Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages
Abstract
Aeromonas hydrophila is a Gram-negative pathogen that causes serious infectious disease in humans. A. hydrophila induces apoptosis in infected macrophages, but the host proinflammatory responses triggered by macrophage death are largely unknown. Here, we demonstrate that the infection of mouse macrophages with A. hydrophila triggers the activation of caspase-1 and release of IL-1β. Caspase-1 activation was abrogated in macrophages deficient in Nod-like receptor family, pyrin domain containing 3 (NLRP3) and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), but not NLR family, CARD domain containing 4 (NLRC4). The activation of the NLRP3 inflammasome was mediated by three cytotoxins (aerolysin, hemolysin and multifunctional repeat-in-toxin) produced by A. hydrophila. Our results indicated that the NLRP3 inflammasome senses A. hydrophila infection through the action of bacterial cytotoxins.
Introduction
The genus Aeromonas is responsible for a significant number of animal and human infections. Aeromonas are opportunistic human pathogens that can cause intestinal and extraintestinal, wound, soft tissue, skin, and blood infections and septicemia. The most common human disease associated with Aeromonas infection is gastroenteritis with diarrhea symptoms. Among the 21 species that have been differentiated based on DNA–DNA hybridization, A. caviae, A. veronii biotype sobria and A. hydrophila are most commonly associated with human infections and account for more than 85% of all clinical isolates 1, 2.
Recently, it has been reported that infection with A. hydrophila induces apoptosis in macrophages 3, 4. Although the cytotoxicity by the bacteria is believed to enable the bacteria to evade eradication and clearance by the macrophages, the host proinflammatory responses triggered by the death of the macrophages remain unknown.
Induction of apoptosis is considered a virulence mechanism of pathogenic bacteria that can cause tissue damage and facilitate bacterial colonization. However, accumulating evidence indicates that infection with many pathogens including bacteria, viruses and fungi induces the proinflammatory cell death of infected macrophages through the activation of caspase-1 5-17. The Nod-like receptor (NLR) family, pyrin domain containing 3 (NLRP3) is critical for caspase-1 activation and the secretion of IL-1β and IL-18 in response to multiple microbial molecules and endogenous danger molecules, whereas the NLR family, CARD domain containing 4 (NLRC4) protein is believed to sense bacterial flagellin and induce caspase-1 activation 18, 19.
Here, we investigated the cell death of macrophages infected with A. hydrophila and demonstrate that the bacteria cause pyroptosis with caspase-1 activation via the NLRP3 inflammasome. Aerolysin, hemolysin (HlyA) and multifunctional repeat-in-toxin (RtxA) produced by A. hydrophila are required to elicit the NLRP3 inflammasome and necrotic cell death. We identify the essential bacterial and host factors for eliciting caspase-1 activation and proinflammatory responses.
Results and discussion
Infection with A. hydrophila induces caspase-1 activation, IL-1β release and pyroptosis
It has been reported that aerolysin produced by A. salmonicida induces caspase-1 activation in CHO cells 20, 21. However, whether whole bacterial infection by A. hydrophila leads to caspase-1 activation in macrophages has not been elucidated. We examined caspase-1 activation in infected primary mouse BM-derived macrophages (BMM) and observed that BMM infected with WT strains of A. hydrophila underwent rapid cell death with membrane swelling, a morphological feature of necrotic cell death (Fig. 1A). Furthermore, the activation of caspase-1 (Fig. 1B) and secretion of IL-1β (Fig. 1C) were induced during infection, whereas lactate dehydrogenase (LDH) was released into culture supernatants, indicating that necrotic cell death is induced upon infection. Taken together, these results indicate that infection with A. hydrophila triggers pyroptosis, a form of proinflammatory cell death in macrophages.
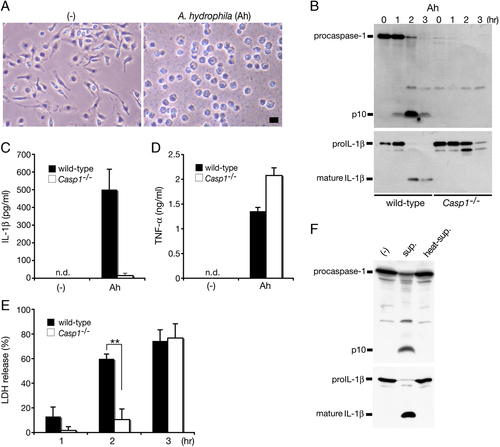
Infection with A. hydrophila (Ah) induces caspase-1 activation, IL-1β release and pyroptosis in infected BMM. BMM from WT or caspase-1-deficient (Casp1−/−) mice were primed with LPS (1 μg/mL; 3 h) and infected with WT A. hydrophila for the indicated times. (A) Phase contrast images of primed WT BMM either uninfected (–) or 1 h post-infection. Bar, 20 μm. (B) Caspase-1 and IL-1β processing in infected WT or caspase-1-deficient primed BMM was analyzed by immunoblotting with anti-caspase-1 or anti-IL-1β antibody to detect active p10 subunit of caspase-1 and mature form of IL-1β (17 kDa). Culture supernatants from primed infected BMM were analyzed 3 h after infection for (C) IL-1β and (D) TNF-α levels by ELISA and (E) LDH release. Data are mean±SD of triplicate samples, n.d., none detected; **p<0.01 (Student's t-test). (F) Caspase-1 and IL-1β processing as detected by western blotting in primed, uninfected WT BMM after incubation for 2 h with either fresh (sup) or heat-treated (100°C for 10 min) bacterial culture supernatants of the WT Ah strain or medium alone. Data are representative of at least two independent experiments.
Processing and secretion of IL-1β during A. hydrophila infection were significantly reduced in caspase-1-deficient macrophages (Fig. 1B and C). By contrast, TNF-α was secreted from both A. hydrophila-infected WT and caspase-1-deficient BMM (Fig. 1D). LDH release was not completely abrogated in caspase-1-deficient macrophages at an early stage of A. hydrophila infection but increased to a level similar to that of WT BMM by 3 h post-infection (Fig. 1E). These results indicate that caspase-1 has a partial effect on LDH release and suggest that A. hydrophila also induces caspase-1-independent cell death.
We next examined the ability of bacterial culture supernatants to induce caspase-1 activation in BMM. Addition of culture supernatant from WT A. hydrophila to LPS-primed WT BMM did indeed result in caspase-1 activation and IL-1β processing, which was abolished after heat treatment of the culture supernatant (Fig. 1F), suggesting that heat-labile toxins secreted from bacteria are involved in caspase-1 activation.
Caspase-1 activation by A. hydrophila occurs via NLRP3-ASC inflammasome
The NLR family member proteins NLRP3 and NLRC4 are known to mediate caspase-1 activation by forming a macromolecular complex (also called an inflammasome) with the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) 18. To explore the mechanisms responsible for the activation of caspase-1 by A. hydrophila, we investigated the roles of NLRP3, NLRC4 and ASC. NLRP3-, NLRC4- or ASC-deficient macrophages were infected with A. hydrophila and examined for caspase-1 activation and IL-1β processing/secretion. Compared with WT BMM, caspase-1 activation and IL-1β processing/secretion during infection were abolished in BMM deficient in NLRP3 or ASC, but not NLRC4 (Fig. 2A and B). We also observed a delay in LDH release from infected NLRP3- and ASC-deficient cells (Fig. 2C) consistent with that observed in infected caspase-1-deficient cells (Fig. 1D). These results demonstrate that NLRP3 and ASC are essential host factors for the activation of caspase-1 during A. hydrophila infection and are partly involved in cell death. To support our finding that the NLRP3-ASC inflammasome is activated by A. hydrophila, we also examined caspase-1 activation and IL-1β processing/secretion in the presence of high concentrations of extracellular potassium, which is known to inhibit NLRP3 inflammasome activation 22. The high extracellular potassium concentration abrogated caspase-1 activation and IL-1β processing/secretion while partially inhibiting LDH release from WT BMM (Supporting Information Fig. 1). This latter finding further supports our notion that A. hydrophila also induces caspase-1-independent cell death.
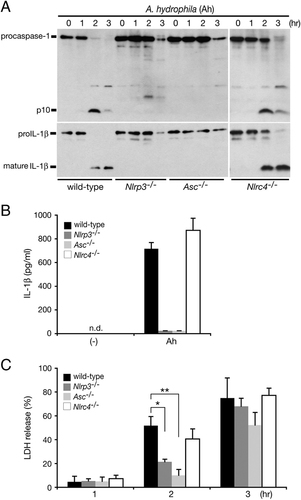
A. hydrophila (Ah) induces caspase-1 activation and IL-1β release via the NLRP3-ASC inflammasome. BMM from WT, NLRP3- (Nlrp3−/−), ASC- (Asc−/−) or NLRC4-deficient (Nlrc4−/−) mice were primed with LPS and infected with WT A. hydrophila. (A) Caspase-1 and IL-1β processing in the infected primed BMM was analyzed at the indicated time points post-infection by immunoblotting with anti-caspase-1 or anti-IL-1β antibody to detect active p10 subunit of caspase-1 and mature form of IL-1β (17 kDa). Culture supernatants from primed infected BMM were analyzed 3 h after infection for (B) IL-1β levels and (C) LDH release. Data are mean±SD of triplicate samples; n.d., none detected; *p<0.05, **p<0.01 (Student's t-test). Data are representative of at least two independent experiments.
Caspase-1 activation is mediated by aerolysin, HlyA and multifunctional RtxA
Purified aerolysin has been shown to induce caspase-1 activation and an aerolysin-deficient A. trota mutant was incapable of inducing caspase-1 activation in human fibroblasts 21. However, an A. hydrophila aerolysin mutant (ΔaerA) remained capable of activating caspase-1 and IL-1β processing/secretion in infected BMM (Fig. 3A and B), suggesting that additional stimulators are involved in caspase-1 activation. To identify these factors, we performed gene deletion mutagenesis experiments and identified two additional cytotoxins, HlyA and multifunctional RtxA. The lack of expression of these cytotoxins was confirmed by RT-PCR (Supporting Information Fig. 2). As shown in Fig. 3A, C and E, A. hydrophila carrying a single mutation in aerolysin (ΔaerA), HlyA (ΔhlyA) or RtxA (ΔrtxA) was still able to induce caspase-1 activation, IL-1β processing/secretion and LDH release at levels comparable to those observed in cells infected with WT A. hydrophila. However, a ΔaerAΔhlyA, but not ΔaerAΔrtxA or ΔhlyAΔrtxA, double mutant displayed a significantly reduced induction of caspase-1 activation, IL-β processing/secretion and LDH release (Fig. 3B, D and F). Moreover, the triple mutant ΔaerAΔhlyAΔrtxA was unable to induce caspase-1 activation and IL-1β release (Fig. 3B and D), although a small amount of LDH was released after infection with the triple mutant suggesting that an as yet identified bacterial factor(s) is involved in cell death. TNF-α was secreted during infection by both WT and triple mutant but the triple mutant elicited a higher level of TNF-α secretion compared with WT (Fig. 3G). This may be due to reduced cell death observed during infection with the triple mutant. The cytotoxic enterotoxin (Act) has been reported to cause apoptosis of murine macrophages and induce host inflammatory responses 23, 24. However, in this study, caspase-1 activation and IL-1β release were not affected by mutation of the act gene either alone or in combination with the deletions described above (data not shown). Taken together, these data suggest that both aerolysin and HlyA play a substantial role in NLRP3-ASC inflammasome activation, whereas RtxA plays a minor and Act is dispensable.
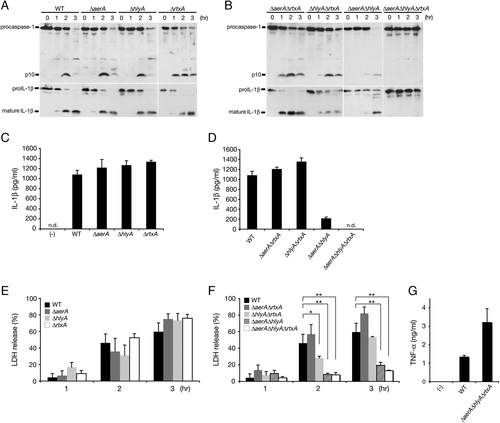
Three cytotoxins of A. hydrophila (Ah) are essential for the induction of caspase-1 activation and cell death. BMM from WT mice were primed with LPS and infected with either WT Ah or with one of the isogenic cytotoxin single, double or triple mutants. (A) Caspase-1 and IL-1β processing in primed infected WT BMM was analyzed by immunoblotting with anti-caspase-1 or anti-IL-1β antibody to detect active p10 subunit of caspase-1 and mature form of IL-1β (17 kDa). Culture supernatants from primed infected BMM were analyzed 3 h after infection for IL-1β levels (C, D), LDH release (E, F) and TNF-α levels (G). Data are the mean±SD of triplicate samples; n.d., none detected; *p<0.05, **p<0.01 (Student's t-test). Data are representative of at least two independent experiments.
Caspase-1 activation does not require LPS priming
In our experiments, we utilized IL-1β secretion as a read-out for inflammasome/caspase-1 activation yet IL-1β expression requires LPS priming. We wanted to examine whether LPS priming was required for caspase-1 activation by A. hydrophila. Since we had identified secreted toxins as the bacterial factors involved in this activation, we next assessed the processing and secretion of IL-18, another caspase-1-activated proinflammatory cytokine in response to bacterial culture supernatants. Caspase-1 activation and IL-18 processing were observed in unprimed BMM incubated with culture supernatant from WT A. hydrophila (Supporting Information Fig. 3), suggesting that priming by LPS is not necessary for caspase-1 activation by secreted cytotoxins.
We also assessed the ability of bacterial culture supernatants from our toxin mutants to induce caspase-1 activation and IL-18 processing in unprimed BMM. As shown in Supporting Information Fig. 3, secreted AerA and HlyA but not RtxA stimulated caspase-1 activation and IL-18 processing. Although further study would be needed to understand the RtxA activity, we speculate that secreted RtxA is unstable or small in amount in bacterial culture supernatants. Collectively, although the relative contributions of each cytotoxin may differ, these data suggest that three cytotoxins produced by A. hydrophila are involved in the activation of caspase-1 and cytokine processing in infected macrophages.
Concluding remarks
The infection of mouse macrophages with A. hydrophila triggered the activation of caspase-1, IL-1β release and pyroptosis. The NLRP3 inflammasome was found to mediate the caspase-1 activation and IL-1β release induced by infection with A. hydrophila. The three cytotoxins aerolysin, HlyA and multifunctional RtxA were indispensable for the activation of the NLRP3 inflammasome. Our data suggest that A. hydrophila induces host proinflammatory responses via caspase-1 activation in infected macrophages.
Materials and methods
Bacterial strains
The WT A. hydrophila ATCC7966 strain was used. Isogenic A. hydrophila mutants, ΔaerA, ΔhlyA, ΔrtxA and double or triple mutants thereof were constructed using allele replacement strategies and the suicide vector pYAK1 provided by Dr. T. Iida (Osaka University, Japan). The bacterial strains were grown in Luria–Bertani broth. Cell-free culture supernatants from overnight cultivation were collected by centrifugation and filtration (0.22 μm). DNA-free total RNA was isolated from bacteria using RNeasy kit (Qiagen). RT-PCR was performed using Super Script III One-Step RT-PCR (Invitrogen).
Mice and preparation of macrophages
C57BL/6 mice were purchased from CREA Japan (Tokyo, Japan) as WT mice. C57BL/6 background caspase-1-deficient mice, NALP3-deficient (Nlrp3−/−) mice 22, NLRC4-deficient (Nlrc4−/−) mice 25 and ASC (Asc−/− or Pycard−/−)-deficient mice 26 were housed in pathogen-free facility. BMM were prepared from the femurs and tibias of the above-mentioned mice and cultured for 5–6 days in 10% FBS-RPMI 1640 supplemented with 30% L-cell supernatant. All animal studies were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of the Ryukyus (Okinawa, Japan).
Reagents
LPS (O55:B5) was purchased from Sigma-Aldrich. The following antibodies were obtained commercially: rabbit anti-mouse caspase-1 (sc-514, Santa Cruz), goat anti-mouse IL-1β (AF-401-NA, R & D Systems) and rabbit anti-mouse IL-18 (5180R-100, BioVision).
Bacterial infection, LDH assay and ELISA
BMM were seeded at a density of 5×105 cells per well in 24-well plates containing 10% FBS-RPMI 1640. LPS (1 μg/mL)-primed cells were infected with A. hydrophila at an MOI of ∼10 per cell. The plates were centrifuged at 600×g for 10 min to synchronize the infection stage and then incubated at 37°C. At the indicated times after infection, LDH activity in the culture supernatant of infected cells was measured using a CytoTox 96 assay kit (Promega) according to the manufacturer's protocol. The following formula was used to calculate LDH release: ((OD490 sample−OD490 spontaneous)/(OD490 maximum release−OD490 spontaneous))×100, whereby OD490 spontaneous represents LDH release into the culture supernatant from uninfected cells and OD490 maximum release denotes LDH release after lysis of the uninfected cells. Cytokines present in the culture supernatants were quantified by ELISA (R &D Systems). The end point of all experiments was defined as 3 h post-infection (hpi) since Aeromonas are extracellular pathogens and multiply quickly in cell culture media.
Immunoblot
BMM were seeded at a density of 2×106 cells per well in 6-well plates and infected with bacteria. The cells were lysed and combined with the supernatant precipitated with 10% trichloroacetic acid. The samples were loaded onto 15% SDS-PAGE, and the cleaved form of caspase-1, IL-1β or IL-18 was detected using anti-caspase-1, anti- IL-1β or anti-IL-18 antibody, respectively. Antibodies detect appropriate subunits as follows: anti-caspase-1, p45 procaspase-1 and p10 active subunit; anti-IL-1β, pro-IL-1β (31 kDa) and mature form (17 kDa); and anti-IL-18, pro-IL-18 (24 kDa) and mature form (18 kDa).
Statistical analysis
Statistical analyses were performed using the Student's t-test. Differences were considered significant at a p-value of<0.05.
Acknowledgements
The authors thank the members of the Suzuki laboratory for their advice. The authors thank R. Flavell and K. Kuida for providing caspase-1-deficient mice. The authors also thank Dr. T. Iida for providing some materials. This work was supported by Grant-in-Aid for Postdoctoral Fellowships for Foreign Researchers (20-08458) (A. J. M.), Grant-in-Aid for Japan Society for the Promotion of Science Fellows (22-3784) (Y. K.), Grant-in-Aid for Scientific Research (C) (21590484) (C. T.) and Grant-in-Aid for Scientific Research on Priority Areas (21022042) (T. S.) from the Japanese Ministry of Education, Culture, Sports and Technology in Japan, the Uehara Memorial Foundation, and the NOVARTIS Foundation (Japan) for the Promotion of Science (T. S.).
Conflict of interest: The authors declare no financial or commercial conflict of interest.