Arginines in the CDR of anti-dsDNA autoantibodies facilitate cell internalization via electrostatic interactions
Abstract
Internalization of autoantibodies against double-stranded DNA (anti-dsDNA) is crucial to the pathogenesis of systemic lupus erythematosus. Anti-dsDNA may bind to cell-surface targets in order to facilitate the subsequent cell penetration of the anti-dsDNA. In this study, we observed that the 9D7 monoclonal anti-dsDNA autoantibody (9D7 mAb) penetrates into Jurkat cells via a novel alternative pathway. Endocytosis inhibitors or a lipid-raft inhibitor did not significantly change the penetration of 9D7 mAb into the Jurkat cells. However, heparin sulfate, chondroitin sulfate B, decaarginine and chondroitinase ABC significantly suppressed the internalization and the 9D7 mAb inhibited the internalization of Tat-GFP. Moreover, the penetration of the 9D7 mAb was significantly reduced in proteoglycan-deficient cells (pgs A-745). Positively charged amino acids including arginine are commonly found in the CDR of the 9D7 mAb. Point mutations to the arginine residues in the CDR of the H chain of the recombinant 9D7 mAb significantly attenuated its DNA-binding and cell-penetration abilities. These findings indicate that cell penetration of anti-dsDNA is due to the electrostatic interactions of arginine residues in the CDR with the negatively charged sulfated polysaccharides on the cell surface.
Introduction
Autoantibodies against double-stranded DNA (anti-dsDNA) are not only important markers of systemic lupus erythematosus (SLE) but also the major players in the pathogenesis of this disease 1. A subset of anti-DNA has been demonstrated to interact with cell-surface targets, penetrate into living cells and migrate to the nucleus to bind to their cognate nucleus antigen 2. These targets include glomerular basement-membrane components 3, 4, ribosomal phosphoproteins 5, myosin 1 6, calreticulin 7, α-actinin 8, 9 and NR2 glutamate receptor 10.
Positively charged amino acids, such as arginine and lysine, are commonly found in the CDR of the human and murine anti-dsDNA 11, 12. Moreover, arginine residues in CDR3 of VH (heavy chain V region) cross-react with dsDNA 12, 13. This subset of autoantibodies has been demonstrated to interact with heparin sulfate 3. In addition, heparin may inhibit the DNA-binding ability of Ab from the kidneys and ameliorate glomerulonephritis in MRL-lpr/lpr mice 14. A 30-amino acid peptide corresponding to the CDR2 and CDR3 of VH of anti-DNA has been observed to be effective in intracellular transport of haptens and macromolecules 15. It has been reported that the arginine-rich basic peptide derived from the HIV-1 Tat protein (positions 48–60) has the ability to facilitate cell internalization and acts as a protein carrier 16. Moreover, penetration of Tat peptides has been shown to be competitively inhibited by sulfated polysaccharides or polyarginine peptides, suggesting that negatively charged sulfated polysaccharides on the cell surface might contribute to the internalization of these peptides 17-19. The internalization of the anti-dsDNA into living cells may also take place via a similar mechanism.
In this study, we employed the 9D7 monoclonal anti-dsDNA autoantibody (9D7 mAb) 5, 20 and Jurkat cells to study the mechanisms of anti-dsDNA internalization. We hypothesized that the positively charged amino acids, especially the arginine residues, in the VH CDR of anti-dsDNA are important sites involved in the interaction with negatively charged macromolecules on the cell surface and facilitating anti-dsDNA internalization. Sulfated polysaccharides and polyarginine peptides were used to compete with anti-dsDNA internalization. Furthermore, we prepared single-chain variable fragment (scFv) from the parental 9D7 mAb and mutants with replacement of arginine residues in the CDR to determine their DNA-binding and cell-penetration abilities. The results of this study indicate that electrostatic interactions of arginine residues in the CDR with the negatively charged sulfated polysaccharides on the cell surface are an alternative pathway for the penetration of anti-dsDNA into living cells.
Results
Anti-dsDNA penetrate into living cells in a dose- and time-dependent manner
To determine the penetration of anti-dsDNA into living cells, Jurkat T cells were incubated with the 9D7 mAb or mouse IgG2b (mIgG2b) at different concentrations and for different times. After washing, fixation and permeabilization, the intracellular 9D7 mAb was stained with fluorescein-conjugated anti-mIgG and analyzed by flow cytometry. Compared with mIgG2b, 9D7 mAb was able to penetrate into Jurkat cells in a dose- (Fig. 1A) and time-dependent manner (Fig. 1B). After incubation with the 9D7 mAb, no positive staining was detected in the cells treated with the fluorescein-conjugated anti-mIgG but without Triton permeabilization (Fig. 1C), suggesting no binding of anti-dsDNA to the cell surface.
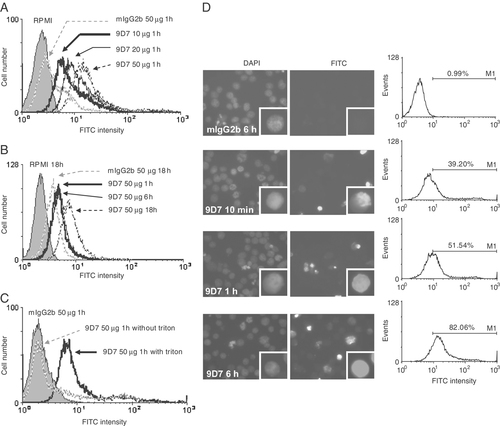
Anti-dsDNA penetrates into Jurkat cells in a dose- and time-dependent manner. (A) The mAb cell penetration was analyzed by flow cytometry after treating Jurkat cells with RPMI or mIgG2b or different concentrations of 9D7 mAb for 1 h or (B) after treating the Jurkat cells with 9D7, mIgG2b or RPMI for different lengths of time. (C) 9D7 mAb penetrated into the cells and did not simply bind to the cell surface. Jurkat cells were treated with 9D7 or mIgG2b for 1 h. The cells were immediately fixed, extensively washed and incubated with or without 0.1% Triton X-100. The penetration of the anti-dsDNA mAb was analyzed by flow cytometry. (D) The Jurkat cells were treated with 9D7 or mIgG2b (50 μg/mL) for different durations. At the indicated intervals, the cells were stained with DAPI (left panel) or FITC-labeled anti-mIgG (middle panel) and analyzed by fluorescence microscopy. An enlarged cell is shown in each pair of inset panels. The percentage of positively stained cells was quantified by flow cytometry in the right panel. Each figure is a representative of three independent experiments.
To confirm the intracellular localization of anti-dsDNA, the Jurkat cells treated with the 9D7 mAb or mIgG2b were stained with DAPI or FITC-labeled anti-mIgG Ab and analyzed by fluorescence microscopy (Fig. 1D). The left panel shows the location of nucleus. At 10 min, 1 and 6 h, the 9D7 mAb was found to have translocated into the nucleus whereas cells treated with mIgG2b did not show positive staining even at 6 h (middle panel). The fluorescence intensity also increased with time (right panel) as revealed by flow cytometry.
Penetration of anti-dsDNA into living cells is not mediated by endocytosis
To determine whether endocytosis is the mechanism for the internalization of anti-dsDNA, Jurkat cells were treated with endocytosis inhibitors (cytochalasin, taxol and nocodazole) before the addition of the 9D7 mAb (Fig. 2A). Results of flow cytometry showed that the inhibitors had no significant inhibitory effects on the cell-penetration ability of the 9D7 mAb. Recently, lipid raft-mediated endocytosis has been reported to play a role in the translocation of metallothionein into HepG2 cells 21 and in the entry of allergens into the mast cells 22. To inhibit the functions of lipid raft, Jurkat cells were pretreated with methyl-beta-cyclodextrin (MBCD). The internalization of the 9D7 mAb was not inhibited by this treatment (Fig. 2B). Moreover, internalization of the 9D7 mAb was not affected in TCR-deficient Jurkat cells (Fig. 2C). These findings suggest that the internalization of anti-dsDNA is not mediated by endocytosis.
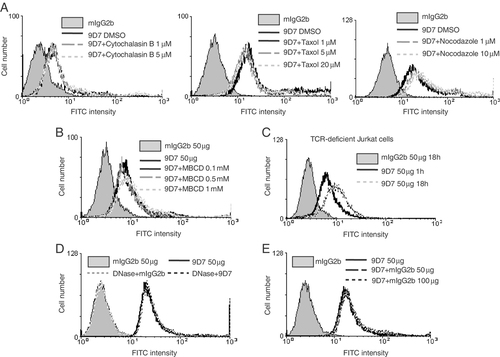
Internalization of anti-dsDNA into cells is independent of receptor-mediated endocytosis. (A) and (B) Jurkat cells were treated with mIgG2b (50 μg/mL) alone or were pretreated with either DMSO or the indicated inhibitors (endocytosis inhibitors: cytochalastin, taxol, nocodazole; a lipid raft inhibitor: MBCD) at the given concentrations for 1 h and then incubated with the 9D7 mAb for a further 1 h. (C) The TCR-deficient Jurkat cells were treated with 9D7 for 1 or 18 h or mIgG2b for 18 h. (D) Jurkat cells and protein samples were separately treated with DNase I for 30 min. The treated cells were then incubated with DNase-treated 9D7 mAb or mIgG2b (50 μg/mL) for 1 h. The untreated cells incubated with untreated antibodies were used as controls. (E) Jurkat cells were untreated or pretreated with mIgG2b at the given concentrations and then incubated with 9D7 (50 μg/mL) for 1 h. (A–E) The mAb penetration was determined by flow cytometry. Each figure is representative of three independent experiments.
To exclude the internalization of anti-dsDNA through immune complex formation, DNase I was used to digest DNA fragments in the cell cultures or in the Ab preparations. This treatment did not affect the penetration of the 9D7 mAb into the cells (Fig. 2D). In addition, internalization of the 9D7 mAb was not competed by mIgG2b (Fig. 2E), suggesting that the penetration of anti-dsDNA is not mediated by immunoglobulin receptors.
To determine the effectiveness of the endocytosis inhibitors and DNase I, the 9D7 mAb was incubated with thymus DNA to form immune complexes as a positive control. These complexes were able to enter into Jurkat cells. However, the penetration of 9D7–DNA complexes was inhibited by the treatment with the inhibitors or DNase I (data not shown), indicating that these compounds were effective.
Anti-dsDNA penetrates into living cells via electrostatic interactions
To determine whether the internalization of anti-dsDNA occurs via the interactions with the negatively charged sulfated polysaccharides on the cell surface, the penetration of the 9D7 mAb into Jurkat cells was measured after treatment with chondroitin sulfate B (Fig. 3A), heparin sulfate (Fig. 3B) and decaarginine (Arg10) (Fig. 3C). These compounds were found to inhibit internalization of the 9D7 mAb in a dose-dependent manner. Similar findings were observed in normal human T cells treated with the 9D7 mAb and Arg10 (Fig. 3D). Inhibition of 9D7 mAb internalization was also observed in the Jurkat cells treated with chondroitinase ABC to digest surface glycosaminoglycan (GAG) (Fig. 3E). The 9D7 mAb inhibited the uptake of Tat-GFP into Jurkat cells, demonstrating that the 9D7 mAb and Tat-GFP may penetrate into Jurkat cells via the similar pathway (Fig. 3F). Moreover, the internalization of the 9D7 mAb was not inhibited at 4°C (Fig. 3G). These findings suggest that the entry of anti-dsDNA into living cells may be mediated through the negatively charged sulfated polysaccharides on the cell surface in an energy-independent manner. To confirm the existence of this form of cell internalization, we determined the penetration of the 9D7 mAb into CHO K1 cells and CHO K1 proteoglycan-deficient cells (pgs A-745), mutants lacking xylosyltransferase required for the initiation of GAG synthesis. There were no apparent changes in the penetration of the 9D7 mAb into CHO K1 cells whereas the penetration of the 9D7 mAb was significantly reduced in pgs A-745 mutant cells (Fig. 4). These findings confirm that the negatively charged sulfated polysaccharides on the cell surface play a major role in the entry of anti-dsDNA into living cells. However, not all autoantibodies involved in lupus may enter living cells via this pathway, as Arg10 did not show inhibitory effects on the penetration of the monoclonal anti-P protein autoantibody (Fig. 3H). The monoclonal anti-P protein autoantibody is a subset of anti-ribosomal P-protein Ab. These Ab penetrate into living cells through the P0 protein on the cell membrane 23.
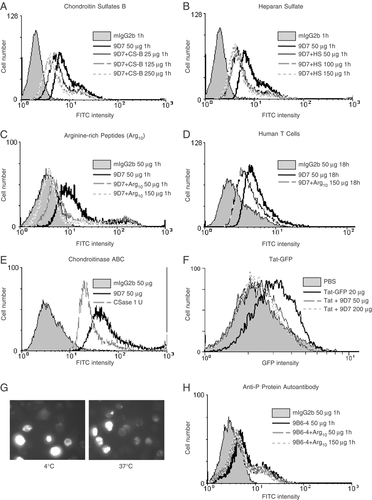
Inhibition of the internalization of anti-dsDNA by chondroitin sulfate, heparan sulfate and arginine-rich peptides. Jurkat cells were pretreated with or without (A) chondroitin sulfates B and (B) heparan sulfate at different concentrations for 1 h before adding 9D7 mAb. The cells were incubated for a further 1 h. Jurkat cells (C) and human T cells (D) were treated with or without Arg10 at different concentrations. 9D7 mAb was added immediately after Arg10 treatment. The cells were incubated for 1 h (C) or 18 h (D). (E) Cells were treated with or without chondroitinase ABC (1 U/mL) at 37°C for 1 h and subsequently incubated with 9D7 mAb at 37°C for 1 h. (A–E) Cells treated with mIgG2b (50 μg/mL) alone were used as control. (F) Jurkat cells were either untreated (PBS), treated with Tat-GFP (20 μg/mL) or co-treated with Tat-GFP (20 μg/mL) and 9D7 mAb (50 or 200 μg/mL) at 37°C for 1 h. (A–F) mAb penetration was analyzed by flow cytometry. (G) 9D7 mAb (50 μg/mL) was incubated with Jurkat cells at 37 or 4°C for 1 h and mAb penetration was analyzed by fluorescence microscopy. (H) Jurkat cells were treated with or without Arg10 at different concentrations. The 9B6-4 mAb was then added immediately after Arg10 treatment. Cells treated with mIgG2b (50 μg/mL) alone were used as control. The cells were incubated for 1 h and then analyzed by flow cytometry. Each figure shown is a representative of three independent experiments.
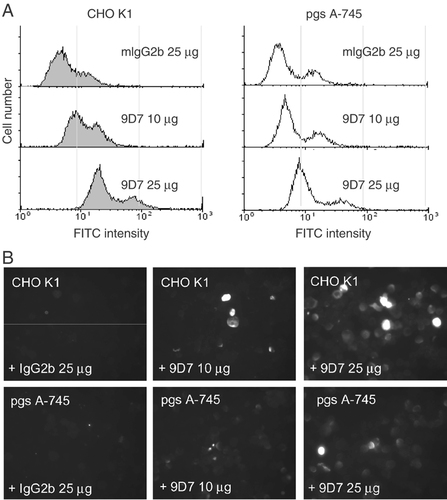
Internalization of anti-dsDNA was significantly reduced in proteoglycan-deficient cells (pgs A-745) but not in CHO K1 (WT) cells. mAb cell penetration was analyzed by either flow cytometry (A) or fluorescence microscopy (B) after treating CHO K1 or pgs A-745 cells incubated with different concentrations of 9D7 mAb or mIgG2b. The CHO K1 cells are shown in gray and the pgs A-745 cells in white in (A).
Amino-acid sequence, properties and 3-D modeling of recombinant anti-dsDNA
As shown in Fig. 5A, there are nine positively charged amino acids (5 arginine, 3 lysine and 1 histidine) in the CDR of the H and L chains of the 9D7 mAb.
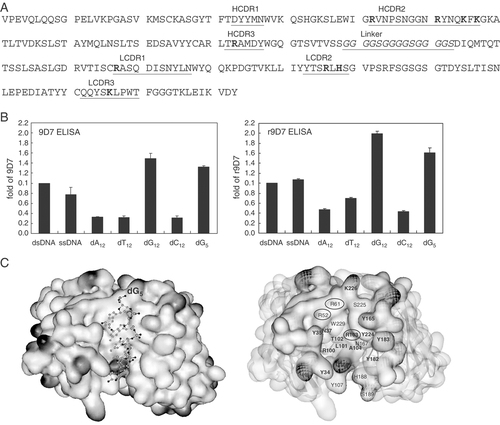
Amino-acid sequence, properties and 3-D structure of scFv recombinant anti-dsDNA. (A) Amino-acid sequence of the H and L chain V regions of the 9D7 mAb. The CDR of the H and L chains and the (GGGGS)3 linker (italicized) are underlined. (B) The binding abilities of the parental 9D7 and the scFv recombinant anti-dsDNA Ab r9D7 from E. coli against dsDNA, ssDNA, oligo-dA12, oligo-dT12, oligo-dG12, oligo-dC12 or oligo-dG5 were determined by ELISA. Binding of dsDNA to 9D7/r9D7 was set at 1 and all binding expressed relative to this standard. Data are represented as mean+SD based on two independent experiments. (C) The molecular interface of the r9D7–oligo-dG5 complex (left panel) and the amino acids of r9D7 in contact with dG5 (right panel) were modeled by the Insight II software. The major amino acids in contact with dG5 are shown in black and the remaining contact sites are shown in gray. The mutant arginine sites are indicated by circles. The shading in the model represents areas of charge.
To investigate the roles of the critical amino acids in the cell penetration and DNA binding of anti-dsDNA, we generated wild-type and different mutants of scFv from Escherichia coli and insect cells. VH and VL (light chain V region) of the 9D7 mAb were cloned from the 9D7 hybridoma cell line and these regions were linked by adding a (GGGGS)3 polypeptide chain between the C terminus of VH and the N terminus of VL (Fig. 5A). The relative binding abilities of the scFv-recombinant 9D7 expressed in E. coli (r9D7) were not significantly different from those of the parental Ab to dsDNA, ssDNA and oligo-dN; however, stronger binding to oligo-dG was observed (Fig. 5B).
Based on these findings and the sequence of the 9D7 mAb (Fig. 5A), the structure of r9D7–oligo-dG complex was modeled using the SWISS-MODEL and Insight II software (Fig. 5C). The oligo-dG5 ligand fitted in a narrow groove formed by the CDR of the H and L chains (left panel). The critical amino acids in VH and VL of the 9D7 mAb for the binding of the Ab to oligo-dG5 are shown in the right panel. The R52, R61 and R103 in VH CDR2 and CDR3 were found to be involved in significant interactions between the 9D7 mAb and nucleic acids. It has been reported that arginine side chains may affect both DNA-binding specificity and affinity 24-28. Furthermore, Arg10 has been demonstrated to inhibit the internalization of the 9D7 mAb (Fig. 3C). We hypothesized that these three arginine residues in CDR2 and CDR3 of the H chain of the 9D7 mAb chain may play an important role in DNA binding and cell penetration.
Roles of arginines in the CDR of anti-dsDNA
The R52A, R61A and R103A mutants were constructed and expressed in E. coli (r9D7) and insect cells (vr9D7). The DNA-binding abilities of the wild-type and mutants were determined by ELISA and found to be decreased in the three mutants, especially those with mutation at R61A or R103A (Fig. 6).
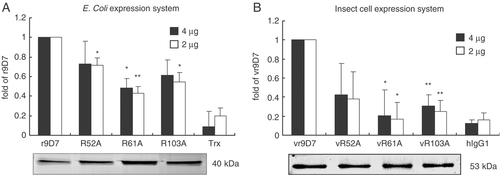
DNA-binding abilities of the scFv recombinant anti-dsDNA Ab (r9D7) mutants. The binding of different amounts of purified r9D7 or its mutants (R52A, R61A and R103A) to dsDNA were determined by ELISA. These proteins were expressed in the BL21 (DE3) E. coli strain (A) or insect cells (vr9D7, vR52A, vR61A, vR103A, (B)). Purified proteins were analyzed by SDS-PAGE and are shown in the lower panel. The negative controls for these two systems (A and B) were Trx and human IgG1 (hIgG1), respectively. Data are represented as mean+SD based on three independent experiments. *p<0.05 and **p<0.01 by the Student's t-test compared with the r9D7 or vr9D7 groups.
Cellular penetration of the wild-type and mutant vr9D7 was analyzed by flow cytometry (Fig. 7A). The penetration ability significantly decreased in the R61A and R103A mutants whereas there was no significant change in the R52A mutant. In addition, we also determine the cross-reactivity of vr9D7 with heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2) 29 and phosphoglycerate kinase 1 (PGK-1) 30 (Fig. 7B). Compared with the wild-type vr9D7, the binding ability to PGK-1 significantly decreased in all mutants. However, the binding abilities to hnRNP A2 were only partially decreased in some of the mutants. The R52A mutant lost about 80% of its PGK-1-binding ability and 60% of its hnRNP A2-binding ability. However, its cell-penetration ability remained unaffected. Although the R103A mutant retained 55% of its binding ability to hnRNP A2, it completely lost the cell-penetration ability (Fig. 7). Therefore, the cross-reactivity of the 9D7 mAb with hnRNP A2 and PGK-1 may not correlate with its penetration ability. These finding also suggest that arginine residues in the CDR of anti-dsDNA play important roles not only in DNA and cross-reactive protein binding but also in cell penetration.
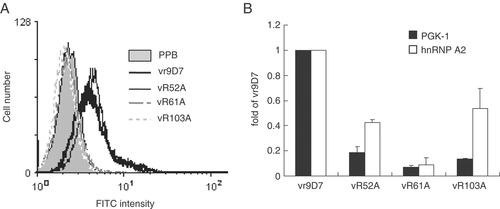
Cellular penetration and protein binding ability of the recombinant anti-dsDNA and its mutants expressed in insect cells. (A) Jurkat cells were treated with 20 μg/mL vr9D7 or its mutants (vR52A, vR61A, vR103A) for 24 h. Cell-penetration abilities of the mAb into Jurkat cells were analyzed by flow cytometry. (B) The hnRNP A2 and PGK-1 binding activities of vr9D7 and its mutants (4 μg/100 μL) were determined by ELISA. Data are represented as mean+SD based on two independent experiments.
Discussion
Multiple organ involvement and variations in manifestations are typical in SLE. The clinical outcomes have been attributed to the specificities of the autoantibodies and the sensitivities of the tissues 31. Anti-dsDNA with high affinity to dsDNA and cross-reactivity with tissue proteins may be correlated with lupus pathogenicity 32. The internalization of a subset of anti-DNA has been reported to occur via receptor-mediated endocytosis 3-10. In addition to myosin 1 6 and calreticulin 7, a nucleoside salvage transporter has been demonstrated to be a cell-membrane receptor for the scFv of 3E10 anti-dsDNA 33. In this study, we observed that the penetration of the 9D7 mAb into Jurkat cells is dose- and time-dependent and not simply surface bound. Moreover, there are no significant changes in the penetration of the 9D7 mAb into Jurkat cells treated with endocytosis inhibitors and a lipid-raft inhibitor or into TCR-deficient cells. These findings indicate that the penetration of anti-dsDNA into living cells might not require a cell-membrane receptor.
Cell-penetrating peptides are able to translocate across the cell membrane in a seemingly energy-independent manner 34. Among the cell-penetrating peptides, the internalization of Tat has been attributed to the interaction with sulfated proteoglycans on the cell surface 18, 19. In this study, we demonstrated that the internalization of the 9D7 mAb is not only inhibited by the negatively charged heparan sulfate and chondroitin sulfate but also competed with the positively charged Arg10. Moreover, the 9D7 mAb competes with the uptake of Tat-GFP, suggesting that the internalization of these molecules may occur via a similar pathway. Although anti-dsDNA internalization mediated by myosin 1 only occurs at 37°C 6, 35, internalization of the 9D7 mAb is not significantly affected at 4°C. In addition, the penetration of the 9D7 mAb was observed to be significantly reduced in pgs A-745 cells with defects in GAG biosynthesis. Based on these findings, we conclude that the penetration of anti-dsDNA into living cell takes place via electrostatic interactions with sulfated proteoglycans on the cell surface in an energy-independent manner.
In this study, we determined the sequence of the 9D7 mAb. There are nine positively charged amino acids (5 arginine, 3 lysine and 1 histidine) in the CDR of the H and L chains. This finding is similar to that of the arginine-rich basic Tat peptide 17-19. However, the number of positively charged amino acids in the CDR of the 9D7 mAb is higher than in the case of F14-6 and H9-3 or 3E10, which, respectively, require calreticulin or ENT2 on the cell surface as receptors for their internalization 7, 33. Moreover, we also characterized the purified r9D7 by fluorescence spectroscopy and circular dichroism. The recorded circular dichroism data showed that estimates for the proportion of α-helical and β-sheet secondary structure elements are consistent with the values expected from the known structure (data not shown). The interaction of r9D7 expressed in E. coli with dsDNA, ssDNA and oligonucleotides was also found to be similar to that of the parental 9D7 mAb, although it showed a stronger binding ability to oligo-dG.
Structure analysis contributes to our understanding of the anti-dsDNA pathogenic potential. The binding mechanism of anti-dsDNA significantly differs from those of other DNA-binding proteins, since anti-dsDNA does not contain known DNA-binding motifs. The primary structure of anti-dsDNA is determined by their mutatable variable region segments of the immunoglobulin genes. Arginine, lysine and asparagine residues in the CDR favor DNA binding. The V regions in the H chain may make major contributions to DNA binding 32. In our computer model of r9D7, arginine residues in the CDR have been demonstrated to be the sites for the anti-dsDNA–DNA interaction. However, the remaining amino-acid residues may also be involved in the interaction. Among the arginine residues, the R61A and R103A of scFv r9D7 mAb significantly inhibited DNA-binding and cell-penetration ability. Although the roles of other amino-acid residues in DNA binding and cell penetration are not clear, arginine should play an important role in these aspects.
We have found that in addition to the Jurkat cells, primary human T cells and CHO K1 cells, the 9D7 mAb is able to enter a wide variety of cell lines, such as RBA-1, RKO, J774, H1299, LLC-1 and 786-O (data not shown). These findings suggest that anti-DNA internalization may not be cell specific. After entering into living cells, anti-DNA may enhance cell growth and proliferation 36 or induce cell death and apoptosis 37-41. In addition, these Ab may also trigger pro-inflammatory events after cell penetration 20, 41-46. In our preliminary study, penetration of anti-dsDNA was demonstrated to be essential to the decrease in IL-2 production in activated Jurkat T cells. It is possible that anti-dsDNA, after penetration into living cells, may induce signal transduction. Furthermore, it may also cross-react with the intracellular mRNA-binding proteins, hnRNP A2 29 and PGK-1 30. In normal T cells, hnRNP A2 and PGK-1 bind to the reiterated AUUUA sequences in the 3′-untranslated region of labile mRNA 47, 48 and, thus, enhance the stability of IL-2 mRNA 49. Therefore, the cross-reaction between anti-dsDNA and hnRNP A2 or PGK-1 may decrease the availability of hnRNP A2 or PGK-1 and in turn reduce the stability of IL-2 mRNA and down-regulate the IL-2 production.
Structural information and functional analysis of lupus antibodies such as DNA-binding ability, cross-reaction with cellular proteins and penetration capability support the use of ligands to inhibit tissue deposition of the autoantibodies. Heparin is effective in improving glomerulonephritis in MRL-lpr/lpr mice 14 and arginine-rich peptides have significant inhibitory effects on the penetration of anti-dsDNA. Heparin-related compounds and arginine-rich peptides may be used as therapeutic agents to block the internalization of these Ab and in turn prevent the subsequent pathogenesis of SLE.
Materials and methods
Monoclonal Ab
The 9D7 anti-dsDNA mAb hybridoma cell line was established by fusing spleen cells from an MRL-lpr/lpr mouse with a BALB/c murine myeloma cell line as previously described 20. The 9D7-cell line was selected based on its production of mAb reactive with calf thymus dsDNA and immunofluorescence staining of HEp-2 cells where it showed a peripheral nuclear pattern. Purification of anti-dsDNA mAb from culture supernatant was carried out by protein A-Sepharose affinity chromatography (GE Healthcare Life Sciences, Sweden) and endotoxins in the purified Ab were removed using the polymyxin B-agarose affinity column (Sigma-Aldrich Co., St. Louis, MO, USA). The concentration of mIgG was determined using a mouse-IgG ELISA kit (Roche, Sandhofer, Germany). The mouse IgG2b (mIgG2b) (MOPC195, Sigma) was used as a nonspecific isotype antibody control and was prepared by the same procedures as the 9D7 mAb.
Cells
The human acute T-cell leukemia (Jurkat), CHO K1 and pgs A-745 mutant cells [18] were obtained from the American Type Culture Collection (Rockville, MD, USA) while the TCR-deficient Jurkat cell line was kindly provided by Dr. Hsien-Yeh Hsu (National Yang-Ming University, Taipei, Taiwan, ROC). The experiments using human materials were approved by the Institutional Review Board, National Yang-Ming University. The human blood samples were obtained with informed consent from healthy donor volunteers in our laboratory. PBMC were purified by Ficoll-Paque (GE Healthcare Life Sciences), and CD3+ T cells were isolated from PBMC by the EasySep human CD3 selection kit (StemCell Technologies, North America) according to the instructions of the manufacturer. The cells were cultured in RPMI 1640 medium (HyClone, Utah, USA) with 10% FCS (HyClone), 1% glutamine and 1% penicillin-streptomycin (GIBCO, Paisley, UK). The cells were maintained in a 5% CO2 humidified incubator at 37°C.
Penetration of 9D7 anti-dsDNA mAb into living cells
The penetration of anti-dsDNA mAb into living cells was determined by the method described by Avrameas et al. 15. Wild-type Jurkat, TCR-deficient Jurkat cells, CHO K1 or pgs A-745 mutant cells (1×106/mL) were incubated at 37 or 4°C in the complete culture medium with different concentrations of the 9D7 mAb, r9D7, mutant r9D7 or mIgG2b. At different time points, the cells were fixed with 1% paraformaldehyde for 25 min. After extensive washing and treating with or without 0.1% Triton-X for 5 min, the cells were stained with FITC-labeled anti-mIgG Ab (Jackson, West Grove, PA, USA) for 1 h. Cell penetration was analyzed using the FACSCalibur flow cytometer (BD Biosciences, Mississauga, Canada). A total of 10 000 events per sample were counted and analyzed.
For fluorescence microscopy, the cells were handled as above and the nucleus was then stained with DAPI (160 nM) (Sigma) for 10 min after incubation with the FITC-labeled anti-mIgG Ab. The cells were next spun onto coverslips by centrifugation at 500 rpm for 5 min and then analyzed by fluorescence microscopy.
Inhibition on cell penetration
Jurkat cells (1×106/mL) were pretreated with cytochalasin B (1 or 5 μM) (Sigma), taxol (1, 5 or 20 μM) (BIOMOL, Plymouth Meeting, PA, USA), nocodazole (1 or 10 μM) (Sigma), MBCD (0.1, 0.5 or 1 mM) (Sigma), chondroitin sulfates B (25, 125 or 250 μg/mL) (Sigma) or heparan sulfate (50, 100 or 150 μg/mL) (Sigma) under serum-free conditions for 1 h before incubation with the 9D7 mAb in the RPMI with 10% FBS. In addition, Jurkat cells or human T cells were co-treated with Arg10 (50 or 150 μg/mL) (Genemed Synthesis, San Antonio, TX, USA) and the 9D7 mAb or 9B6-4 mAb (50 μg/mL). After treatment with the inhibitors, the cells were incubated for different durations and the penetration of the 9D7 mAb into the cells was detected by staining and flow cytometry.
Penetration of the 9D7 mAb after DNase I treatment
The Jurkat cell cultures, 9D7 mAb and mIgG2b were treated separately with DNase I (20 μg/mL) (Sigma) for 30 min at 37°C. After treatment, the Jurkat cells were incubated with the DNase-treated 9D7 mAb or mIgG2b (50 μg/mL) at 37°C for 1 h. Penetration of the 9D7 mAb was analyzed by staining and flow cytometry.
Penetration of the 9D7 mAb after chondroitinase ABC treatment
To digest the linkage between N-acetylgalactosamine and glucuronic acid or iduronic acid on the cell surface, Jurkat cells at the density of 1×106/mL in RPMI 1640 supplemented with 0.1% BSA were treated with chondroitinase ABC (1 U/mL) (Sigma) at 37°C for 1 h. Jurkat cells with and without this treatment were separately incubated with the 9D7 mAb or mIgG2b (50 μg/mL) at 37°C for 1 h. Penetration of the 9D7 mAb was analyzed by staining and flow cytometry.
Competitive inhibition of internalization of Tat-GFP
Tat-GFP was kindly provided by Dr. Yeu Su (National Yang-Ming University). Jurkat cells were co-treated with Tat-GFP (20 μg/mL) and the 9D7 mAb (50 or 200 μg/mL) at 37°C for 1 h. Internalization of Tat-GFP was analyzed by flow cytometry.
Cloning and sequencing of scFv anti-dsDNA mAb
The H and L chain rearranged transcripts of 9D7 anti-DNA mAb were cloned using RT-PCR. The cDNA was prepared by reverse transcription of the mRNA isolated from 9D7 cells. PCR was performed with multiple degenerate sense primers designed for amplification of the leader sequences of mouse H and L chains 50. The antisense primers were designed to hybridize to the sequences coding for amino acids 122–116 of the murine κC region and 130–120 of CH1 of all murine Ig except IgG3. The conditions for PCR were 1 min denaturation at 95°C, 1 min annealing at 60°C and 1 min extension at 72°C for 28 cycles. The amplified fragments were isolated, ligated into pGEM-T (Promega, Madison, WI, USA) and sequenced (Mission Biotech, Taipei, Taiwan).
The sense primers for the signal peptides of the H and L chains were redesigned without degeneracy to match known sequences. The cDNA of the H and L chains were reamplified by PCR. The primers were also engineered to create the peptide linker sequence ((GGGGS)3) between the heavy and light chains. The specific PCR primers used to amplify the variable region of full-length H and L chain cDNA transcripts were: 9D7 heavy chain sense primer: 5′-CCA TAG GTA CCA GAG GTC CAG CTG CAA CAG TCT GGA-3′, antisense primer: 5′-GCC AGA GCC ACC TCC GCC TGA ACC GCC TCC ACC GGA GAC GGT GAC TGA GGT TCC TTG-3′; 9D7 L chain sense primer: 5′-TCA GGC GGA GGT GGC TCT GGC GGT GGC GGA TCG GAT ATC CAG ATG ACA CAG ACT ACA-3′, antisense primer: 5′-CCA TAG TCG ACT TTG ATT TCC AGC TTG GTG CCT CC-3′. The oligonucleotides were synthesized by Genemed Synthesis.
Generation and mutagenesis of scFv r9D7
Full-length scFv DNA was excised from pGEM-T and ligated into the E. coli expression vector pET23a with a thioredoxin (Trx) fusion protein at the N terminus. Correct orientation of the DNA in pET23a-Trx was confirmed by restriction enzyme mapping. The oligonucleotides were synthesized (Genemed Synthesis) to introduce the desired mutations: R52A (CDR2) primer: 5′-TGG ATT GGA GCT GTT AAT CC-3′; R61A (CDR2) primer: 5′-GGT GGT AAT GCG TAC AAC CA-3′; R103A (CDR3) primer: 5′-AGA TTG ACT GCA GCT ATG GA-3′. Each mutagenic oligonucleotide was annealed to a single-stranded template and extended by T4 DNA ligase (Promega) to generate the mutant strand. Mutations were confirmed by dideoxynucleotide sequencing (Mission Biotech).
Expression and purification of scFv r9D7 in E. coli
Purified plasmids containing full-length scFv DNA were expressed in E. coli. The wild-type and mutant clones of the scFv 9D7 pET23a-Trx plasmids (2 μg) were transformed into BL21 (DE3). After induction with 1 mM IPTG for 3 h, the culture was harvested. Proteins were purified by His-binding resin (Novagen, Madison, WI, USA) and refolded using a linear gradient of urea from 6 M downwards in PBS buffer.
Expression and purification of scFv-Fc r9D7 mAb in insect cells
Full-length scFv DNA was amplified using PFU DNA polymerase (Promega) and specific primers: 9D7 specific sense primer: 5′-CCA TAG TTA ACA TGG AGG TCC AGC TGC AAC AGT CTG GA-3′ and antisense primer: 5′-CCA TAA GAT CTT TTG ATT TCC AGC TTG GTG CCT CC-3′ (Genemed Synthesis). The amplified products were cloned in-frame into the vector pBacPAK9 (Clontech, Palo Alto, CA, USA) containing the human IgG1 Fc-coding sequence, which was kindly provided by Dr. Shie-Liang Hsieh (National Yang-Ming University). The Sf21 cells were co-transfected with 500 ng of the scFv-Fc pBacPAK9 vector and 100 ng Bsu36 I digested BacPAK6 viral DNA (Clontech) and recombinant viruses were then isolated by plaque assay according to the protocol of the manufacturer. The scFv-Fc fusion protein was recovered from the filtered supernatant of the recombinant virus-infected sf21 cells using protein A-Sepharose beads (GE Healthcare Life Sciences). The bound scFv-Fc 9D7 protein was eluted with glycine buffer (pH 3) and dialyzed against PBS.
Computer modeling of recombinant anti-dsDNA Ab (r9D7)
A stereo-view of the r9D7 was modeled based on homology to known antibody structures using the SWISS-MODEL software (http://swissmodel.expasy.org). The r9D7–dG5 interactions were fitted based on the best energy requirement using the Insight II software (San Diego, CA, USA). This model provided useful information predicting the interactions between the antibody and nucleic acids. Figures were prepared using the WebLab Viewer Lite (San Diego, CA, USA).
DNA- and protein-binding assay
The DNA-, hnRNP A2- and PGK-1-binding activities of the mAb were measured by ELISA. For the DNA-binding assay, polystyrene microtiter plates were precoated with 150 μL of 0.5 mg/mL protamine chloride (Sigma) per well for 2 h and then washed with PBS (pH 7.2). Calf thymus dsDNA, ssDNA (Sigma), oligo-dA5, oligo-dT5, oligo-dG5, oligo-dC5 and oligo-dG12 (Research Biolabs, Singapore) (10 μg/100 μL/well) were individually incubated in the microwells overnight at room temperature. The ssDNA was produced from the dsDNA by heating at 95°C for 10 min followed by flash cooling to 4°C.
To measure the hnRNP A2- and PGK-1-binding activities, polystyrene microtiter plates were precoated with 100 μL of recombinant hnRNP A2 29 or PGK-1 30 (5 μg/mL in 50 mM sodium carbonate buffer, pH 9.6) at 4°C for 18 h. After washing with PBS (pH 7.2) containing 0.05% Tween-20, the plates were blocked with 200 μL of 2% BSA in PBS for 2 h. The plates were washed again. Samples of the 9D7 mAb or r9D7 (4 μg/100 μL) were added and incubated for 1 h. Then, 100 μL of diluted HRP-conjugated goat anti-mIgG or anti-human IgG (Jackson) was added and incubated for another 30 min. To detect recombinant proteins from the E. coli expression system, 100 μL of diluted anti-Trx Ab (Invitrogen, Carlsbad, CA, USA) was added to each well and incubated for 1 h before HRP-anti-mIgG treatment. The plates were washed and 100 μL of a freshly prepared substrate solution (1.37 mg/mL of 2,2′-azinodi-13-ethylbenz-thiazoline sulfonic acid in 100 mM phosphate buffer, pH 4.2, containing 0.6 μL/mL 30% H2O2) was added to each well. After incubation for 15 min, the absorbance was measured at 405 nm.
Statistical analysis
Results were expressed as mean±SD. Differences were assessed by the Student's t-test. p<0.05 was considered statistically significant.
Acknowledgements
This work was supported by grants from the National Science Council (95-2320-B-010-022-MY3), VGHUST Joint Research Program, Tsou's Foundation (VGHUST97-P6-31) Yen Tjing Ling Medical Research Foundation (CI 95-8) and Taipei City Hospital, ROC to K. H. Sun.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




