Transgenic expression of Spi-C impairs B-cell development and function by affecting genes associated with BCR signaling†
Abbreviations
BLNKB-cell linker
BtkBruton's tyrosine kinase
EµIgH intronic enhancer
Fofollicular
IµIgH sterile transcript
KLHkeyhole lymphocyte hemocyanin
MZmarginal zone
NP4-hydroxy-3-nitrophenylacetyl
pro-B cellprogenitor B cell
T1/2/3transitional 1/2/3
TDT-cell-dependent
Abstract
Spi-C is an Ets family transcription factor closely related to PU.1 and Spi-B. Expression of Spi-C is developmentally regulated in the B-cell lineage, but its function remains unknown. To determine the function of Spi-C in B-cell development, we generated mice expressing a B-cell-specific Spi-C transgene under the control of the IgH intronic enhancer. Spi-C transgenic mice had 50% fewer B cells than wild-type littermates. Flow cytometric analyses showed that splenic transitional B cells and bone marrow pre-B or immature B cells from transgenic mice were dramatically reduced compared with those of wild type. Both nonspecific and Ag-specific serum IgM levels were significantly increased in transgenic mice, while serum IgG levels were significantly decreased compared with wild type. Spi-C transgenic B cells proliferated poorly after stimulation by anti-IgM or anti-CD40 in vitro, although they responded normally to LPS stimulation. Using real-time RT-PCR, we found that several BCR signaling-related mediators were downregulated at pre-B-cell and mature B-cell stages in transgenic mice, while an inhibitor of BCR signaling was upregulated. Taken together, these data indicate that ectopic expression of Spi-C can impair B-cell development and function by affecting genes associated with BCR signaling.
Introduction
In mammals, B cells develop in defined stages from hematopoietic stem cells to immature B cells in bone marrow after birth. The earliest committed B cell, termed the progenitor B (pro-B) cell, is characterized by the rearrangement of D-J segments in the immunoglobulin heavy chain (IgH) locus and the expression of B220 and IL-7 receptor on the cell surface. The IgH locus then continues to rearrange its V region gene segments until productive V-DJ alleles are generated. The product of this rearrangement, the µ chain, assembles with the surrogate immunoglobulin light chains (IgL), VpreB, and λ5, together with the signaling molecules Igα and Igβ, to form a pre-B-cell receptor (pre-BCR) on the cell surface. Signaling through the pre-BCR initiates rearrangement at either κ or λ light chain loci in pre-B cells, which results in the assembly and surface expression of the functional BCR and progression to immature B cells. These newly formed B cells leave bone marrow and migrate to the spleen as short-lived, transitional B cells. Based on variable expression of cell surface markers, the transitional B-cell population can be divided into at least two subsets. The earliest transitional 1 (T1) B cells may give rise to the late T2 B cells, which either serve directly as precursors for mature follicular (Fo) B cells, or may transit through a newly defined T2-pre-marginal zone (MZ) stage before becoming mature MZ B cells 1-4. A late transitional stage termed T3 has also been described 3.
BCR signaling is critically important at many stages of B-cell development. For example, during the early stages of B-cell lymphopoiesis in bone marrow, signals transduced through the pre-BCR are necessary for positive selection and expansion of precursor subpopulations 5-7. Mature B cells in the periphery require the presence of BCR and presumably low levels of BCR signaling for survival 8. Deficiency of BCR signaling mediators, such as B-cell linker (BLNK), Lyn, B220, and Bruton's tyrosine kinase (Btk) causes defects in B-cell development at the pre-B cell or transitional B-cell stage and eventually results in fewer mature B cells in the periphery (reviewed in 9).
Many transcription factors have been identified that play roles in controlling various stages of B-cell development or function, such as PU.1, Spi-B, Ikaros, E2A, EBF, Pax-5, IRF-4, Rel, and OCA-B 10, 11. PU.1, Spi-B, and Spi-C are members of the Ets transcription factor family, exhibiting structural similarity in their DNA-binding domains. PU.1 (encoded by Sfpi1) is expressed in most hematopoietic cells, including B lymphocytes, macrophages, mast cells, neutrophils, and early erythroblasts 12-17. Sfpi1−/− mice die during embryonic development at day 18.5 and produce no lymphoid or myeloid cells, demonstrating an essential role in the early stages of hematopoietic development 18. Low PU.1 levels are required for B-cell lymphopoiesis, whereas high PU.1 levels suppress the B-cell fate and promote myeloid cell development 19. Spi-B is expressed in B cells, T cells, and plasmacytoid dendritic cells 20, 21. B cells are produced in normal numbers in Spib−/− mice, but proliferate poorly and die in response to BCR crosslinking in vitro, suggesting that Spi-B plays a role in B-cell function rather than development 22. Spi-C (also known as Prf, for PU.1-related factor) is expressed in developing B cells as well as in macrophages 23, 24. However, its function remains unclear. Our laboratory recently demonstrated that Spi-C opposes PU.1 activity in progenitor B cells 25. This result suggests that Spi-C has the potential to modulate PU.1 activity to promote B-cell differentiation.
To investigate the biological function of Spi-C in B cells, we generated transgenic mice that express Spi-C under the control of the mouse IgH intronic enhancer (Eµ). We observed that ectopic expression of Spi-C resulted in a significantly reduced number of B cells in the spleen. Further analyses showed impaired development at the pro-B-cell to pre-B-cell transition, significantly reduced immature B-cell numbers in the bone marrow, and reduced frequencies of transitional B cells in the spleen of transgenic mice. We found that the serum IgM level in nonimmunized transgenic mice was significantly increased compared with wild type, while the serum IgG level was significantly decreased. In the immune response to T-cell-dependent (TD) Ag, the specific IgM level was also significantly increased in transgenic mice, whereas the specific IgG level was significantly decreased. Transgenic B cells proliferated poorly and died after stimulation by anti-IgM or anti-CD40 in vitro, although they responded comparably with wild-type B cells in response to lipopolysaccharide (LPS) stimulation. Using real-time RT-PCR, we discovered that transcripts encoding BCR signaling mediators were altered in pre-B cells and mature B cells of transgenic mice compared with wild type. Taken together, these data indicate that Spi-C acts in vivo in B-cell development and function, and its ectopic expression can impair these activities by affecting genes associated with BCR signaling.
Results
Characterization of transgenic mice
To determine whether Spi-C has biological function in the B-cell lineage, we generated transgenic mice that ectopically express murine Spi-C under the control of the mouse IgH intronic enhancer (Fig. 1A). Four founder lines were generated, and for each line Spi-C transcript levels in total splenocytes were compared between transgenic and wild-type littermates using real-time RT-PCR (data not shown). Two founders expressed elevated levels of Spi-C transcripts. One founder line (66 155) was used for the experiments described in this study, as its phenotype was similar to but more pronounced than the other line (data not shown). The Spi-C transgenic phenotype was not affected by backcrossing to C57Bl/6 mice for six generations.
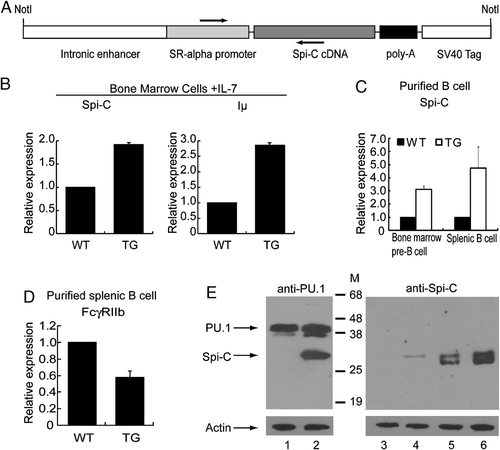
Characterization of Eµ-Spi-C transgenic mice. (A) The Spi-C transgene construct contains the mouse IgH intronic enhancer (Eµ), SR-α promoter, full-length mouse Spi-C cDNA, and polyadenylation signals. The arrows indicate primers for PCR identification of Eµ-Spi-C transgenic founder mice. (B) Real-time RT-PCR analysis of Spi-C and Iµ transcripts in cultured bone marrow pro-B cells from 66155 line of transgenic and wild-type littermates (n=2). (C) Real-time RT-PCR analysis of Spi-C in bone marrow pre-B cells and splenic B cells from 66155 line of transgenic and wild-type littermates (n=2). (D) Real-time RT-PCR analysis of FcγRIIb transcripts in splenic B cells from 66155 line of transgenic and wild-type littermates (n=2). (E) Immunoblotting analysis of Spi-C expression in splenic B cells from 66155 line of transgenic mice. Lysates from MIGR1-infected pro-B cells (lanes 1 and 3), MIG-Spi-C-infected pro-B cells (lanes 2 and 4), wild-type B cells (lane 5), and Spi-C transgenic B cells (lane 6) were probed with anti-PU.1 Ab (top left panel) or anti-Spi-C Ab (top right panel). As a loading control, all lysates with probed with anti-β-actin antibody (lower panels). Anti-PU.1 antibody is partially cross-reactive to Spi-C 25.
As shown in Fig. 1B and C, Spi-C transcript levels in ex vivo and cultured B cells of transgenic mice were 2–5 fold higher than from wild-type littermates. We previously documented that cultured pro-B cells ectopically expressing Spi-C had increased levels of IgH sterile transcripts (Iµ) and reduced transcript levels of the FcγRIIb gene 25. Consistently, Iµ transcript levels in cultured bone marrow pro-B cells from transgenic mice were nearly three-fold higher than in wild type (Fig. 1B). FcγRIIb transcript levels in splenic B cells of transgenic mice were significantly lower than in wild type (Fig. 1D). Expression of the Spi-C transgene was further confirmed by immunoblotting using an antibody against Spi-C (Fig. 1E). We found that the concentration of Spi-C protein in splenic B cells from transgenic mice was increased approximately two-fold compared with the wild type.
Transgenic mice have fewer B cells in bone marrow and spleen
We observed that spleens of transgenic mice were reduced in size compared with wild-type littermates. The total spleen cell count of transgenics was 36.6±20.0×106 compared with 92.2±29.1×106 for their wild-type littermates (n=15, p<0.01). The percentage of B220+CD43− mature B cells from transgenic mice was 17.9±8.2% compared with 33.6±13.1% for wild type (n=5, p<0.05), indicating that there were fewer B cells in transgenic mice.
To examine the B-cell phenotype in transgenic mice, we performed flow cytometric analyses of cells from bone marrow, spleen, and peritoneal cavity. The results showed that in bone marrow and spleen of transgenic mice, the percentage of CD19+B220+ cells was significantly lower than in wild type (Fig. 2A and B). Furthermore, the frequency of CD19+FcγRII/III+ cells in bone marrow and spleen of transgenic mice was significantly reduced compared with wild type (Fig. 2A and B), and FcγRII/III staining was less intense (Fig. 2C), consistent with Fig. 1D and our previous report that ectopic expression of Spi-C reduces FcγRIIb levels 25. B220+IgM+ cells in bone marrow and spleen of transgenic mice were also significantly reduced in frequency compared with wild type (Fig. 2A and B).
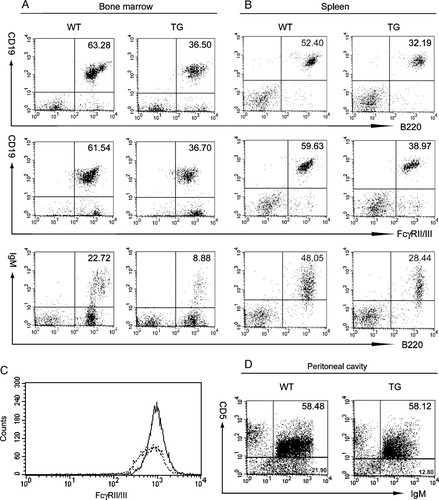
Flow cytometric analysis of B cells in bone marrow and spleen from Eµ-Spi-C transgenic and wild-type mice. Single-cell suspensions of bone marrow (A) or spleen (B) were gated for size and granularity and analyzed with the indicated Abs. Plots are representative of at least three individual experiments. Numbers indicate the frequencies of double positive cells within the lymphocyte gate. (C) Reduced expression of FcγRII/III in B cells from Eµ-Spi-C transgenic mice. The staining intensity of FcγRII/III is shown for gated CD19+ splenic B cells from wild-type mice (solid line, mean 1013.9) and Eµ-Spi-C mice (dotted line, mean 783.2). (D) Wild-type and Eµ-Spi-C mice have similar frequencies of B-1a peritoneal B cells. Peritoneal cavity cells were stained with the indicated antibodies and analyzed for the frequency of B-1a (CD5+IgM+) or B-1b/B-2 cells (CD5−IgM+).
It was recently reported that Spi-C is highly expressed in splenic B-1a cells but expressed at reduced levels in peritoneal B-1a cells 26. To determine whether ectopic Spi-C expression in transgenic mice affects B-1 cell development, we examined the frequency of CD5+IgM+ B-1a cells and CD5−IgM+ B-1b/B-2 cells in the peritoneal cavity. There was no significant difference in the percentage of CD5+IgM+ B-1a cells in the spleen and peritoneal cavity of transgenic versus wild-type mice (1.9 versus 1.9% in spleen, n=4; 40.5 versus 39.4% in peritoneal cavity, n=4) (Fig. 2D). In contrast, the frequency of CD5−IgM+ B-1b/B-2 cells in the spleen of transgenic mice was significantly reduced when compared with wild type (44.5 versus 63.8%, n=4). CD5−IgM+ B-1b/B-2 cells in the peritoneal cavity of transgenic mice were moderately reduced in transgenic versus wild-type mice (on average 36.4 versus 43.3%, n=4) (Fig. 2D). Taken together, these results suggest that transgenic expression of Spi-C affects B-2 (and possibly B-1b) cell development, but does not detectably affect B-1a cell development. We expect that the reduced frequency of CD19+B220+ cells in transgenic spleens is caused mainly by the decrease in number of B-2 cells.
Transgenic mice have fewer splenic transitional B cells and exhibit a difference in bone marrow B-cell development
Next, we sought to determine the earliest stage of splenic B-cell development affected by Spi-C. Allman et al. showed that the developmental marker AA4.1 and variable surface levels of IgM and CD23 can be used to delineate T1, T2, and T3 populations 3. Using this scheme, we found that immature transitional B cells (B220+AA4.1+) in spleens of transgenic mice were dramatically reduced compared with wild type (Fig. 3A). As shown in Table 1, the absolute numbers of T1, T2, T3, MZ, and mature Fo B cells were significantly lower in transgenic mice than wild type. Interestingly, when gated on the reduced B220+AA4.1+ population, the frequency of T1 cells was reduced in transgenic mice compared with wild type, while the frequency of T2 and T3 B cells were significantly higher than in wild type. When gated on B220+AA4.1− mature B cells, the frequencies of MZ and Fo B cells were comparable between transgenic and wild-type mice (Table 1). These results suggest that differentiation from transitional B cells to mature B cells is not blocked in transgenic mice. However, ectopic Spi-C expression caused a defect in transitional B-cell development.
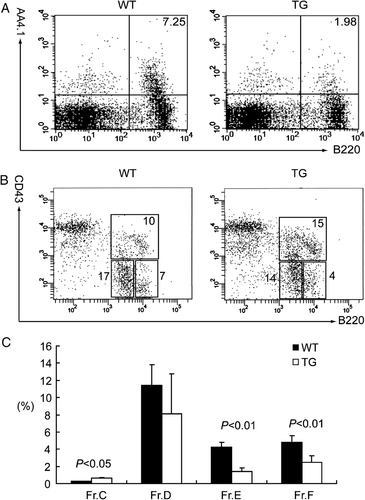
Flow cytometric analysis of B-cell developmental stages in spleen (A) and bone marrow (B and C). (A) The frequencies of immature transitional B cells in spleens from transgenic and wild type were analyzed with anti-B220 and anti-AA4.1 Abs. (B and C) B-cell developmental stages in bone marrow were analyzed with anti-B220, anti-CD43, anti-BP-1, and anti-IgM Abs according to the classification by Hardy et al. 27. (B) The frequencies of B220+CD43+ cells (including pre-pro-B and pro-B cells, upper box), B220lowCD43− cells (including pre-B and immature B cells, lower left box) and B220highCD43+ cells (mature recirculating B cells, lower right box) in bone marrow from Eµ-Spi-C transgenic and wild-type mice are indicated and are representative of at least three individual experiments. (C) The frequencies of pro-B cells (Fraction C, B220+CD43+BP-1+), Pre-B cells (Fraction D, B220dullCD43−IgM−), newly generated B cells (Fraction E, B220dullCD43−IgM+), and mature recirculating B cells (Fraction F, B220brightCD43−IgM+) in bone marrow from transgenic and wild type are indicated in the Y-axis of bar graph (n=3).
| Splenic B cell subpopulationa) | WT (n=5) | TG (n=5) | ||
| No.b) | %c) | No. | % | |
| T1 (sIgMhighCD23−) | 1.14±0.18 | 44.2±6.5 | 0.08±0.02** | 27.4±4.2** |
| T2 (sIgMhighCD23+) | 0.49±0.13 | 19.1±5.3 | 0.07±0.03** | 26.7±2.0* |
| T3 (sIgMlowCD23+) | 0.26±0.06 | 9.8±1.4 | 0.05±0.02** | 19.2±2.6** |
| MZ (sIgMhighCD23−) | 2.94±0.42 | 14.1±1.0 | 1.08±0.52* | 16.2±8.4 |
| Fo (sIgM+CD23+) | 14.90±3.41 | 68.5±5.8 | 5.07±3.38* | 65.1±8.7 |
- a) a)Splenic B-cell subpopulations were classified according to the report by Allman et al. 1. Transitional B cells, including T1, T2, and T3, were gated on B220+AA4.1+ cells; MZ and Fo B cells were gated on B220+AA4.1− cells.
- b) b)The absolute number (×106) of each subpopulation in the spleen is shown as mean and standard error.
- c) c)The percentage of each subpopulation gated as described above in (a) is shown as mean and standard error. *p<0.05, **p<0.01.
The reduction in transitional B cells in the spleen of transgenic mice suggested that B-cell development might be affected in bone marrow. To investigate this possibility, we compared B-cell developmental stages in the bone marrow of transgenic and wild-type mice using the scheme described by Hardy et al., which resolves B220+ B lineage cells in bone marrow into different fractions based on cell surface expression of CD43, BP-1, and IgM 27. As shown in Fig. 3B, B220+CD43+ cells (including pre-pro and pro-B cells) were significantly increased in transgenic mice compared with wild type, whereas B220+CD43− cells (including pre-B and immature B cells) were significantly reduced in transgenic mice compared with wild type. The flow cytometry results show that B-cell numbers in the bone marrow of transgenic mice were significantly increased at the pro-B-cell stage (Fraction C, B220+CD43+BP-1+), but reduced at the pre-B cell stage (Fraction D, B220dullCD43−IgM−), the newly generated B-cell stage (Fraction E, B220dullCD43−IgM+), and the mature recirculating B-cell stage (Fraction F, B220brightCD43−IgM+) (Fig. 3C). In conclusion, B-cell development in transgenic mice is affected starting in the bone marrow.
Impaired IgG and enhanced IgM responses to TD Ag in transgenic mice
To determine whether the Spi-C transgene alters Ig production in nonimmunized mice, we compared the serum IgM, IgG, IgE, and IgA levels between transgenic and wild-type mice by ELISA. As determined by ratio paired t-test, the IgM level in transgenic mice was significantly higher than with wild type (Fig. 4A), whereas the total IgG level in transgenic mice was significantly lower compared with wild-type (Fig. 4B). There was no significant difference for IgA levels between transgenic and wild-type mice (data not shown). IgE Ab levels in both wild-type and transgenic mice were below the limit of detection of our assay.
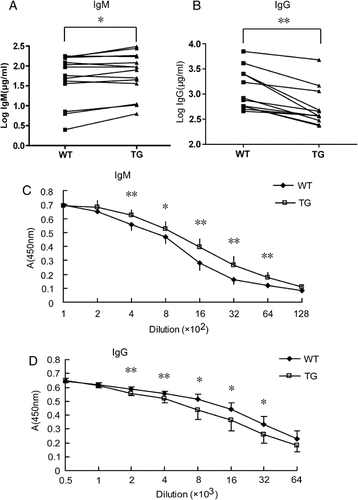
Analysis of Ig isotype levels in transgenic mice. (A and B) The serum IgM (A) and IgG (B) concentration in 12 pairs of nonimmunized transgenic and wild-type littermate mice were measured by ELISA. Data from each pair are linked by a straight line. The Ig concentrations are shown after log transformation. (C and D) NP-specific IgM (C) and IgG (D) levels in serially diluted sera from eight pairs of immunized transgenic and wild-type littermate mice were estimated by ELISA. Data are shown as units of absorbance at 450 nm. *p<0.05, **p<0.01.
To determine the effect of ectopically expressed Spi-C on antibody response to TD Ag, transgenic and wild-type littermates were immunized i.p. with 100-µg of 4-hydroxy-3-nitrophenylacetyl–keyhole lymphocyte hemocyanin. Sera were collected at day 14 post immunization and assayed for NP-specific IgM and IgG Abs by ELISA. We found that the relative level of NP-specific IgM was about two-fold higher in transgenic mice than in wild type (Fig. 4C). In contrast, the relative level of NP-specific IgG was about two-fold lower in transgenic mice than in wild type (Fig. 4D). Therefore, transgenic mice exhibit enhanced IgM and impaired IgG responses to TD Ag.
B-cell proliferation in vitro
The effect of transgenic Spi-C expression on pre-B cells, immature B cells, and transitional B cells suggests an effect on BCR signaling, which is necessary for normal B-cell development. To determine proliferation of transgenic B cells in response to exogenous signals, we stimulated splenic B cells from individual mice with anti-IgM, anti-CD40, or LPS, followed by measurement of proliferation by 3H-thymidine uptake. Strikingly, B cells from transgenic mice were significantly less proliferative in response to stimulation by anti-IgM or anti-CD40 antibodies compared with wild type (Fig. 5). However, the proliferation of B cells from transgenic and wild-type mice was similar in response to LPS stimulation. Analysis of apoptosis performed as described in Materials and methods confirmed that two- to four-fold fewer transgenic B cells survived at day 3 after stimulation with anti-IgM or anti-CD40 as compared with wild type (data not shown), which correlated with the reduced levels of 3H-thymidine incorporation. The viability of either unstimulated or LPS-stimulated transgenic B cells was similar to that of wild-type B cells (data not shown). These results suggest that splenic B cells from transgenic mice proliferated poorly and died in response to anti-IgM or anti-CD40 stimulation. These results suggest that BCR signaling might be affected by ectopic Spi-C expression in transgenic mice.
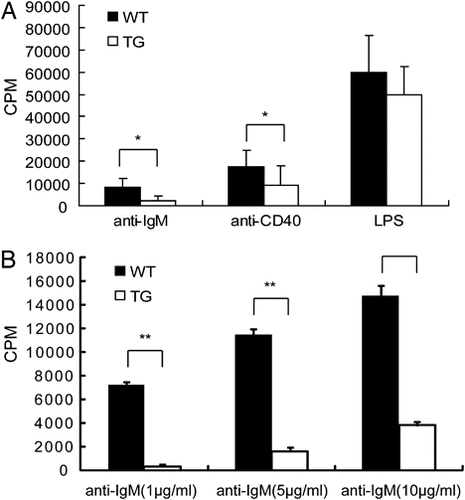
B-cell proliferation assayed in vitro. (A) Splenic B cells from transgenic and wild-type mice were incubated for 3 days with 10 µg/mL of anti-IgM, anti- CD40, or LPS. (B) Splenic B cells from transgenic and wild-type mice were incubated for 3 days at different concentrations of anti-IgM. Four independent experiments were performed in triplicate. Results are shown as mean CPM of incorporated 3H-thymidine. *p<0.05, **p<0.01.
Spi-C affects expression of genes associated with BCR signaling
We previously showed that Spi-C could oppose PU.1 activity in pro-B cells 25. PU.1 and Spi-B have been reported to play important roles in peripheral B-cell development and function 22, 28. PU.1 and Spi-B directly regulate the expression of several genes associated with BCR signaling such as BLNK, B220, Btk, P2Y10, and Grap2 10, 29-32. Inactivating mutations in genes encoding BLNK, B220, Btk, and Lyn are sufficient to cause defects in B-cell development at the pre-B-cell or transitional B-cell stage, resulting in fewer mature B cells in the periphery (reviewed in Reference 9). Spi-C has DNA-binding specificity identical to PU.1 and Spi-B and therefore might regulate many of the same genes as these transcription factors 25. In order to explore the underlying mechanisms accounting for reduced B-cell proliferation in Spi-C transgenic mice, we examined levels of BLNK, Lyn, B220, Btk, P2Y10, and Grap2 transcripts. The levels of BLNK, Lyn, B220, Btk, and P2Y10 transcripts were significantly decreased in bone marrow pre-B and immature B cells enriched from transgenic mice compared with the wild type (Fig. 6A). Interestingly, these genes are positive regulators of BCR signaling. In contrast, Grap2, which functions as an inhibitory adaptor protein in BCR signaling by inhibiting inductive BLNK phosphorylation and its recruitment to Igα, was increased in transgenic pre-B cells compared with wild type (Fig. 6A). Next, we examined the levels of these transcripts in sorted splenic Fo B cells (B220+AA4.1-IgM+CD23+). Analysis of gene expression in wild-type and transgenic Fo B cells showed results similar to those in bone marrow pre-B cells (Fig. 6B). Taken together, these results suggest that BCR signaling was affected in both pre-B cells and mature B cells in Spi-C transgenic mice.
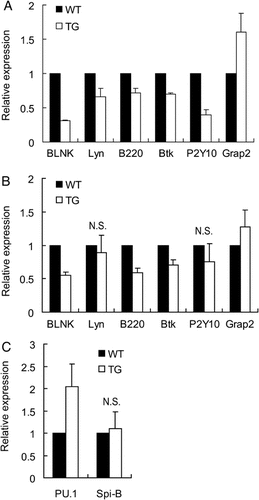
(A and B) Real-time RT-PCR analysis of genes involved in BCR signaling in B cells from transgenic and wild-type mice (A). Analysis of pre-B cells enriched from bone marrow by negative selection of CD43+ cells (n=3). (B) Analysis of Fo B cells (B220+AA4.1-IgM+CD23+) purified from spleen by flow cytometric sorting. (C) Real-time RT-PCR analysis of transcription factors PU.1 and Spi-B in Fo B cells purified from spleen by flow cytometric sorting. All results were significant (p<0.01) except those labeled NS.
We hypothesized that Spi-C might exert its effects on target genes by downregulating PU.1 or Spi-B in transgenic mice. As shown in Fig. 6C, PU.1 transcripts were increased, while Spi-B transcripts were unaffected in sorted splenic Fo B cells from Spi-C transgenic mice. Thus, we conclude that decreased transcription of genes associated with BCR signaling in transgenic mice is not mediated by reduction of PU.1 or Spi-B transcript levels.
Spi-C is expressed at the highest level at the transitional B-cell stage in normal development
It was previously reported that Spi-C is modulated during B-cell development, with low protein levels detected in pre-B cells and higher levels in mature B cells 23. To further determine where Spi-C is expressed during normal B-cell development, we performed real-time RT-PCR analysis on RNA prepared from sorted C57Bl/6 B cells. Bone marrow cells were sorted based on the expression of cell surface markers into pro-B cells (B220+CD43+) and pre-B/immature B cells (B220+CD43−). Splenocytes were sorted into transitional B cells (B220+AA4.1+) and mature B cells (B220+AA4.1−). As shown in Fig. 7, Spi-C transcripts were increased more than two-fold from the pro-B to the pre-B-cell stage. In the spleen, Spi-C transcripts were dramatically increased about ten-fold in transitional B cells compared with pre-B cells, but were reduced in mature B cells to a level comparable to pre-B cells. Therefore, within the B-cell lineage, Spi-C transcripts are expressed at the highest levels in transitional B cells, suggesting that Spi-C normally plays a role at the transitional stage of B-cell development.
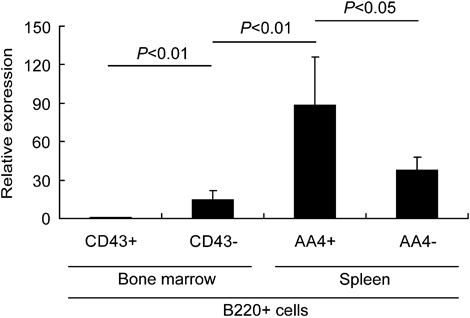
Real-time RT-PCR analysis of Spi-C transcript levels in wild-type B lineage cells sorted into the indicated populations. RNA was prepared from sorted cells (gated on B220+) in three separate cell sorting experiments. The results shown represent the mean and standard error of three separate real-time RT-PCR analyses performed and analyzed as described in Materials and methods.
Discussion
In order to investigate a potential biological function of the Ets transcription factor Spi-C in B-cell development, we generated transgenic mice that ectopically express Spi-C under transcriptional control of the IgH intronic enhancer. Real-time RT-PCR and immunoblotting verified that Spi-C is elevated ∼two-fold in transgenic mice compared with wild type. Ectopic expression of Spi-C resulted in a severe impairment of B-cell development and function. Spi-C transgenic mice had half the number of B cells as wild-type littermate mice, defective Ig production, and reduced B-cell proliferation in response to anti-IgM or anti-CD40 stimulation in vitro. Analysis of gene expression revealed that ectopic Spi-C expression altered levels of transcripts encoding BCR signaling mediators. We conclude that ectopically expressed Spi-C impairs B-cell development and function by affecting genes associated with BCR signaling.
This project was initiated because of our previous observation that Spi-C opposes the activity of PU.1 on target genes in B cells including the IgH intronic enhancer and the FcγRIIb promoter 25. PU.1 functions as an activator of FcγIIb transcription, but as a repressor of IgH transcription, and Spi-C regulates these two genes opposite to PU.1 25, 33. PU.1, Spi-B, and Spi-C have identical DNA-binding specificities and therefore could potentially regulate transcription of the same set of target genes 23, 24. If Spi-C opposes Spi-B function as well as PU.1, then the phenotype of Eµ-Spi-C transgenic mice might have similarities to the phenotype of Spib−/− mice. Our results reveal that this prediction is at least partially correct. Spib−/− mice have impaired antibody responses 22, 34, as do Eµ-Spi-C transgenic mice (Fig. 4). Furthermore, B cells from Spib−/− mice have reduced ability to proliferate in response to anti-IgM stimulation 28, as do B cells from Eµ-Spi-C transgenic mice (Fig. 5). Therefore, the phenotype of Eµ-Spi-C transgenic mice might be due in part to Spi-C opposing Spi-B, as well as PU.1. Interestingly, Eµ-Spi-C transgenic mice have increased IgM responses in spite of having reduced overall numbers of B-2 cells and IgG responses. We speculate that IgM titers might be increased in Eµ-Spi-C transgenic mice as a consequence of Spi-C directly activating Ig µ transcription (Fig. 1B).
PU.1 and Spi-B are thought to have partially redundant functions in B cells, since the phenotype of Spib−/− mice is made considerably more severe by a 50% reduction of PU.1 (Sfpi1+/−Spib−/− mice) 28. PU.1 and/or Spi-B have been shown to regulate transcription of the genes encoding Rel 35, BLNK 33, B220 29, Btk 30, 33, P2Y10 31, and Grap2 32, all of which have important functions in modulating BCR signaling or survival and proliferation in response to BCR signaling. Ectopic expression of Spi-C did not decrease Rel transcription in Eµ-Spi-C transgenic B cells, possibly because transcripts encoding PU.1 were upregulated by about two-fold (Fig. 6C and data not shown). Since Spi-C opposes PU.1 activity on several target genes 25, increased PU.1 levels might modulate the Eµ-Spi-C phenotype. However, ectopic expression of Spi-C was sufficient to reduce the levels of transcripts encoding BLNK, B220, Btk, and P2Y10 (Fig. 6A and B), all of which play important roles in transducing BCR signals 9. In contrast, ectopic expression of Spi-C increased transcripts encoding Grap2, an inhibitor of BCR signaling 32. Therefore, ectopic expression of Spi-C causes a pattern of gene expression changes that would be expected to result in reduced BCR signaling.
Mutation of the genes encoding BLNK, Lyn, or Btk causes severe defects in B-cell development because of reduced levels of BCR signaling 9. Therefore, reduced levels of BLNK, Lyn, and Btk gene expression could account for the reduction of BCR-dependent proliferation caused by ectopic Spi-C expression (Fig. 5). Reduction of BCR signaling by ectopic Spi-C expression provides a coherent explanation of the phenotype of Eµ-Spi-C transgenic mice. We found that pre-B cells, immature B cells, and T1 B cells, all stages of B-cell development particularly sensitive to BCR signaling, were the most dramatically affected by ectopic Spi-C expression. In contrast, the frequency of pro-B cells was increased by ectopic Spi-C expression (Fig. 3C). We previously demonstrated that ectopic expression of Spi-C in cultured pro-B cells causes an increased rate of proliferation, which might explain the increase in frequency of pro-B cells in vivo 25. When gated on the splenic transitional B-cell population (AA4.1+), T2 and T3 cells were increased in frequency by transgenic expression of Spi-C, relative to wild type (Table 1). In addition, the relative frequencies of Fo mature and MZ B cells were minimally affected by transgenic Spi-C expression. Taken together, these data suggest that transgenic expression of Spi-C causes creates a bottleneck in production of immature pre-B cells in the bone marrow. This results in fewer cells transitioning to the spleen, and as a consequence, reduced T1 frequencies. However, after the T1 stage, the frequencies of later transitional stages and then mature stages begin to recover.
Spi-C is reported to be expressed in a stage-specific manner during B-cell development. Unexpectedly, the level of Spi-C expression was higher in mouse spleen than in mature B-cell lines 23, 24. Our data resolve this apparent contradiction by revealing that Spi-C is expressed at highest levels in the splenic AA4.1+ transitional B-cell population (Fig. 7). The high levels of transcripts in transitional B cells suggest that Spi-C may normally play a role at this stage. Unlike mature Fo or MZ B cells, transitional B cells proliferate poorly in response to BCR signaling. Furthermore, BCR engagement in T1 cells is thought to signal for apoptotic cell death 36. Since our data show that Spi-C affects genes associated with BCR signaling, we speculate that Spi-C function might account for differences in the function of BCR signaling in transitional versus mature B cells. More experiments will be necessary to test this hypothesis.
Taken together, our data indicate that Spi-C is a member of a select group of transcription factors that regulate B-cell maturation. The transgenic mouse model that we have described will be useful in identification of new genetic pathways involved in the B-cell development and function. Our data suggest that PU.1, Spi-B, and Spi-C represent a regulatory network of Ets transcription factors that is essential for normal development and function of B cells.
Materials and methods
Generation and breeding of transgenic mice
A full-length murine Spi-C cDNA with an N-terminal hemagglutinin epitope tag was cloned between SacI and EcoRV sites of the pEµ-SRα vector 37. After removal of prokaryotic plasmid sequences from the construct using NotI, the gel-purified linearized fragment was microinjected into pronuclei of FVB/N strain murine oocytes by the Transgenic Mouse Facility at the University of Cincinnati. Four positive founders were obtained and identified by PCR with primers spanning the IgH intronic enhancer and Spi-C cDNA sequence. The founders were backcrossed to wild-type C57BL/6 to produce independent transgenic lines that were maintained as heterozygotes. Mice were bred in-house by the Laboratory Animal Medical Services at the University of Cincinnati. In total, 6–12 wk-old transgenic mice and their wild-type littermates were sacrificed for experiments.
Generation and culture of pro-B cells
Bone marrow cells from Spi-C transgenic and wild-type littermates were plated onto a monolayer of irradiated (2000 rad) S17 stromal cells after lysis of RBC. Cultures were fed with fresh IL-7-containing complete IMDM medium every 4 days and analyzed by flow cytometry to confirm expression of CD19. MIGR1 and MIG-Spi-C retrovirus infected pro-B-cell lines were cultured under the same conditions 25.
Enrichment of pre-B cells and mature B cells
Bone marrow Pre-B cells or splenic B cells were enriched by negative selection with biotinylated anti-CD43 (S7) and streptavidin-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA) from the spleen of 6–8-wk-old Spi-C transgenic and wild-type littermate mice. Purity was analyzed by staining with APC-conjugated anti-B220 (RA3-6B2) and was always ≥95% (data not shown). Splenic Fo B cells (B220+AA4.1-IgM+CD23+), splenic transitional cells (B220+AA4.1+), bone marrow fraction A-C B cells (B220+CD43+, and bone marrow fraction D-F B cells (B220+CD43−) were purified by flow cytometric sorting as described below.
Immunoblotting and flow cytometry
Whole-cell lysates from magnetically enriched mouse splenic B cells were probed with anti-Spi-C antibody (Aviva Systems Biology, San Diego, CA) and visualized with horseradish peroxidase (HRP) conjugated anti-rabbit secondary antibody and a SuperSignal West Pico kit (Pierce, Rockford, IL). Whole-cell lysates prepared from MIGR1 and MIG-Spi-C retrovirus infected pro-B-cell lines were probed with anti-PU.1 (T-21, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-β-actin (Santa Cruz) antibodies as negative and positive controls. Mouse peritoneal cells, bone marrow cells, or splenocytes were washed after hypotonic lysis of RBC with ammonium chloride solution and stained with appropriate mAb, including biotin-conjugated anti-CD5 (53-7.3), anti-CD19 (1D3), anti-CD43 (S7), anti-IgM (II/41), anti-FcγRII/III (2.4G2); FITC-conjugated anti-BP-1 (6C3), anti-CD23 (B3B4); PE-conjugated anti-CD93 (AA4.1); and APC-conjugated anti-B220 (RA3-6B2). These mAb were purchased from BD Pharmingen (San Diego, CA). PE-Cy5.5-conjugated anti-IgM (II/41) was purchased from eBioscience (San Diego, CA). Biotinylated mAbs were revealed with PE- or PerCP-conjugated streptavidin (BD Pharmingen). The stained cells were analyzed on a FACSCalibur system (BD Immunocytometry Systems, San Jose, CA). Cell sorting was performed using a FACSVantage SE cell sorter with FACSDiva upgrade (BD Immunocytometry Systems). Purity of sorted cells was always ≥95%.
B-cell proliferation assay
Magnetically enriched splenic B cells (5×104) from Spi-C transgenic and wild-type littermates were cultured in triplicate wells of 96-well plates in complete IMDM medium alone or in the presence of 1, 5, or 10 µg/mL anti-mouse IgM (µ chain specific) (Jackson Immunoresearch, West Grove, PA), 10 µg/mL anti-mouse CD40 (1C10) (Biolegend, San Diego, CA), or 10 µg/mL LPS (Sigma, Saint Louis, MO). Cells were incubated for 3 days at 37°C, 5% CO2. Sixteen hours before the end of incubation, 1 µCi of 3H-thymidine was added per well. The cells were harvested onto glass fiber filter paper, and 3H-thymidine incorporation was determined by a beta counter (Rack-beta, LKB Instruments, Houston, TX). Apoptosis of enriched splenic B cells with or without stimulation was assessed by flow cytometry with the Annexin V-PE and 7-AAD apoptosis detection kit (BD Pharmingen) according to the manufacturer's instructions.
Real-time RT-PCR
Total RNA was extracted from sorted or magnetically enriched B cells using RNA-Bee reagent (Tel-Test, Friendswood, TX). cDNA synthesis was performed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). All real-time RT-PCR reactions were performed in triplicate on a SmartCycler system (Cepheid, Sunnyvale, CA) as previously described 33. At the end of each reaction, the specificity of amplification was confirmed by gel electrophoresis. The expression levels of target genes relative to G6PDH controls were calculated using the ΔΔCt method 38. The primers used were: Iµ, 5′-ACC TGG GAA TGT ATG GTT GTG GCT T-3′ and 5′-ATG CAG ATC TCT GTT TTT GCC TCC-3′; FcγRIIb, 5′-CCC AAG TCC AGC AGG TCT TTA CC-3′ and 5′-CCC AAT GCC AAG GGA GAC TAA AT-3′; Spi-C, 5′-AAG TCT TTG GAG AAC AGC CTC GCT-3′ and 5′-AAA GGG AGG AAG AGG CAG GAG AAA-3′; BLNK, 5′- TCG ACG TTT GCA GAC CAG GAG-3′ and 5′- GGT GTA CGG CTG CTT GGA ATC-3′; Lyn, 5′- CGA GCT GCT AAC GTC CTG GTC-3′ and 5′- TGA AGC AGC CGA AGT TGA TGG-3′; B220, 5′- TTG GAA AGT GCA GAA ACA GAA GAT-3′ and 5′- CTT GCC TCC ATC CAC TTC ATT AT-3′; Btk, 5′- GCT CTG TAG GC TCC AAG TTT C-3′ and 5′- ATC TCT CAT ACG GCA TCT TCC-3′; P2Y10, 5′- CAG TTG ACA AGG AGT AGT GGA TGC-3′ and 5′- GGA GAC CCG GGA TGA ATA TGA AG-3′; Grap2, 5′- ACC GGA AGC TGT CAG ACC ACC T-3′ and 5′- TCC AAG GCC GCA GTG TCC ATC-3′; PU.1, 5′-CGG ATG TGC TTC CCT TAT CAA AC-3′ and 5′-TGA CTT TCT TCA CCT CGC CTG TC-3′; Spi-B, 5′-CCG AGG GGA GGG GAT CTG AGG-3′ and 5′-GGA GGA GAA CTG GAA GAC GCC G-3′; G6PDH, 5′-GAA CAT CAT CCC TGC ATC CA-3′ and 5′-CCA GTGA GCT TCC CGT TCA-3′.
Immunizations
To determine the immune response against TD Ag, mice were injected i.p. once with 100 µg of alum precipitated NP coupled to keyhole lymphocyte hemocyanin (KLH) at a ratio of 19:1 (Biosearch Technologies, Novato, CA). Alum was purchased from Pierce (Imject). Two weeks after injection, sera were collected from immunized mice.
ELISA
The serum IgM, IgG, IgE, or IgA levels in nonimmunized mice were analyzed by a standard sandwich ELISA. Diluted sera or appropriate isotype standards were added to 96-well microtiter plates coated with anti-mouse Ig capture Abs specific for the aforementioned Ig isotypes. After incubation at 37°C for 1.5 h, the plate was washed with 0.05% Tween-20 PBS and H2O using an automated plate washer (ELx50, BioTek Instruments, Winooski, VT). HRP (HRP)-conjugated anti-mouse Ig detection Abs specific to IgM, IgG, IgE, or IgA were added to the plate. After washing unbound detection Abs, ABTS substrate (Roche Molecular Biochemicals, Indianapolis, IN) was added and colorimetric detection was performed with a Spectramax plus automated microplate reader with a 405 nm filter and Softmax Pro software (Molecular Devices, Sunnyvale, CA). The concentrations of IgM, IgG, IgE, and IgA in the sera were calculated using a standard curve generated from the absorbance readings of known mouse Ig isotype standards. The antibodies were purchased from the following manufacturers: goat anti-IgE, rabbit anti-IgA, goat anti-IgA–HRP, purified mouse IgA, rabbit anti-IgM, purified mouse IgM, and purified mouse IgG2b (Bethyl, Montgomery, TX); goat anti-IgE–HRP, purified mouse IgE, and goat anti-Ig (Southern Biotech, Birmingham, AL); goat anti-IgM–HRP (Sigma); and goat anti-IgG–HRP (Biomedia, Burlingame, CA). The method to determine the levels of NP-specific IgM or IgG after immunization was similar to the above description. Briefly, serial dilutions of sera from transgenic and wild-type mice were added to NP16-BSA (Biosearch Technologies) coated plates, and the relative binding of IgM and IgG was determined using HRP-conjugated goat anti-IgM and IgG. The ratios of binding to NP16-BSA were calculated using OD450 in the linear ranges of the assays.
Statistical analysis
The Statistical Consulting Center at Wright State University determined the appropriate statistical analysis for the serum Ig ELISA results from nonimmunized mice. ELISA results were analyzed using a ratio paired t-test in which the Ig concentrations from transgenic and matched wild-type littermates were log transformed followed by a paired t-test analysis. All other statistical analyses were performed using a paired or independent two-sample Student's t-test. p-values <0.05 were considered significant. Unless otherwise indicated, error bars represent standard deviation of the mean.
Acknowledgements
We acknowledge Dr. Jerry Adams (Walter and Eliza hall Institute, Melbourne, Australia) for his gift of the pEµ-SRα vector; Jon Neumann of the University of Cincinnati Transgenic Mouse Facility for generating Eµ-Spi-C transgenic founder mice; and the flow cytometry core of the Cincinnati Children's Hospital for assistance in cell sorting. We thank Meghana Kamath for critically reading the manuscript. This work was supported by Ohio Cancer Research Associates grant 5407 (to R. P. D.) and National Institutes of Health grants AI052175 (to R. P. D.) and ES014676 (to C. E. S.).
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




