s.c. injection of 1 × 107 lethally irradiated mouse B16 melanoma cells (cell line from ATCC Promochem, Wesel, Germany) or mock injected with PBS twice at intervals of 7 days. After another 7 days mice were intracutaneously injected with 1 × 106 or 1.5 × 106 intact B16 melanoma cells into each flank of the back skin. Tumor size was measured with a caliper.
TNCB-induced contact hypersensitivity
TNCB sensitization was carried out as described earlier [25, 43]. Briefly, taut+/+ and taut−/− mice were sensitized by administration of 5% TNCB solution (80 μL of a 4:1 mixture of acetone/olive oil) on the shaved abdomen and 7 days later challenged by application of 1% TNCB (30 μL of a 1:9 mixture of acetone/olive oil) on both sides of the ears. Control group mice were treated with 1% TNCB on the ears only. Ear thickness was measured with a micrometer (Oditest®; Kroeplin, Germany) and data are expressed as change in ear thickness compared with that before treatment. Nonspecific thickness increase (irritant reaction in control group mice) was subtracted.
SEB stimulation
For in vitro studies 1×106 whole lymph node cells/mL were cultured for 3 days in the presence of different amounts of SEB (Toxin Technologies, Sarasota, FL, USA), and cellular survival was determined by Trypan blue dye exclusion. For in vivo studies 50 μg SEB was injected i.p. and 1 day later, mice were sacrificed and lymph nodes were isolated. AnnexinV binding was analyzed by FACS after staining with CD4-Pacific blue (BD Pharmingen), CD8-allophycocyanin (Biolegend) Vβ8-PE (BD Pharmingen), Vβ6-PE (BD Pharmingen), AnnexinV and 7-AAD (e-Bioscience, NatuTec GmbH Frankfurt, Germany) with a LSRII flow cytometer (Becton Dickinson, Heidelberg, Germany). For investigation of TCM-cell generation mice were injected twice with 50 μg SEB intraperitoneally at an interval of 4 weeks. Five weeks after the second injection SEB-specific TCM cells were quantified by FACS analysis.
Histology
Ear tissue was collected 48 h after TNCB challenge, lymph nodes and spleen were isolated from untreated mice. Tissues were fixed in paraformaldehyde (PFA) and sections were stained with hematoxylin and eosin.
Adoptive transfer
Taut+/+ and taut−/− animals were sensitized with 5% TNCB and 5 days later CD3+ T cells were isolated from draining lymph nodes and spleen by negative selection (MiltenyiBiotec) according to manufacturer's instructions. T cells were loaded with 1 μM of CFSE and equal amounts of labeled taut+/+ or taut−/− cells were i.v. injected into C57BL/6N WT mice (9 × 106 cells per mouse in PBS). The consecutive day recipient mice were challenged with 2% TNCB solution administered to the shaved abdominal skin; controls were treated with solvent alone. Six days after transfer, mice were sacrificed and lymph node cells were analyzed by FACS. Eight regions (R1–R8) relevant to the numbers of T-cell divisions showing halving of the fluorescence intensity of the CFSE dye were defined. The recovery of cells was counted as the number of CFSE+ cells from all fractions (R1–R8) per acquired events multiplied by total number of isolated cells (frequency of recovered cells = (ΣCFSE+ cells/total acquired events) × total lymph node cell number). Recovered cell numbers from TNCB challenged recipient mice were normalized to recovered cell numbers from accordant not-challenged recipients, enumerated as 100%.
Statistics
Unpaired t-test, or analysis of variance (ANOVA) as appropriate, has been employed to analyze for significant differences in the respective parameters between taut+/+ and taut−/− mice, or treated lymphocytes. P < 0.05 has been considered to be statistically significant.
Acknowledgements
We thank Ulrike Schmidt for excellent technical assistance and Martin Röcken for critically reading the manuscript. This work was supported by SFB 685 (S.K. and T.B.), DFG grant BI 696/3-3 and 696/5-1 (T.B.), SFB 575 (U.W. and D.H.), DFG Graduate Program 1033 (U.W. and D.H.), SFB 766 (F.L.), and DFG grant 1279/3-1 (T.W.).
Conflict of interest: All authors declare no financial or commercial conflicts of interest.
References
Abbreviations
-
- CHS
-
- contact hypersensitivity responses
-
- SEB
-
- staphylococcal enterotoxin B
-
- TCM cell
-
- central memory T cell
-
- TNCB
-
- trinitrochlorobenzene




