Importance of TLR2 in the direct response of T lymphocytes to Schistosoma mansoni antigens
Abstract
The immunomodulatory effect of Schistosoma mansoni antigens has often been attributed to interaction with PRR expressed on APC. Our previous work has shown that S. mansoni-soluble egg antigen (SEA) can induce, together with a Th2 response, TGF-β-dependent Foxp3 expression in naïve CD4+ T cells from NOD mice. We found that SEA can directly upregulate the expression of surface-bound TGF-β in purified CD4+ T cells in the absence of accessory cell interactions. In this study, we show that the C-type lectin receptors DEC-205 and galectin-3 were involved in the direct interaction between S. mansoni antigens and CD4+ T cells. SEA was able to enhance CD4+ T-cell secretion of bioactive TGF-β in response to TLR2 ligand stimulation, in the absence of APC. We also show that TLR2 expressed on CD4+ T cells was important for the Foxp3 expression induced by SEA.
Introduction
Infection with the trematode worm Schistosoma mansoni often marks the onset of profound changes to the immune system of the host. Various soluble antigen fractions from S. mansoni, either from the worm (soluble worm antigen) or from the egg (S. mansoni-soluble egg antigen (SEA)), have been used in vitro and in animal models to study the actions of parasite antigens on many different cell types. The immunomodulatory effect of the parasite extracts has also been shown to be beneficial as a preventative treatment in several animal models of autoimmune disease 1. We have demonstrated that both live infections and soluble extracts from S. mansoni can prevent type 1 diabetes 2, 3, and more recently, we have provided evidence of a role for SEA-induced Foxp3+ Treg in diabetes prevention in NOD mice 4. We have shown that SEA can induce Foxp3 expression in naïve CD4+ T cells from NOD mice. We also found that SEA, in the absence of APC, can directly upregulate on NOD CD4+ T cells expression of C-type lectin receptors (CLR) and surface-bound TGF-β, as determined by the presence of the latency-associated peptide (LAP) of TGF-β 5. TGF-β is a central cytokine in the induction and maintenance of Treg but also in the induction of inflammatory Th17 cells 6. The regulation of TGF-β bioactivity, coupled with the presence of pro-inflammatory cytokines (such as IL-1, IL-6, IL-21 and IL-23), determines the balance between inflammation and regulation 7, 8.
Given that in our system SEA can only induce Foxp3 expression in NOD, but not in C57BL/6 naïve CD4+ T cells 4; in this study, we explored links between PRR expression and TGF-β regulation by SEA in the T cells in order to formulate a mechanism behind the dissimilar effects induced by SEA in CD4+ T cells from the two strains of mice. As it has been shown that the internalization of S. mansoni antigens by multiple CLR regulates the response of APC to TLR-induced signals 9, and because of the ability of SEA to upregulate galectin-1 and -3 mRNA expression directly in T cells 4, we examined the expression and the function of specific CLR on T cells. The modulation of TLR signaling by S. mansoni antigen has been most widely studied on DC and MΦ 9-13. TLR2 has been shown to recognize molecules within SEA 14, 15, and it has been suggested that it may participate in the control of immunopathology during S. mansoni infection, in part through effects on the priming and expansion of Treg 16. However, another report found that TLR2 appears to be dispensable in the control of pathology and infection 17, and TLR2 has been shown not to be required to modulate the activation of DC or their ability to induce a Th2 response 18, 19. Given the interest in T-cell expression of TLR and other PRR 20, 21, including expression on Treg 22, 23, together with our observation of a direct effect of SEA on T cells, we investigated the involvement of PRR in modulating the interaction of S. mansoni antigens with T lymphocytes. In particular, we studied the role of TLR2 and the CLR DEC-205 and galectin-3 in the ability of SEA to induce TGF-β and Foxp3 expression in CD4+ T cells. We have also examined PRR expression on both NOD and C57BL/6 mouse T cells in order to explain the dissimilar effects induced by SEA in the two strains.
Results
Differential expression of PRR and upregulation of SEA-induced LAP in NOD and C57BL/6 T cells
We have shown that only NOD and not C57BL/6 mouse naïve CD4+ T cells can upregulate Foxp3 expression when cultured in the presence of BMDC with SEA, and we demonstrated that SEA could directly upregulate TGF-β and CLR expression in CD4+ T cells in the absence of APC 4. We suggested that a direct interaction between S. mansoni antigens and PRR on CD4+ T cells was important in the ability of NOD mouse cells to upregulate Foxp3. To address why CD4+ T cells from the two mouse strains responded in a different way to SEA stimulation, we investigated whether PRR expression was dissimilar between the strains. In response to SEA, only purified CD4+ T cells from NOD mice upregulated surface expression of galectin-3 and DEC-205 (Fig. 1A and B). In addition, SEA induced upregulation of LAP only on NOD mouse CD4+ T cells (Fig. 1C). The induction of LAP on NOD mouse CD4+ T cells lends support to the finding that only NOD mouse naïve CD4+ T cells express Foxp3 in response to SEA in a polarization assay using BMDC.
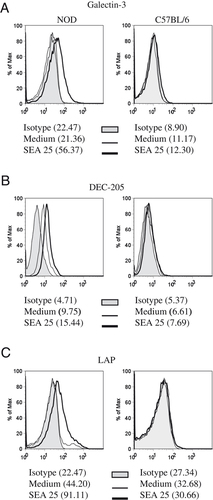
Differential expression of PRR and upregulation of LAP in NOD and C57BL/6 T cells. Splenic CD4+ T cells from NOD or C57BL/6 were purified to >98% CD4+CD3+ cells. Cells were stimulated with anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL) in the presence of SEA (25 μg/mL). Surface expression of (A) galectin-3, (B) DEC-205 and (C) LAP, as determined by FACS. Data are representative of three independent experiments.
TLR have previously been shown to be involved in S. mansoni antigen recognition by DC 12, 14-17, and for this reason, we analyzed TLR expression in CD4+ T cells from NOD and C57BL/6 mice. We found that both the mRNA and surface protein levels of TLR1 and TLR6 are significantly higher in NOD mouse CD4+ T cells (Fig. 2A and B). Conversely, there were no significant differences in TLR2, TLR4, DEC-205 or galectin-3 expression between strains, either by mRNA or protein (Fig. 2A and B, and data not shown). Consistent with these data, anti-CD3 driven proliferation of NOD CD4+ T cells surpassed that of C57BL/6 CD4+ T cells when enhanced by Pam3CysK4 and FSL-1, which are respectively, TLR1/2 and TLR6/2 ligands (Fig. 2C). The differences between the two strains were also reflected in the cytokine secretion following TLR2 ligation. Upon anti-CD3 stimulation in the presence of TLR ligation, NOD mouse T cells secreted more IL-2, IL-6 and IL-17, than C57BL/6 T cells (Supporting Information Fig. 1). Taking into consideration the involvement of TLR2 12, 14-17 in S. mansoni-induced immunomodulation, the disparate response by NOD and C57BL/6 CD4+ T cells to TLR2 ligation may provide a partial explanation for the differential response to SEA in the two strains.
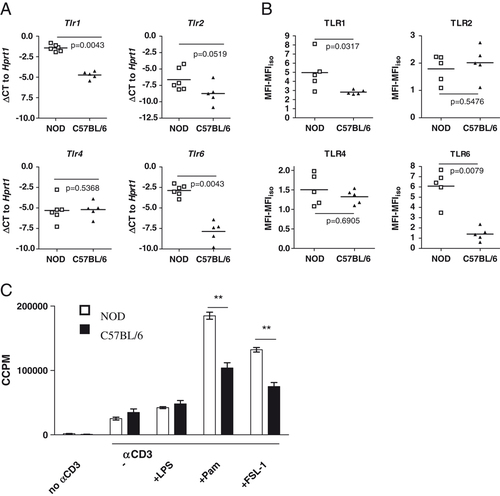
Differential expression of TLR in NOD and C57BL/6 T cells. Splenic CD4+ T cells from NOD or C57BL/6 were purified to >98% CD4+CD3+ cells. (A) mRNA expression levels of TLR were assessed by RT-PCR, and the data shown are the mean delta CT (ΔCT) values of duplicate reactions normalized to Hprt1. (B) Surface TLR expression as determined by flow cytometric analysis. Non-viable cells were excluded on the basis of 7-AAD uptake, and the background MFI obtained using isotype control antibodies was subtracted. Data in A and B are values from five or six individual mice, analyzed by Mann–Whitney U test, two-tailed. (C) Cells were stimulated with anti-CD3 (2 μg/mL) in combination with different TLR ligands (100 ng/mL). Data show mean ±SEM of three mice and are representative of two independent experiments. **p<0.01 by unpaired t-test, two-tailed.
SEA induced bioactive TGF-β secretion in CD4+ T cells, which was enhanced by TLR2
The interaction between PRR expressed on innate immune system cells and S. mansoni antigens has been studied by several groups 9, 17, 18. As has been described by others 20, 21 we found that the expression of several TLR and CLR is not limited to APC. FACS analysis revealed expression and staining for TLR1, TLR2, TLR4 and TLR6 (Fig. 2A and B) in CD4+ T cells from NOD and C57BL/6 mice. Having shown that SEA can directly regulate the expression LAP (Fig. 1C) on CD4+ T cells, we investigated the ability of SEA to induce bioactive TGF-β in NOD mouse CD4+ T cells. For the first time, we were able to show that SEA directly induced the secretion of bioactive TGF-β from purified CD4+ T cells stimulated with anti-CD3 and anti-CD28 in absence of APC (Fig. 3A). This effect was not inhibited by the addition of anti-TLR2 (data not shown).
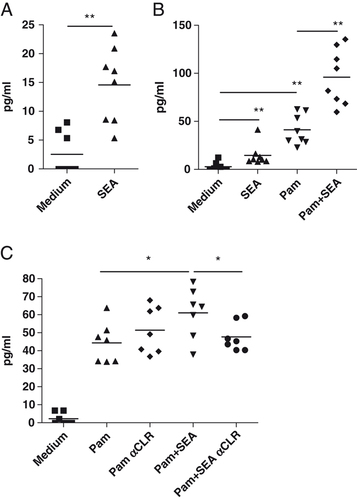
SEA induces bioactive TGF-β expression in T cells and enhances bioactive TGF-β secretion in CD4+ T stimulated through TLR2. T cells (2×105) were cultured for 48 h with 4×105 anti-CD3/anti-CD28-coated microbeads and 50 U/mL IL-2 in the presence of (A) 20 μg/mL SEA and/or (B and C) 1 μg/mL Pam3CysK4 (Pam). Blocking CLR, DEC-205 and galectin-3 (αCLR), significantly reduces bioactive TGF-β secretion in TLR2- and SEA-stimulated CD4+ T cells. All blocking antibodies were used at 10 μg/mL and were added 30 min prior to SEA (C). Supernatants from individual mice (n=7–8) T cells cultures were analyzed as described in the Materials and methods section for the presence of bioactive TGF-β and are presented as mean values for individual mouse samples. Statistical analysis by Wilcoxon-matched pairs test, two-tailed (*p<0.05, **p<0.01).
After confirming the ability of TLR2 ligation to modulate the proliferation and cytokine secretion of T cells (Fig. 2C and Supporting Information Fig. 1), we decided to investigate whether TLR2 stimulation also drives TGF-β secretion by CD4+ T cells. We examined the effect of TLR2 ligands on T cells stimulated with anti-CD3 and anti-CD28 in the absence of APC. We found that TLR2 (Pam3CysK4 and MALP-2) but not TLR4 (LPS) ligands induced the secretion of bioactive TGF-β by CD4+ T cells (Supporting Information Fig. 2, or data not shown). We furthermore found that SEA had an additive effect in conjunction with TLR2 stimulation, enhancing the secretion of bioactive TGF-β by Pam3CysK4 (Fig. 3B). All together these findings highlight the importance of TLR expression on T cells and the ability of SEA to modulate cytokine secretion by T cells as well as APC 10.
Finally, having shown that SEA can directly upregulate the expression of both galectin-3 and DEC-205 on CD4+ T cells (Fig. 1A and B), we decided to investigate the role of these two CLR in the ability of SEA to enhance bioactive TGF-β by CD4+ T cells. Figure 3C shows that blocking CLR (galectin-3 and DEC-205) specifically reduced the ability of SEA to enhance bioactive TGF-β secretion by Pam3CysK4. In the absence of TLR2 stimulation, blockade of galectin-3 and DEC-205 did not decrease SEA-induced bioactive TGF-β and only mildly decreased the expression of LAP on CD4+ T cells (Supporting Information Fig. 3).
These data demonstrate for the first time that TLR2 and CLR, but not TLR4, modulate functional changes in CD4+ T cells in the absence of APC mediation.
Pam3CysK4, but not SEA, induces IL-17 in naïve CD4+ T cells
The generation of both Th17 and Foxp3+ Treg is dependent on TGF-β (at least in mice), and the presence of IL-6 has been shown to shift the balance in favor of Th17 induction 7. We found that although both SEA and Pam3CysK4 stimulated bioactive TGF-β production from CD4+ T cells (Fig. 3A and B), the addition of the TLR2 ligand Pam3CysK4 to co-cultures of naïve T cells and BMDC induced IL-17 rather than Foxp3 expression (Fig. 4A and B). Altogether with IL-6, other cytokines, such as IL-1 and IL-23, have been shown to play an important role in the inhibition of TGF-β-dependent Foxp3 Treg induction 7, 24. When we analyzed the supernatants from these polarization assays for cytokine secretion by ELISA, Pam3CysK4, but not SEA, induced IL-17 together with IL-1, IL-6 and IL-23 (Fig. 4C). These data are also consistent with the ability of Pam3CysK4 to induce not only TGF-β but also IL-17 and IL-6 production by NOD-purified CD4+ T cells (Supporting Information Fig. 1).
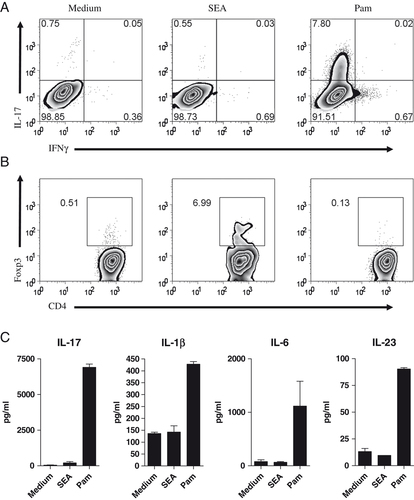
Pam3CysK4 drives a Th17 response from CD4+ naïve T cells. (A) Naïve CD4+ T cells (5×105) were cultured with 1×105 BMDC, soluble anti-CD3 (0.5 μg/mL), with or without 10 μg/mL SEA or 10 ng/mL Pam3CysK4 (Pam), and intracellular cytokine production was assessed on day 5 by intracellular staining. (B) Naïve T cells (1×105) and BMDC (2×104) were cultured as in A for 5 days, and stained for intracellular Foxp3 expression. (C) Supernatants from the cultures shown in A were analyzed by ELISA. Data show mean±SD (n=3) and are representative of three independent experiments.
Foxp3 induction in NOD mouse naïve T cells by SEA is TLR2 dependent
Many studies have shown an involvement of TLR2 in S. mansoni immunomodulation and we therefore examined the role of TLR2 engagement in Foxp3 induction by SEA. We have previously shown that SEA can upregulate Foxp3 expression in CD4+ T cells in a TGF-β-dependent manner 4. Figure 5A and B show that preventing TLR2 or CLR ligation in a T-cell polarization assay reduced Foxp3 expression in naïve CD4+ T cells cultured in vitro with SEA. Anti-TLR2 but not anti-TLR4 affected Foxp3 induction (Fig. 5A and B). Interestingly, TLR2 stimulation by SEA was only important for Foxp3 Treg induction, as the Th2 induction by SEA was not affected by adding anti-TLR2 to the cell culture (data not shown). These observations are consistent with our data showing that SEA enhanced the secretion of bioactive TGF-β induced by TLR2 ligation in CD4+ T cells (Fig. 3B) and that Foxp3 induction by SEA was TGF-β dependent 4.
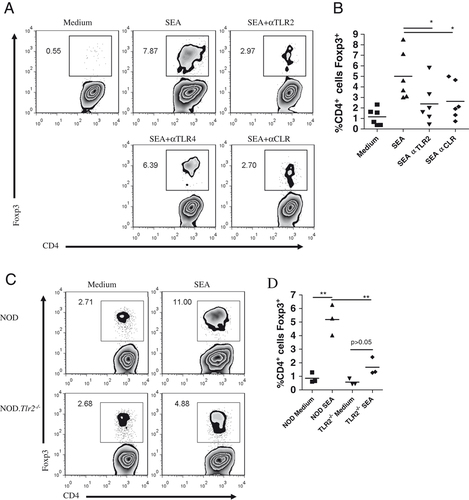
The induction of Foxp3 in naïve T cells by SEA requires TLR2 and is influenced by DEC-205/galectin-3. (A) Representative FACS plots showing Foxp3 intracellular staining, and (B) summary data from six independent experiments using blocking antibodies. Naïve CD4+ T cells (1×105) were cultured with 2×104 BMDC, soluble anti-CD3 (0.5 μg/mL) and 10 μg/mL SEA in the presence of antagonistic antibodies for TLR2, TLR4 and/or the CLR DEC-205 and galectin-3 for 5 days. All blocking antibodies were used at 10 μg/mL and were added at least 30 min prior to SEA. *p=0.0313 by Wilcoxon matched pairs test, two-tailed. (C) Naïve T cells from NOD.Tlr2−/− mice were co-cultured with wild-type NOD BMDC under different conditions for C and D, one experiment each. (C) Naïve T cells (5×105) from either NOD or NOD.Tlr2−/− mice were cultured with wild-type NOD BMDC (1×105) and 0.5 μg/mL anti-CD3 for 5 days in the presence of 25 μg/mL SEA. On day 5, the cells were harvested and stained for analysis of intracellular Foxp3 expression. (D) Naïve T cells (1×105) from either NOD or NOD.Tlr2−/− mice were cultured in triplicate samples with BMDC (2×104), anti-CD3 (0.5 μg/mL) and/or SEA (25 μg/mL). On day 4, cells were stained for Foxp3 and analyzed using flow cytometry. **p<0.01 by two-tailed, unpaired t-test.
To further demonstrate the importance of TLR2 expression on CD4+ T cells for SEA-mediated induction of Foxp3+ T cells, we used naïve CD4+ T cells from TLR2-deficient NOD mice in a polarization assay together with wild-type NOD BMDC. Figure 5C and D show that in the absence of TLR2 on T cells, Foxp3 induction by SEA was significantly reduced. Altogether, these data underline the important role of TLR2 expression on CD4+ T cells for Foxp3 induction by SEA.
Discussion
Many studies have examined the effects of schistosome-derived products for their in vitro effects on DC phenotype and function 3, 10, 25. In this study, we highlighted the importance of CLR and TLR in the T-cell response we observed to SEA. Schistosome-derived products have been shown to bind both CLR and TLR on APC with roles identified for both TLR2 and DC-SIGN in the human and TLR2 in mice 14-17, 26. In this study, we provided evidence that in addition to its effects on DC, SEA also mediated effects directly on CD4+ T cells, inducing surface-bound LAP and expression of the CLR DEC-205 and galectin-3 on T cells from NOD but not C57BL/6 mice (Fig. 1A and B). DEC-205 has been used to identify a population of tolerogenic DC specialized for the induction of Foxp3+ Treg 27, and for the first time, we showed that a parasite product can increase the surface expression of the CLR DEC-205 directly on T cells. The upregulation of surface expression of galectin-3 is particularly interesting given the recent observation that galectin-3, recruited intracellularly to the immunological synapse, negatively regulates TCR-mediated CD4+ T-cell activation 28. Generation of Treg has been associated with suboptimal T-cell activation, resulting from antigen presentation by immature or sub-optimally primed DC 29, 30. The ability of galectin-3 to influence the immunological synapse together with the ability of SEA to modulate DC maturation may play a key role in favoring the development of Foxp3-expressing cells in our T-cell/DC co-cultures 25.
As there are several reports of TLR expression on T cells 20, 21, 31, we also explored whether TLR played a role in SEA-mediated responses in T cells. The finding that the TLR profile in CD4+ T cells from NOD and C57BL/6 was dissimilar in expression and function (Fig. 2 and Supporting Information Fig. 1) also suggested a role for TLR in the inability of SEA to induce Foxp3 expression in C57BL/6 CD4+ T cells. We were able to demonstrate that SEA can increase TGF-β secretion in TLR2-stimulated CD4+ T cells (Fig. 3B), and we further showed for the first time that CD4+ T cells can secrete modest but significant levels of bioactive TGF-β directly in response to SEA (Fig. 3A). It is interesting to observe that by blocking DEC-205 and galectin-3, the secretion of TGF-β by CD4+ T cells stimulated with a TLR2 agonist and SEA was significantly decreased. On the other hand, in the absence of strong TLR2 stimulation, blockade of galectin-3 and DEC-205 decreased SEA-induced LAP, but did not reduce the secretion of bioactive TGF-β (Supporting Information Fig. 3). Altogether, these data not only demonstrated an involvement of DEC-205 and galectin-3 in the T-cell response to SEA, but also suggest the involvement of unidentified receptor(s) in the ability of SEA to induce TGF-β directly from CD4+ T cells.
Finally, we demonstrated the importance of TLR2 in the direct interaction between SEA and T cells, using T cells from TLR2-deficient NOD mice in a polarization assay together with BMDC from wild-type NOD mice. In the absence of TLR2 expression on naïve CD4+ T cells, there was a reduced ability to upregulate Foxp3 in response to SEA.
We also showed the involvement of galectin-3, DEC-205 and TLR2 by using blocking antibodies in the DC/T-cell polarization assay, where we observed a significant reduction of SEA-induced Foxp3. In this system, PRR expressed on both DC and T cells may modulate the interaction with SEA.
It is furthermore interesting to note that while both SEA and a TLR2 agonist drove biologically active TGF-β production, in the DC/T-cell co-culture system the TLR2 agonist additionally induced IL-6, IL-1β and IL-23. The TLR2 agonist therefore induced a Th17 response rather than Foxp3 expression. Integration of signals from TLR2 and its co-receptors, including CLR as well as other TLR, may result in synergistic or distinctive patterns of cytokine production, which may depend on whether the ligand derives from bacteria, fungi or helminths. Recently, it has been found that even viral antigens can stimulate TLR2, but do so differently than canonical TLR2 ligands, activating type 1 interferon production 32.
In summary, our study has focused on the direct interaction between S. mansoni-soluble antigens and PRR expressed on T lymphocytes and demonstrates for the first time that SEA is able to influence the phenotype of T cells through its interactions with both TLR2 and CLR DEC-205 and galectin-3. Finally, our work demonstrated that the interaction of PRR with microbial products is not exclusive to cells of the innate immune system and that the modulation of CLR and TLR expressed on T lymphocytes is also important to understand the immune response to pathogens.
Materials and methods
Mice
Female NOD/Tac mice were housed and barrier bred in the Pathology Department, University of Cambridge animal facilities (Cambridge, UK). TLR2-deficient C57BL/6 mice 33 were backcrossed ten generations to NOD/Caj mice and intercrossed to generate TLR2-deficient homozygous NOD mice 34. Female C57BL/6 mice were purchased from Charles River Laboratories. Mice were used between 6 and 8 wk of age. All work was conducted under UK Home Office project license regulations after approval by the Ethical Review Committee of the University of Cambridge.
Cell culture
Cells were cultured in IMDM (Invitrogen) supplemented with 2 mM L-glutamine, 50 μM 2-ME, 100 U/mL penicillin–streptomycin (All Sigma) and 10% FBS (Gibco). Cells were grown in a humidified 5% CO2 atmosphere at 37°C.
SEA, antibodies and TLR agonists
Preparation of SEA was described previously 4. Anti-CD3 (2C11) and anti-CD28 (37.51) were purchased from BD Pharmingen. The following antibodies were used in tissue culture experiments at 10 μg/mL: anti-TLR2 (T2.5) and anti-TLR4-MD-2 (MTS510) from eBioscience; anti-DEC-205/CD205 (NLDC-145, Dendritics) and anti-galectin-3 (B2C10, Santa Cruz). Defined TLR agonists were purchased from Imgenex (MALP-2, TLR2/6), or Invivogen: ultrapure LPS from Salmonella minnesota (TLR4), Pam3CysK4 (TLR1/2) and FSL-1 (TLR2/6).
Flow cytometry
Monoclonal antibodies against the following molecules were used: CD3ε (2C11) and CD4 (RM4-5) from BD Pharmingen; Foxp3 (FJK-16s), CD25 (PC61), CD44 (IM7), TLR1 (eBioTR23), TLR2 (6C2 or T2.5), TLR-4-MD-2 (MTS510), galectin-3 (eBioM3/38) from eBioscience; and DEC-205/CD205 (NLDC-145) from AbD Serotec. Biotinylated polyclonal goat anti-human LAP (BAF246), anti-mouse TLR6 (BAF1533) and normal goat serum (BAF108) (R&D Systems) were used with streptavidin-Alexa647 (Molecular Probes). Intracellular staining was performed using a Foxp3 staining set (eBioscience). Non-specific binding was blocked using 2.4G2 anti-FcγR supernatant (prepared in house). For live cell discrimination, 7-aminoactinomycin D (BD Pharmingen) was used. Data were acquired on a FACScalibur (BD Pharmingen) or on a CyanADP (Beckman Coulter) and analyzed using FlowJo software (TreeStar).
Cell sorting
Cells were isolated from splenic cell suspensions using immunomagnetic selection on an AutoMACS Pro (Miltenyi). T cells were isolated using CD4-microbeads, achieving >98% purity of CD4+CD3ε+ cells. Naïve T cells (>98% pure) were further sorted from AutoMACS selected CD4+ cells on the basis of CD4+CD44lowCD25− using a MoFlo (Beckman Coulter).
BMDC culture and naïve T-cell polarizations
Immature DC were differentiated from bone marrow precursors using GM-CSF (PeproTech) as previously described 4. Naïve CD4+CD44lowCD25− T cells were co-cultured with BMDC in a 5:1 ratio in the presence of 0.5 μg/mL anti-CD3. Cells were either cultured in flat-bottomed 96-well plates, using 1×105 T cells and 2×104 BMDC for 4–5 days, or in flat-bottomed 24-well plates, using 5×105 T cells and 1×105 BMDC for 5 days.
RNA isolation and real-time RT-PCR
RNA was isolated using an RNeasy Plus Micro kit (Qiagen), which included genomic DNA removal. Extracted RNA (100 ng) was reverse transcribed, linearly amplified using a Whole Transcriptome kit and analyzed in real-time using SYBR green PCR (All Qiagen). cDNA was analyzed in duplicate reactions with amplification of target gene and housekeeping gene (hypoxanthine phosphoribosyl transferase 1 (Hprt1)) transcripts performed on the same reaction plate on a 7500 fast real-time PCR system (Applied Biosystems). Relative gene expression was normalized to Hprt1 according to the delta cycle threshold method (ΔCT), CTGene1−CTHprt1 35. Proprietary QuantiTect Primer Assays for all genes were purchased from Qiagen.
TGF-β bioassay
The MLE/PAI cell line is derived from mink lung epithelial cells and contains firefly luciferase under PAI-1 promoter control. Cells were cultured with experimental supernatants and were compared with a standard curve generated using recombinant human TGF-β1 (R&D). Cells were lysed and luciferase activity was measured using a Luciferase Reporter Assay (Biotium) 36.
ELISA
Cytokine secretion was measured using DuoSet sandwich ELISA according to the manufacturer's instructions (R&D).
Statistics analysis
Statistical analyses were performed using GraphPad Prism 4 software. The non-parametric tests, Mann–Whitney U and Wilcoxon matched pairs test, were employed to assess independent and paired data, respectively. An unpaired t-test was used to analyze parametric data sets. Tests performed and calculated two-tailed p values are indicated in the individual figure legends.
Acknowledgements
The authors thank the BBSRC, The Wellcome Trust and the MRC for their support of our projects. They also thank Dr. Clare Bryant for helpful discussion and advice. Oliver Burton is funded by a Herchel Smith Fellowship from Williams College (MA, USA).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




