B-cell clonal diversification and gut-lymph node trafficking in ulcerative colitis revealed using lineage tree analysis†
Abbreviations
CDCrohn's disease
GLgermline
IBDinflammatory bowel disease
SHMsomatic hypermutation
UCulcerative colitis
Abstract
In studies of inflammatory bowel diseases (IBD), research has so far focused mainly on the role of T cells. Despite evidence suggesting that B cells and the production of autoantibodies may play a significant role in IBD pathogenesis, the role of B cells in gut inflammation has not yet been thoroughly investigated. In the present study we used the new approach of lineage tree analysis for studying immunoglobulin variable region gene diversification in B cells found in the inflamed intestinal tissue of two ulcerative colitis patients as well as B cells from mucosa-associated lymph nodes (LN) in the same patients. Healthy intestinal tissue of three patients with carcinoma of the colon was used as normal control. Lineage tree shapes revealed active immune clonal diversification processes occurring in ulcerative colitis patients, which were quantitatively similar to those in healthy controls. B cells from intestinal tissues and the associated LN are shown here to be clonally related, thus supplying the first direct evidence supporting B-cell trafficking between gut and associated LN in IBD and control tissues.
Introduction
Ulcerative colitis (UC), one of the idiopathic inflammatory bowel diseases (IBD), is a chronic relapsing inflammatory disorder that may lead to significant impairment of gastrointestinal structure and function. UC usually involves the large intestine and extends proximally from the rectum upwards in a continuous fashion. Histologically, inflammation is usually limited to the mucosal layer and may involve ulceration and crypt abscess formation. UC has also been linked to an increased risk of gastrointestinal malignancy 1.
Despite recent progress in IBD research, in human subjects as well as a wide variety of experimental animal models, many questions concerning the immunological and genetic basis of the disease remain unanswered. The mucosa-associated lymphoid tissue of the gastrointestinal tract has the important tasks of, on the one hand, recognizing harmful pathogens and antigens within the gut and mounting an appropriate immune response against them and, on the other hand, knowing when and how to dampen or suppress that response once the assault has been cleared or when commensal flora or self-antigens are encountered 2. The pathogenesis of IBD appears to be related to a disruption of that finely tuned and regulated balance which exists within the mucosa-associated lymphoid tissue, resulting in deregulated and exaggerated local immune responses. This imbalance is most probably a result of complex genetic, environmental, and immunological susceptibility factors 3.
Experimental models of intestinal inflammation indicate that mucosal inflammation is probably mediated either by excessive effector T-cell function or deficient regulatory T-cell function. Exaggerated T helper 1 cell response associated with increased secretion of IL-12, IFN-γ and/or TNF results in a Crohn's Disease (CD)-like colitis in these experimental models, and exaggerated T helper 2 cell response associated with increased secretion of IL-4, IL-5 and/or IL-13 results in UC-like colitis 3. On the other hand, defects in various regulatory cells, including T cells that produce TGF-β and/or other suppressive cytokines and inhibit the effector cell response, also seem to play a significant role 2-5.
While the prime culprits appear to be T cells, particularly CD4+ T cells, there is little doubt that other cell populations play a part in the pathogenesis of IBD. Mucosal epithelial cells and their ability to recognize intestinal microflora have been implicated in the initiation or propagation of mucosal inflammation in IBD, and abnormalities in the innate immune response involving antigen-presenting cells, macrophages and natural killer cells have also been described 1, 3-6.
Despite the relatively little attention they have received in recent years, B cells and the production of autoantibodies may also play a significant role in IBD pathogenesis as well. Tolerance toward commensal microflora is broken in active IBD 7 and autoantibodies directed against intracellular components of epithelial cells, anti-neutrophil cytoplasmic antibodies, as well as antibodies directed against intestinal bacteria are produced in the colonic tissue of UC patients 8, 9. Antibodies against certain self-antigens, such as tropomyosin, have been detected in some patients with UC 10, while some patients with CD have developed antibodies against mannan, a component of the yeast Saccharomyces cerevisiae cell wall. Enhanced mannan exposure seems to stimulate specific immune responses in a subgroup of CD patients with genetically determined low mannan binding lectin concentrations 11.
McCabe et al. 12 examined the B-cell repertoire in intestinal mononuclear cells from normal individuals and patients with IBD. They found that while genes from all seven heavy chain variable region (VH) gene families were expressed, differences in VH usage in lamina propria intestinal B cells exist between CD, UC and normal individuals. VH1 mRNA transcripts were present at higher levels than their genomic representation and transcripts for VH1 and VH4 appeared to have higher levels in the active stage of disease. They concluded that within the polyclonal intestinal B-cell response, VH usage is skewed, and this may be relevant to the antigenic and autoimmune responses in IBD 12. Other studies showed VH3 and VH5 usage as well 12, 13. A recent study using a murine model of CD showed that an expanded B-cell population blocked regulatory T cells and exacerbated the experimental ileitis 14.
The B lymphocyte immune response involves affinity maturation of the cells' antigen B-cell receptors, involving somatic hypermutation (SHM) of receptor genes and antigen-driven selection of the resulting mutants 15-18. SHM of immunoglobulin (Ig) variable region (IgV) genes is million-fold faster than normal somatic mutation, and uses different mechanisms 19-21; it depends on transcription, activation-induced cytidine deaminase and DNA mismatch repair, and is mechanistically related to class switch recombination 22. It is as yet unknown how SHM is triggered, although germinal center initiation and its structural organization 23-25 are known to require both cognate and co-stimulatory interactions with T cells 26, 27, and are assumed to involve B cells that are already participating in the response. SHM may be triggered, and possibly be also maintained in germinal center B cells, by immune complexes formed by IgG antibodies bound to the antigen 15, 19, 28, 29. It is particularly not clear how the processes of SHM and selection interact dynamically and temporally to shape the memory B-cell repertoire, on which the system depends for fast antigen elimination in subsequent encounters 30-33, although several models have been suggested based on imaging studies 34-36. Bioinformatical approaches utilized so far in the study of affinity maturation include the analysis of the frequencies of specific types of mutation 37-45 and mathematical models exploring the dynamical interactions between SHM and clonal selection 46-51 and their spatial segregation 52-55.
Previous histological and molecular studies of autoimmune diseases have found that autoimmune B-cell clones undergo SHM and antigen-mediated selection 55-58. A major contribution to the understanding of the pathogenesis of IBD and particularly UC can be achieved by analysis of the B-cell clones participating in immune responses in the gut, in particular their Ig variable region gene diversity. A significant step toward understanding the role of B cells in UC has been made by Thoree et al. 59, who demonstrated clonal relationships between B cells derived from UC intestinal tissues and peripheral blood.
In this study we focused on studying IgV gene diversification in B cells found in the inflamed intestinal tissue of UC patients and B cells from mucosa-associated lymph nodes (LN). We have used the novel methodology of generation of Ig gene lineage trees, deduced from the point mutations found in Ig gene sequences derived from UC and control intestine samples. Mutational lineage trees have been created using our IgTree© program (Barak et al., submitted). Lineage tree characteristics (described below) were analyzed using our MTree© program 60, 61, and compared with samples taken from healthy intestinal tissue of intestinal carcinoma cases. The results of our analysis confirm that diversification processes do occur in B-cell clones found in the inflamed and healthy intestinal tissues 59. Most importantly, our finding of clonal relationships between B cells from inflamed gut and associated LN implies that those B cells are continuously trafficking between the gut and the associated LN.
Results
Ongoing Ig gene diversification in B-cell clones
Inflamed colon and mucosa-associated LN tissues of two UC patients were chosen. As controls we used histologically normal colon tissues of three colon carcinoma cases (Table 1). All tissues were formalin-fixed and paraffin-embedded. H&E staining of sections was carried out for case revision (Fig. 1A). Amplification of FR2-JH regions was carried out with total genomic DNA as a template. Two hundred and thirty sequences obtained from UC samples and 91 sequences from control intestine were extracted, out of which 144 and 61 sequences from UC and control, respectively, were analyzed. Germline (GL) genes were identified by alignment with published human IgV genes using IMGT V-QUEST and SoDA, and accordingly grouped and aligned using ClustalW, as explained in the Materials and methods. An example of a clonal alignment is given in Supporting Information Fig. 1. Lineage trees were then generated for each clone, giving 30 lineage trees in total (Table 1); sample trees are shown in Fig. 2.
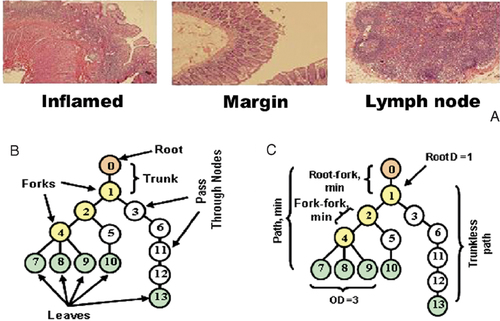
Sample lineage tree, tree properties and tissue staining used for lineage tree analysis. (A) A sample of H&E staining of tissue sections used for lineage tree analysis. (B) Sample lineage tree: nodes in the tree can be either the root node (always the node numbered zero in our definition, colored orange here), leaves (sequences of cells that had no descendants at the time of observation, node numbers 7, 8, 9, 10, 13, colored green), or internal nodes. Internal nodes can be either split nodes or forks – those with more than one child (numbered 1, 2, 4, colored yellow); or pass-through nodes – those with exactly one child (numbered 3, 5, 6, 11, 12, colored white). The trunk length is the distance between the root to the first split node, which equals 1 in this case. (C) Tree properties: all six properties used in this study, demonstrated on the sample lineage tree from Fig. 1B. Tree properties and their definitions are listed in Table 2. In this example, RootD=1, DRSN-min (minimum root to fork distance)=1, DASN-min (minimum fork to fork distance)=1, PL-min (minimum path length)=4, OD-avg=2.3 (average of OD values of split nodes 1 and 2=2 and split node 4=3) and DLFSN-avg (average trunkless path length)=3.4 (average of trunkless path lengths from node ♯1 to leaves 7, 8, 9, 10=3 and to leaf 13=5).
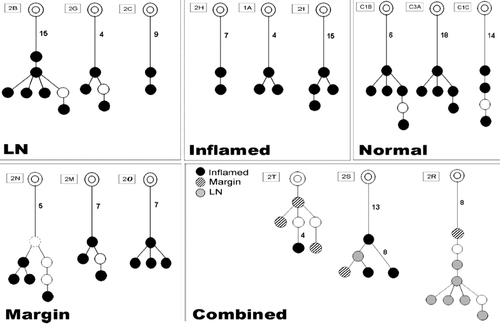
Lineage trees of related IgH variable region sequences from normal healthy intestinal tissue (from patients with colon carcinoma diagnosis) and different areas of intestinal tissue from UC patients. In the trees for LN, inflamed, normal, and tissue resection margins, black nodes represent the observed sequences. White nodes represent intermediate mutations. Deduced forks are marked with dashed circles. The numbers next to the lines represent the number of mutations. Lines in which numbers of mutations are not indicated denote one mutation. “Combined” indicates lineage trees of related IgH variable region sequences isolated from two or three groups of sequences obtained from the different locations of one IBD patient and combined into one tree. In the combined tree drawing, black nodes represent sequences obtained from inflamed intestinal tissue, striped nodes represent tissue resection margin sequences and light gray nodes represent LN sequences. The additional graphical features of the trees are as per the other drawings. Serial numbers for each tree are stated in rectangles, corresponding to Table 1, right column.
| Patient | Age | Sex | Diagnosis and procedure | Clinical data | Sampled tissue | Clone | ♯ of seq.b) | Length (bp) | ♯ leaves | Tree name | ||
| V | D | J | ||||||||||
| ♯1 | 26 | M | UC Total colectomy | Acute disease, 10 months. Pelvic infection. | Inflamed | 1–45 | 6–25 | 4 | 8 | 168 | 2 | 1A |
| Tissue resection margins | 3–49 | 6–25 | 5 | 5 | 191 | 1 | 1B | |||||
| 3–7 | 6–25 | 4 | 3 | 179 | 1 | 1C | ||||||
| ♯2 | 32 | M | UC Total colectomy | Seventeen years of disease. Resist to most treatments (6–7 bowel movements). Other autoimmune symptoms as arthritis Raynault | LN 8 | 1–8 | 4–23 | 4 | 13 | 186 | 2 | 2A |
| 4–31 | 2–2 | 2 | 13 | 173 | 4 | 2B | ||||||
| 3–7 | 6–25 | 4 | 7 | 191 | 1 | 2C | ||||||
| 3–15 | 2–8 | 2 | 5 | 194 | 1 | 2E | ||||||
| 3–23 | 6–13 | 4 | 4 | 191 | 1 | 2F | ||||||
| 1–69 | 4–23 | 4 | 15 | 187 | 2 | 2G | ||||||
| 1–3 | 4–4 | 4 | 1 | *b) | ||||||||
| Inflamed region 2 | 1–f | 3–10 | 5 | 7 | 199 | 1 | 2H | |||||
| 3–30 | 3–9 | 4 | 7 | 197 | 2 | 2I | ||||||
| 1–3 | 4–4 | 4 | 6 | 171 | 1 | 2J | ||||||
| 1–2 | 3–16 | 5 | 5 | 180 | 1 | 2K | ||||||
| 4–34 | 5–12 | 4 | 1 | 214 | *b) | |||||||
| Tissue resection margin area 5 | 1–45 | 3–22 | 4 | 10 | 209 | 1 | 2L | |||||
| 3–9 | 6–13 | 4 | 6 | 200 | 2 | 2M | ||||||
| 3–15 | 3–10 | 4 | 5 | 182 | 3 | 2N | ||||||
| 3–53 | 6–25 | 1 | 7 | 164 | 3 | 2O | ||||||
| 1–24 | 2–15 | 4 | 10 | 181 | 2 | 2P | ||||||
| 4–34 | 5–12 | 4 | 8 | 214 | 1 | 2Q | ||||||
| 4–31 | 2 | 2 | 1 | 173 | *b) | |||||||
| 1–3 | 4–4 | 4 | 1 | *b) | ||||||||
| Combined trees | 4–31 | 2 | 2 | 14 | 173 | 4 | 2R | |||||
| 1–3 | 4–4 | 4 | 10 | 171 | 3 | 2S | ||||||
| 4–34 | 5–12 | 4 | 9 | 214 | 3 | 2T | ||||||
| Control-1 | 46 | M | CC, right hemicolectomy | First diagnosis anemia | Colon | 3–23 | 3–10 | 4 | 7 | 188 | 1 | C1A |
| 1–69 | 4–23 | 4 | 10 | 192 | 3 | C1B | ||||||
| 1–3 | 4–4 | 4 | 16 | 176 | 1 | C1C | ||||||
| Control-2 | 49 | F | CC, right hemicolectomy | Large B-cell lymphoma | Colon | 3–7 | 3–9 | 4 | 11 | 200 | 1 | C2A |
| 3–7 | 2–2 | 4 | 4 | 185 | 1 | C2B | ||||||
| Control-3 | 80 | M | CC, right hemicolectomy | Anemia, prostatic hypertrophy | Colon | 3–30 | 6–6 | 4 | 13 | 187 | 3 | C3A |
| 1–2 | 3–16 | 5 | 3 | 185 | 1 | C3B | ||||||
- a) a) UC – ulcerative colitis; CC – colon carcinoma; note that each line represents a separate lineage tree.
- b) b) Denotes the number of distinct sequences found for the clone.
- c c) The sequences marked with * were not included in the tree quantitative measurements, as they have appeared only once in the data, but have been included in the combined trees.
Combined trees demonstrate B-cell trafficking between different gut locations
Some individual lineage trees from different intestinal areas were found to be related to each other, that is, they possessed the exact same VDJ segments and junctional regions (Table 1). This means that the same B-cell clone was present in different areas. In these cases, we combined the related tress into single lineage trees for the complete clones (Fig. 2). These clonal relationships show a mixing between B cells from different tissue samples, implying that B cells are continuously trafficking between the gut and the associated LN.
To the best of our knowledge, this is the first time clonal relationships between gut and LN B cells are reported. Thoree et al. 59 have previously demonstrated clonal relationships between B cells from UC intestinal tissues and peripheral blood. Together, these results suggest that B-cell clones traffic between tissues more extensively than previously recognized.
Ig gene diversification in UC B-cell clones is normal
We have examined IgH lineage trees obtained from different gut locations: inflamed tissues, tissue resection margins and an associated LN. The inflamed tissues were those parts of the samples in which inflammation was evident. This examination has revealed considerable heterogeneity: complex and branched trees appear along with narrow trees, similarly to control trees. In addition, lineage tree shapes were evaluated not only qualitatively and intuitively but also quantitatively, using our unique rigorous computer algorithm MTree© 57, 58. According to this method, defined distances between key tree nodes (root, split nodes or “forks”, and leaves) reflect dynamical features of clonal evolution including the mutation rate of IgH variable regions, the affinity between target antigen and the B-cell receptor of the Ig clone, and antigen-driven selection pressure.
A thorough statistical analysis correlating tree characteristics with dynamic parameters of the B-cell response such as mutation rate, using a computer simulation of the humoral immune response (Shahaf et al., submitted), has concluded that seven tree characteristics possess the highest correlation values with the biological parameters. These tree properties are the length of the tree's Trunk (T), the minimum root to leaf path length (PL-min or Path-min), the average distance from a leaf to the first split node/fork (DLFSN-avg or Trunkless Path-avg), the average outgoing degree, that is, the average number of branches coming out of any node (OD-avg), the root's outgoing degree (RootD or number of branches coming out of the root), minimum distance between adjacent split nodes/forks (DASN-min or Fork to Fork distance-min) and minimum distance from the root to any split node/fork (DRSN-min or Root-Fork distance-min, Table 2). The average trunkless path, minimum fork-to-fork and root-to-fork distances do not have a value (i.e., are undefined) for trees that do not have any forks. Therefore, comparisons involving these tree properties did not include all trees; average trunkless path comparisons included two, three and six control, LN and UC trees, respectively; minimum root-to-fork comparisons included one, two and six control, LN and UC trees, respectively; and minimum fork-to-fork was not included in the comparisons and analysis, as there was only one UC tree that had a defined value for this tree property.
We compared the dynamics of clonal evolution, as reflected by lineage tree properties, in different locations in UC patient intestines (inflamed tissues, tissue resection margins and associated LN), and the controls. Corresponding with our observation that tissue resection margins of UC patients also looked inflamed, although inflammation severity was lower than that of the inflamed tissues, the measurements of lineage trees generated from both groups were similar without any significant differences. Hence we merged tree measurement results of both UC tissues. We compared measurements of trees obtained from UC intestine, UC LN and control intestine (Fig. 3). The insights derived from these comparisons were as follows. The trunk length, minimum path length and minimum distance between the root and any fork of UC LN trees were shorter than those of both UC and control intestine, although the small number of data points did not allow detection of statistical significance. These short small trees may represent other unrelated clones passing through the LN, that upon averaging reduce the average trunk length, minimum path length and minimum root-to-fork distance values (Table 2 and Fig. 3). Minimum root-to-fork comparisons were assessed for two control trees, two LN trees and six UC trees. Measurements of lineage trees from UC patients did not significantly differ from those of controls (Fig. 3), suggesting that although UC is a chronic condition, Ig diversification processes are normal.
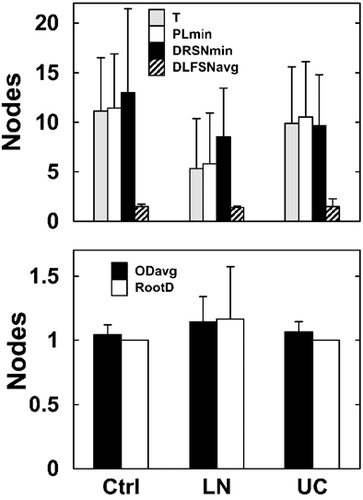
Comparison of lineage tree property measurements between normal control, UC LN (lymph node) and UC intestinal tissues (including the inflamed areas and tissue resection margins, labeled as UC). T – trunk; PL-min – minimum path length; DRSN-min – minimum distance from root to fork; DLFSN-avg – average trunkless path length; OD-avg – average outgoing degree; RootD – the root's outgoing degree. Trunkless path comparisons included two, three and six control, LN and UC trees, respectively. Root-to-fork comparisons included one, two and six control, LN and UC trees, respectively. All other tree property comparisons included all trees. Minimum fork-to-fork distance was not included in the comparisons and analysis, as there was only one UC tree that had a value for this tree property. Error bars represent the data standard deviations. The Mann–Whitney test was used for all comparisons, and the FDR correction for multiple comparisons was applied.
| Tree variable definition | Abbreviation | Correlation to secondary B-cell response dynamic parametersa) |
|---|---|---|
| Length of tree trunk from root to the first fork, that is, the number of mutations shared by all leaves | Tb) | Affinity ↓ |
| Path length, where a path is defined from the root to a leaf, hence PL-min gives the minimum number of mutations per leaf | PL-min | Affinity ↓ Selection ↓ |
| The average distance from a leaf to the first (closest to root) fork, i.e. the trunkless path: DLFSN(leaf i)=PL(leaf i)−T | DLFSN-avg | Affinity ↓ Selection ↓ |
| Outgoing degree representing the average number of children per node. Measured over all nodes, including pass-through nodes | OD-avg | Affinity ↑ Selection ↑ Mutation ↓ |
| The root's outgoing degree, that is, the number of branches emerging from the root. RootD=1⇔ T>0 | RootD | Affinity ↑ |
| The minimum distance between adjacent forks, that is, between two consecutive forks on the same path | DASN-min | Affinity ↓ Selection ↓ |
| The minimum distance from the root to any fork | DRSN-min | Affinity ↓ |
| Selection ↓ |
- a) a) Correlation of tree variables with the dynamic parameters of the secondary B-cell response dynamic parameters (Shahaf et al., submitted): ↑ denotes a direct correlation – as the value of dynamic parameter increases, the tree characteristic increases; ↓ denotes an inverse correlation – as the value of the dynamic parameter decreases, the value of the tree characteristic increases.
- b) b) T was not included in the analysis in this study, as statistical analysis showed that it cannot be compared with the other tree properties using the methods used for the other tree properties, due to small amounts of data.
Discussion
Immune responses in the gut are very common due to the large number of antigens and endogenous normal flora in the gastrointestinal lumen. Increased immune responses are generated when harmful antigens and pathogens are present. It is thought that a background of inflammation and a disruption of the internal immune system balance in the gut characterize IBD. Although much research effort has been focused on IBD, very few studies addressed the immunological aspects of IBD, and fewer still specifically addressed the role of B cells in these diseases.
The present study focused on the clonal expansions of B lymphocytes that occur in the gut of UC patients and compared them to expansions occurring in immunologically healthy intestinal tissues. In spite of extensive technical difficulties, we have successfully amplified DNA segments of up to 220 bp of IgH variable regions from formalin-fixed, paraffin-embedded tissues by PCR. However, the most novel methodological aspect of our study was the generation of lineage trees according to point mutations found in Ig gene sequences derived from UC and control intestine samples, in order to follow the expansion of those B-cell clones that dominate the active responses. We compared samples derived from three types of tissue locations: inflamed intestinal tissues, tissue resection margins (around inflamed tissues) and the associated LN of UC cases, to each other and to control intestinal tissue.
Lymphocytes may be found in the gut within Peyer's Patches, mucosa-associated LN or gut lumen. It is reasonable to assume that lymphocytes may traffic among their different locations in gut. Thoree et al. 59 have previously demonstrated clonal relationships between B cells from UC intestinal tissues and peripheral blood; however, prior to the current study, there was no evidence of clonal relationships between B cells in different areas in and around UC intestinal tissues. In the present study, we have found evidence of clonal relationships between cells in different tissue samples: lineage trees that combine clonally related sequences from distinct locales (inflamed area, tissue resection margins and LN) in the gut show a mixing of Ig gene sequences from the different locales, confirming that sequences with the exact VDJ classification are most likely clonally related, and suggesting trafficking of B cells between the UC intestinal tissues and the associated LN. Our findings are, to the best of our knowledge, the first ones directly supporting B-cell trafficking between gut and associated LN in UC. This, of course, raises the question of whether the diversification of such B-cell clones had started in the LN or in the gut. The clones we have observed are too highly intermixed to suggest a clear answer to this question. It is possible that there were multiple interactions between LN and gut lymphocyte populations throughout the response.
UC patient trees included heterogeneously shaped trees, similarly to the trees in the control samples. Tree shape is an outcome of the stage of the corresponding immune response. It is possible that large branched trees reflect active clones whereas the small and narrow shapes of some others are probably related to clones participating in waning immune responses, or secondary responses subjected to high selection pressures, as selection tends to “prune” the trees. Therefore, regardless of where a clone's diversification has started, it is reasonable to assume that in UC intestinal tissues (inflamed and tissue resection margins areas), the shape heterogeneity reflects the variability of immune responses, where active responses are observed along with waning responses.
In addition to qualitative observations on lineage tree shapes, quantitative measurements of distances between key nodes on these trees (such as root, leaves and forks; properties are defined in Table 2) were performed, to compare between immune responses that occur in the intestinal tissues of UC patients versus responses in control intestinal tissues. Seven tree characteristics that best correlate with B-cell response dynamic parameters were measured. No significant differences in tree shape properties between UC and control trees have been detected, suggesting that the diversification processes of IgH variable region genes are similar in all groups.
LN trees were shorter, though this difference was not significant due to the small number of data points and high data variability, resulting from the highly stochastic mutation process. This suggests, however, that our samples may contain clones from other immune responses, which may or may not be related to the UC inflammation, trafficking through the LN, thus giving highly variable trunk length and minimum path length values. Supporting this assumption, the number of distinct sequences sampled per clone was as large in the LN as in the controls (9.5±4.7 and 9.1±4.7 for LN and controls, respectively), and larger than sequence numbers sampled in inflamed regions or tissue resection margins (6.6±1.1 and 6.8±2.5, respectively). Alternatively, the shorter LN trees may reflect the different compositions of the two tissues. The majority of gut B cells are likely to be involved in the inflammatory process for a long time, and hence they possess longer trunks; while many of the cells in an LN follicle may be B cells of either activated or naïve status, not necessarily belonging to clones involved in the gut inflammatory response, and may have been sampled at a different stage of activation.
Analysis of mutational motifs around mutated nucleotides is used to identify specific motifs preferred or avoided by the mutational mechanism, triggered by activation-induced cytidine deaminase. In this study we observed some, but not all of the motifs reported in the literature, probably due to the small size of our dataset; small differences were observed in mutational motifs and amino acid substitutions (Supporting Information: Results and Figs. 2, 3). In summary, we have presented a thorough analysis of Ig gene sequences from two UC patients and three controls. The study revealed normal Ig gene diversification, indications of antigen-driven selection, and – for the first time – trafficking between gut and associated LN, in B-cell clones from UC patients. These results are a step toward elucidating the mechanisms controlling the fine balance between protection from pathogens and prevention of chronic inflammations, in particular, in UC.
Materials and methods
Tissues
Inflamed colon and associated LN tissues were obtained from two UC cases. As controls we used histologically normal colon tissues from two cases of colon carcinoma (Table 1). All samples were formalin-fixed, paraffin-embedded tissues chosen from the pathology institute archives in the Sheba Medical Center (Fig. 1A).
DNA extraction
The DNA was extracted from 2–5 sections of each formalin-fixed, paraffin-embedded tissues by Paraffix™ DNA extraction kit (Syntezza).
IgH variable region amplification by PCR
The FR2 to J region of IgH variable region genes was amplified by semi-nested PCR using 50–150 ng of genomic total DNA as a template and Paraffix™ PCR tubes (Syntezza). The primer sequences are given in the Supporting Information.
The PCR program was the same in both rounds. It included an activation step of 95°C for 15 min followed by five cycles of 95°C for 1 min, 60°C for 2 min and 72°C for 1 min and 50 s. In the next five cycles the annealing temperature was reduced to 55°C. Twenty-seven cycles with 50°C of annealing temperature were followed. The final step was 72°C for 10 min. In some of the reactions the temperature changes were longer than the default – 0.1°C/s from annealing to elongation and 1.0°C/s from denaturation to annealing. PCR products were separated on a 2% agarose gel.
Cloning and sequencing
PCR products of 220–280 bp were eluted from the gel by the Genelute kit (Sigma) and cloned into the pGEM-T-easy-vector (Promega). DNA plasmids were extracted from white colonies in liquid cultures and sequenced by the SP6 universal primer (HY LABS services).
Lineage tree construction and quantitative analysis
GL genes for each sequence were identified by alignment using V-QUEST (http://imgt.cines.fr/IMGT_vquest/share/textes/) and the SoDA algorithm 62 (http://dulci.org/soda). Sequences were accordingly grouped into clonally related sequence groups and aligned within each group with the most likely GL segments using ClustalW (http://www.ebi.ac.uk/clustalw). Ig gene sequences were transformed into mutational lineage trees using the program IgTree© [63, 64, and Barak et al., submitted], implementing a distance method-based algorithm that finds the most likely tree with a high probability.
A lineage tree is defined, graphically, as a rooted tree where each node corresponds to a B-cell receptor gene sequence (Fig. 1B). For two nodes X and Y, we say that Y is a child of X if the sequence corresponding to Y is a mutant of the sequence corresponding to X, which differs from X by only one mutation, and is one mutation further than X away from the original (GL) gene, that is, the root. Two B cells with identical receptor genes will thus correspond to the same node. A lineage tree depicts the maturation process of a B-cell clone at the moment of observation – it consists only the Ig sequences of cells that were sampled at that moment, and their ancestors back to the root, which were not necessarily sampled at the time of observation. Nodes in the tree can be either the root node, leaves (terminal sequences) or internal nodes, which can be either split nodes (branching points or forks) or pass-through nodes.
Trees were measured using the program MTree© 60, 61, implementing a rigorous algorithm for quantifying the shape properties of the trees in terms of graph theory, such as the number of leaves, number of nodes and various distances on the tree (Fig. 1C). Wherever applicable, minimum, maximum or average tree properties (per tree) were used.
Statistical analysis
Comparison between lineage trees was done using the Mann–Whitney U-test, as normal distributions could not be assumed. To correct for the fact that we perform multiple comparisons (on six different tree properties) between experimental groups, we used the FDR method 65. Only differences that were found to be significant under this correction were considered as meaningful in our studies.
Acknowledgements
The authors are grateful to Hanna Edelman for help in manuscript preparation. This work was part of Neta S. Zuckerman's studies toward a PhD degree in the Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, and was supported in parts by the following grants: Israel Science Foundation grants 759/01 and 546/05, an Israel Cancer Research Fund project grant, a Systems Biology prize grant from Teva Pharmaceuticals and indirectly through sharing of computer resources by a Human Frontiers Science Program Young Investigator Grant, and a Swedish Foundation for Strategic Research grant (to R.M.); a Human Frontiers Science Program Research Grant (D.K.D.W. and R.M.); The Ministry of Science and Technology PhD scholarship for advancing women in science, The Yeshaya Horowitz Association through the Center for Complexity Science PhD scholarship, Bar Ilan University President's PhD scholarship and a Dean's excellence PhD scholarship of The Mina and Everard Goodman Faculty of Life Sciences (to N.S.Z.).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




