The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4+ NKT cells
Abstract
While most CD4+ T cells are MHC class II-restricted, a small subset, including the CD1d-restricted ‘invariant’ NKT (iNKT) cells, are selected on non-classical MHC-I or MHC-I-like molecules. We previously showed that the sequential activity of two zinc finger transcription factors, Gata3 and Thpok, promotes the differentiation of conventional, MHC II-restricted thymocytes into CD4+ T cells. In the current study, we show that a Gata3-Thpok cascade is required for the differentiation of CD4+ iNKT cells. Gata3 is required for iNKT cells to express Thpok, whereas Thpok is needed for proper NKT cell differentiation, and notably for NKT cells to maintain CD4 and terminate CD8 expression. These findings identify the sequential activity of Gata3 and Thpok as a hallmark of CD4+ T-cell differentiation, regardless of MHC restriction.
Introduction
While the vast majority of CD4+ T cells are MHC II-restricted, small but functionally important contingents of CD4+ cells are restricted by other MHC or MHC-like molecules. The most abundant of these cells recognize lipid-bound CD1d molecules, express markers typical of NK cells, including the surface antigen NK 1.1, and can be identified in vitro through their binding to tetramers of α-galactosylceramide (Gal-ceramide)-bound CD1d 1. NKT cells express an invariant TCR-α chain and a limited repertoire of TCR-β chains (hence their name, invariant NKT cells, iNKT), and NKT-cell development is largely dependent on the transcription factor PLZF 2, 3. Although they are selected by MHC I-like CD1d molecules, iNKT cells typically do not express CD8, and appear as CD4+CD8− (CD4 single positive (SP)) or CD4−CD8− (double negative (DN)). This is unlike subsets of MHC I-restricted cells that acquire effector function in the thymus and adopt a CD8-SP phenotype 4, 5. Currently, it is unclear why, unlike conventional MHC I-restricted thymocytes, iNKT precursors differentiate into CD4+ rather than CD8+ cells.
The transcription factors Gata3 and Thpok are both required for the generation of CD4+ T cells from CD4+CD8+ (double positive, DP) thymocytes 6. Gata3 is expressed throughout T-cell differentiation and is required at multiple branch points during T-cell development or effector differentiation 7. Gata3 expression is transiently up-regulated during the differentiation of CD4-lineage thymocytes 8; it is required at an early step of CD4+ T-cell development, and has been proposed to promote the expression of genes specifically expressed in CD4+ T cells, or required for their differentiation 9. Thpok is not expressed in DP thymocytes and its up-regulation during CD4-lineage differentiation requires Gata3 9-11. Thpok is required for the commitment of MHC II-restricted thymocytes to the CD4-lineage, notably by repressing the expression of the transcription factor Runx3 and of cytotoxic genes, and by promoting CD8 silencing 12-15.
In the current study, we found that Thpok is expressed at a high level in CD1d-restricted NKT cells in the thymus and peripheral lymphoid organs. Thpok is required for the differentiation of CD4+ iNKT cells and for their repression of CD8, a function reminiscent of the one it serves in MHC II-restricted T cells. We further document that Thpok expression in iNKT cells is highly dependent on Gata3. We conclude from these findings that a similar cascade, sequentially involving Gata3 and Thpok, promotes the differentiation of MHC II- and CD1d-restricted thymocytes into CD4+ T cells.
Results and discussion
We previously described a bacterial artificial chromosome reporter transgene in which the first Thpok coding exon (exon 2) has been replaced by a GFP cDNA 9, 15 (Supporting Information Fig. 1, thereafter referred to as ThpokGFP transgene). In two independently derived mouse lines, this transgene expressed GFP in essentially all CD4+ T cells and mature CD4-SP thymocytes but not in CD8-SP thymocytes, or in naïve or memory spleen or LN CD8+ T cells 9. However, we found low-level GFP expression in activated ThpokGFP CD8+ cells (Fig. 1A), in agreement with a recent report 16. Immunoblot analyses demonstrated expression of endogenous Thpok protein (Fig. 1A), although at levels well below those in CD4+ cells.
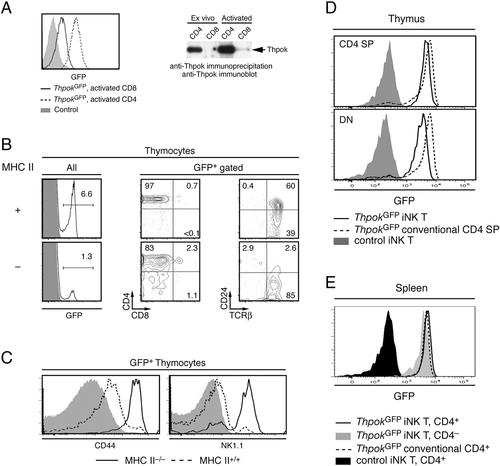
Expression of the ThpokGFP reporter in iNKT cells. (A) GFP expression on ThpokGFP CD8+ (plain line) or CD4+ (dashed line) T cells 7 days after activation, overlaid on background fluorescence from similarly activated control cells (grey-shaded) (left). Expression of Thpok protein was analyzed by immunoprecipitation and immunoblot on freshly isolated (left) and in vitro activated (day 7) CD4+ and CD8+ T cells (right). (B and C) Thymocytes from MHC II-deficient or -sufficient mice carrying the ThpokGFP reporter were analyzed by multicolor flow cytometry. (B) GFP fluorescence (plain lines) is overlaid over background fluorescence from control non-transgenic mice (grey shaded). Two parameter contour plots gated on GFP-expressing cells show CD4 versus CD8 (middle) and TCR-β versus CD24 expression. (C) Overlaid histograms show expression of CD44 (left) and NK1.1 (right) on GFP+ cells from MHC II-deficient (plain lines) and -sufficient (dashed lines). Grey-shaded histograms show staining with an isotype-control antibody. (D) Overlaid histograms show GFP fluorescence (plain lines) on CD4-SP (top) and DN (bottom) iNKT thymocytes from ThpokGFP mice, and background fluorescence (grey-shaded) on the same populations from control mice. Subsets were defined by expression of CD4, CD8, and CD24, and reactivity with α-Gal-ceramide-loaded CD1d tetramer, as shown in Supporting Information Fig. 2A. The dashed line shows GFP fluorescence on conventional CD4-SP cells from ThpokGFP mice. (E) GFP fluorescence is shown on CD4+ (plain line) and CD4− (grey-shaded) iNKT cells, and on conventional CD4+ T cells (dashed lines), all from the spleen of ThpokGFP mice, as gated in Supporting Information Fig.2B. The black-filled histograms depict background fluorescence on CD4+ iNKT cells from control mice. Data (A–E) are representative of at least two experiments.
This suggested that Thpok expression in the T-cell lineage was not strictly limited to MHC II-restricted cells. To further explore this possibility, we introduced the ThpokGFP reporter into MHC II-deficient mice. In such mice, a small subset of thymocytes expressed GFP, of which most were TCRhi CD24lo (Fig. 1B). These cells shared two additional characteristics: they did not express CD8 and were CD44hi (Fig. 1B, C). Invariant NKT (iNKT) cells, which recognize CD1d-bound lipids 17, form the main subset of CD4+ T cells in MHC-II-deficient mice and are TCRhi CD24lo CD44hi, prompting us to assess whether they express Thpok. Supporting this idea, most GFP+ thymocytes in MHC-II-deficient ThpokGFP mice expressed the NKT cell marker NK1.1 (Fig. 1C). Indeed, most iNKT thymocytes, identified using α-Gal-ceramide-loaded CD1d tetramers, expressed the Thpok reporter (Fig. 1D and Supporting Information Fig. 2), and so did peripheral iNKT cells (Fig. 1E).
iNKT cells derive from DP thymocytes 18, cease CD8 expression early during their differentiation, and therefore become CD4-SP cells. Subsequently, a subset of them terminates CD4 expression and becomes CD4−CD8− DN thymocytes. These DN iNKT cells also expressed GFP, although slightly less than their CD4-expressing counterparts (Fig. 1D). This differs from MHC II-restricted cells, which all express both Thpok and CD4. Future studies will determine the mechanistic basis for this difference. Unlike CD4-differentiating thymocytes and naïve CD4+ T cells 13, 15, iNKT cells co-express Runx3 and Thpok, with similar Runx3 levels in CD4+ and DN subsets (Y. X. and R. B., unpublished results); whether or not Runx3 affects CD4 expression in iNKT cells remains to be determined, although we and others previously found that Thpok antagonizes the Cd4-silencing function of Runx3 14, 15, 19.
To examine whether Thpok is required for iNKT-cell development, we evaluated iNKT populations in Thpok-deficient mice 9. While there was no reproducible difference in the frequency of CD1d-specific cells between Thpok-deficient and Thpok-sufficient thymi, Thpok-deficient iNKT cells failed to express CD4, and a subset of them expressed low levels of CD8 (Fig. 2A). iNKT cells were also found in normal frequency in the spleen and liver of Thpok-deficient mice, where they also displayed a DN or CD8-expressing phenotype (Fig. 2B and data not shown). Thus, unlike the related zinc finger transcription factor PLZF 2, 3, Thpok is not needed for the development of iNKT cells, but it is required for their continued CD4 expression. Of note, Thpok-deficient iNKT cells expressed less CD8 than conventional CD8+ cells, and a subset of them expressed CD8α but not CD8β (Fig. 2B, right and Supporting Information Fig. 3A). This suggests that so far unidentified transcription factors, expressed in conventional CD8+ cells but not in iNKT cells, promote CD8 expression. In addition, Thpok-deficient iNKT cells had impaired expression of markers of effector function, including NK1.1 and Granzyme B (Fig. 2C and D), although not of CD44 (Supporting Information Fig. 3B), indicating that Thpok controls multiple aspects of iNKT-cell differentiation. The reduced Granzyme B expression by Thpok-deficient iNKT cells (Fig. 2D) was especially noticeable since Thpok represses Granzyme B expression in conventional T cells 12, 15. Thus, Thpok affects multiple aspects of iNKT cells development in addition to their coreceptor expression. A recent report similarly found that Thpok promotes IFN-γ expression in iNKT cells 20, whereas it has the opposite effect in conventional CD4+ cells 15.
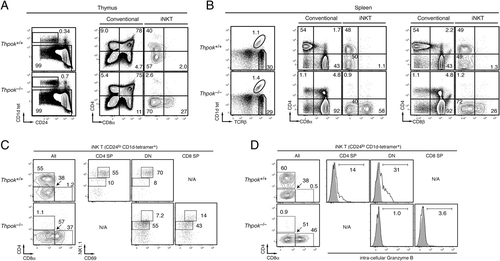
Thpok is required for the differentiation of CD4+ iNKT cells. (A) Conventional and iNKT thymocytes, defined on the basis of α-Gal-ceramide-loaded CD1d binding and expression of CD24 (left) are analyzed for CD4 and CD8α expression (right). (B) Contour plots (right) display expression of CD8α versus CD4 or CD8β versus CD4 on conventional T cells and iNKT cells in the spleen, gated as shown in the top left plots. Data (A and B) are representative of seven mice of each genotype analyzed in four separate experiments. (C and D) Expression of surface CD69 and NK1.1 (C), or of intra-cellular Granzyme B (D) is analyzed on iNKT thymocytes from wild-type and Thpok-deficient defined on the basis of CD24 expression and CD1d-tetramer binding as in (A) and gated for CD4 and CD8α expression as shown in the leftmost plots. Data are representative of three mice of each genotype analyzed in two separate experiments. Grey-shaded histograms in (D) show staining with an isotype control.
Similar to their Thpok-deficient counterparts, Gata3-deficient iNKT cells have reduced CD4 expression 21. We had previously proposed a sequential model for Gata3 and Thpok function during the development of MHC II-restricted CD4+ T cells, whereby Gata3 is required for Thpok expression and Thpok promotes CD4-commitment and CD8 silencing 9. Consequently, we wondered whether Gata3 was required for Thpok expression in iNKT cells. To assess this, we evaluated the expression of Thpok mRNA by RT-PCR in iNKT cells purified from Gata3-sufficient mice and from mice in which floxed Gata3 alleles were deleted in DP cells by a Cd4-Cre transgene (referred to as Gata3ΔDP mice)22. These analyses showed a 90% reduction in Thpok expression in Gata3-deficient relative to wild-type iNKT cells (Fig. 3A). To determine whether this resulted from a homogenous reduction of Thpok expression, we introduced the ThpokGFP transgene into Gata3ΔDP mice. Consistent with our previous finding that Gata3 is required for Thpok expression, there was little or no GFP fluorescence in conventional thymocytes (CD44lo NK1.1−) in ThpokGFP Gata3ΔDP mice (Supporting Information Fig. 4A). In iNKT thymocytes, Gata3 disruption made GFP expression barely detectable, compared with their Gata3-sufficient counterparts (Fig. 3B, columns 2 and 4, and Supporting Information Fig. 4B). We conclude from these experiments that Gata3 promotes Thpok expression in iNKT cells.
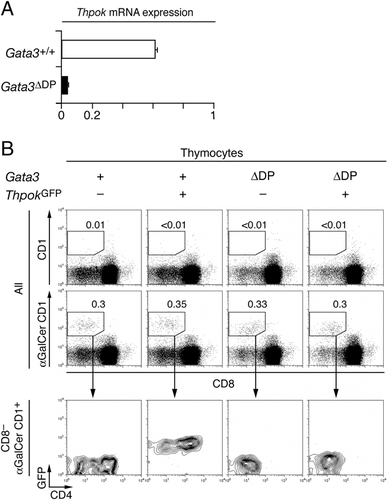
Gata3 promotes Thpok expression in both iNKT and conventional CD4+ T cells. (A) Expression of Thpok mRNA was measured by real-time RT-PCR on sorted iNKT thymocytes (CD24lo CD1d-tetramer+) from Gata3+/+ and Gata3ΔDP mice. Values were normalized to β-actin expression in the same samples and are displayed relative to Thpok mRNA expression in conventional CD4-SP thymocytes, set to 1. Data are representative of two distinct determinations from two separate sorts. (B) iNKT-cell populations are defined on two parameters plots of CD8 expression and α-Gal-ceramide-CD1d binding; the top row show absence of binding of unloaded CD1d tetramers as a specificity control (top panels). Two parameter plots show CD4 versus GFP expression on gated iNKT cells. Note the reduced CD4 levels on Gata3ΔDP iNKT thymocytes and their detectable residual GFP expression (rightmost plot). Representative of three experiments (the other two identifying iNKT thymocytes by their expression of NK.1.1).
As previously reported 21, Gata3 disruption resulted in the disappearance of the CD4+ iNKT subset. We observed a minor re-expression of CD8 on Gata3ΔDP iNKT thymocytes (data not shown and Fig. 3B), with substantial biological variability among mice. This re-expression was not nearly as pronounced as on Thpok-deficient iNKT cells, and it is possible that this is due to the small residual Thpok expression in Gata3-deficient iNKT thymocytes (Supporting Information Fig. 4B). Of note, Gata3 disruption results in drastically reduced peripheral iNKT-cell numbers 21, whereas Thpok disruption had no such effect, indicating that Gata3 serves other functions in addition to promoting Thpok expression. This is supported by the altered development of Gata3-deficient iNKT thymocytes, illustrated by the premature expression of NK1.1 by Gata3- but not Thpok-deficient iNKT thymocytes (Ref. 21 and Supporting Information Fig. 3B).
The expression of Thpok by CD1d-selected iNKT cells indicates that Thpok can be expressed in thymocytes in the absence of MHC-II co-engagement of TCR and CD4. The selection of CD1d-restricted iNKT cells differs from that of conventional T cells in two critical aspects 1, 23. First, selecting CD1d molecules are expressed by DP thymocytes but not by the thymic epithelium 24. Second, the selection of iNKT cells requires homotypic interactions between SLAM family receptors and signaling through the adaptor SAP, none of which is involved in selection of conventional T cells 25. Future work will determine whether either factor promotes Thpok expression by iNKT cells. Because CD1d does not bind CD8, the expression of Thpok by iNKT cells is consistent with the ‘kinetic signaling’ model of lineage choice, which proposes that CD8-independent TCR signaling in thymocytes promotes CD4 differentiation, and therefore results in Thpok expression 26.
The expression of Thpok in iNKT cells depends on Gata3, similar to what we previously reported in MHC II-restricted thymocytes 9. However, while Gata3-deficient MHC II-restricted thymocytes fail to differentiate into mature T cells, iNKT cells develop despite Gata3 disruption, allowing us to dissect effects of Gata3 on cell development from those on Thpok expression. As a result, we could identify a Gata3 requirement for Thpok expression in iNKT cells, distinct from that for selection. Such a demonstration had not been possible in MHC II-restricted thymocytes, which in absence of Gata3 are arrested before the stage at which they would normally express Thpok 9, 27. We had previously shown that Gata3 was recruited to the Thpok locus in MHC II-restricted thymocytes 9. Recent ‘Chipseq’ large scale analyses have found that Gata3 binds the same site, close to the 3′ extremity of Thpok intron 1 in iNKT cells (within DNase I hypersensitivity site A in Ref. 9) (J.Z., William E. Paul and Keji Zhao, unpublished data). In MHC II-restricted thymocytes, this activity of Gata3 is part of a broader network of positive and negative transcriptional regulators that determine Thpok expression 6, 28, and it is likely that this is the case in iNKT cells as well.
Previous studies have led to the idea that Thpok is up-regulated as a result of MHC II signaling in thymocytes 10, 11, 13, 14. We now add an important amendment to this paradigm, namely that Thpok is also expressed in iNKT cells. As in conventional MHC II-restricted thymocytes T cells, Thpok is required for proper repression of CD8 expression by CD1d-restricted iNKT thymocytes and for their development into CD4+ cells, and its expression is Gata3-dependent. We propose that the sequential activation of Gata3 and Thpok is a characteristic of CD4+ T cells, regardless of their antigenic specificity.
Materials and methods
Mice
Thpok−/−, Gata3ΔDP, and ThpokGFP mice were previously described 9, 22. MHC II-deficient mice were from Jax 29. Mice were housed in specific pathogen-free facilities and analyzed between 4 and 12 wk of age. Animal procedures were approved by relevant NIH Animal Care and Use Committees.
Antibodies
The following mAb were obtained from BD Pharmingen and used for staining: TCR-β (H57-597), CD4 (RM4.4 or GK1.5), CD8 (53–6.7), CD24 (M1/69), CD44 (IM7), CD69, and NK1.1 (PK136). PBS-57-loaded and control (unloaded) mouse CD1d tetramers were from the NIH tetramer core facility.
Cell preparation and staining
Cells were prepared and analyzed by flow cytometry according to previously described procedures, using either an LSR II flow cytometer (BD Biosciences) or a modified (Cytek) FACSCalibur cytometer (BD Biosciences) 9. Cell sorting was performed as described using FACSVantage or FACSAria instruments (BD Biosciences) 9. CD4 or CD8 peripheral cells were purified using Dynal beads (Invitrogen), activated with antibodies against CD3 and CD28 and cultured under ‘Th2’ condition as described 15.
Gene and protein expression analyses
Gene expression was analyzed by RT-PCR using Taqman reagents, probes (Zbtb7b Mm00784709_s1) and an ABI PRISM 7500HT sequence detection system, all from Applied Biosystems, following published procedures 12, 15. Gene expression values were normalized to Actb in the same sample. For protein analyses, cells were lyzed in 1% Triton-containing buffer; anti-Thpok immunoprecipitation and immunoblotting was carried out as previously described 9.
Acknowledgements
The authors thank Ehydel Castro for expert mouse technical assistance, Barbara Taylor for help with flow cytometry, Takeshi Egawa for insight on the control of Cd4 expression, Renaud Lesourne, Paul Love and Bill Paul for helpful discussions and reagents, and B.J. Fowlkes, Paul Love and Al Singer for reading the manuscript. This work was supported in part by the Intramural Research Programs of the National Cancer Institute, Center for Cancer Research, and of the National Institute of Allergy and Infectious Diseases, NIH and by NIH Grant R01 AI038339.
Conflict of interest: The authors declare no financial or commercial conflict of interest.