IκBα is required for marginal zone B cell lineage development
Abstract
Inactivation of members of the nuclear factor-κB (NF-κB) family results in the decrease or defect of marginal zone B (MZB) cells. It is not known which inhibitors of the NF-κB family (IκB) are required for MZB cell development. Here, we show that mice with B cell-specific inactivation of the main NF-κB inhibitor IκBα have a marked decrease of MZB cells and their presumed precursors. They exhibited increased mortality rates after blood-borne bacterial infection, indicating the importance of MZB cells for bacterial clearance. In contrast, response to T cell-dependent and -independent antigens resulted only in minor changes in immunoglobulin production. Our data demonstrate the importance of the intact NF-κB/IκBα pathway for proper MZB cell development.
Abbreviations:
-
- cKO:
-
conditional knockout
-
- FOB:
-
follicular B
-
- MZB:
-
marginal zone B
-
- NFB:
-
non-follicular B
Introduction
Marginal zone B (MZB) cells are now recognized as a distinctive naive B lymphoid lineage developing separately from mature follicular (FO) and B1 B cells. Although they are defined primarily on the basis of their location in the splenic marginal zone, they can be distinguished by a characteristic pattern of cell-surface markers IgMhiIgDloCD21hiCD23lo (reviewed in 1, 2). MZB cells are particularly sensitive to triggering by CpG DNA of microbial origin and play a pivotal role in the clearance of blood-borne bacterial infections 1. In humans, postsplenectomy infections are associated with an extraordinarily high mortality underlining the critical MZB cell function for immune defense 1. There appear to be two major driving forces of normal MZB cell development: (i) the specificity of the B cell receptor 3–10 and (ii) signaling through NF-κB p50 and Notch2 6, 11–16.
Analysis from knockout mice of various transcription factor NF-κB family members demonstrated that inactivation of NF-κB p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB and c-Rel results in the decrease or defect of MZB cells 11, 15–18. Recent data show that lack of p100, the inhibitory precursor of the p52 subunit, markedly elevated MZB cell numbers 19. Notch2 signaling is required for MZB cell development, since conditional B cell-specific inactivation of Notch2 and its interaction partner Rbpj results in a severe loss of MZB cells in mice 12, 14. Furthermore, the suppressor of Notch-Rbpj signaling Msx2-interacting nuclear target protein (MINT) and the transcriptional coactivator Mastermind-like (MAML) required for Notch-induced transcription have been implicated in MZB cell development 20, 21. Recently, the Notch-ligand Delta-like 1 expressed on non-B cells in the spleen has been shown to be required for MZB cell development in mice 22. Interestingly, one recent study provided in vivo evidence for the genetic interaction between Notch2 and NF-κB p50 during development of MZB cells 16.
NF-κB transcription factors form complexes by homo- and heterodimerization of their family members p50, p52, p65, RelB, and c-Rel 23, 24. The activity of NF-κB is tightly controlled through association with the inhibitors of NF-κB (IκB), which sequester them in the cytoplasm. The IκB family member IκBα is phosphorylated and degraded in response to external stimuli, thereby liberating NF-κB subunits from retention in the cytoplasm. It is not known so far, which IκB are required for proper MZB cell development.
Results
Generation of mice with IκBα-deficient B cells
To generate mice with tissue-specific deletion of IκBα, loxP sites were introduced into the IκBα locus of mouse embryonic stem (ES) cells by homologous recombination as previously described 25. To selectively disrupt the IκBα gene in B cells, IκBαf/f mice were crossed with CD19-Cre mice, which induced efficient B lineage-specific deletion of the loxP-flanked target sequence. Southern blot analysis of DNA from purified splenic B cells showed efficient deletion of the IκBα gene (Fig. 1A). Immunoblotting of splenic B cells of control mice with one or two loxP-flanked alleles revealed IκBα expression at comparable levels. In contrast, no expression of IκBα was detected in splenic B cells from IκBα conditional knockout (cKO) mice (Fig. 1B). To analyze the consequences of B cell-specific inactivation of IκBα for the NF-κB DNA binding activity we performed electromobility shift assays (Fig. 1C). Splenic B cells of cKO mice revealed constitutive NF-κB activation compared to control B cells with two loxP-flanked alleles. The NF-κB complex was mainly composed of p50 homodimers (labeled as (p50)2) and p50/p65 heterodimers as demonstrated by supershift analysis with antibodies against NF-κB subunits (Fig. 1C).
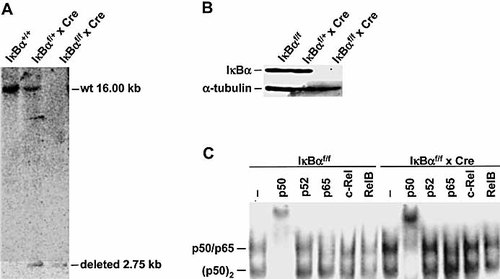
B cell-specific IκBα deficiency. (A) B cell-specific deletion of IκBα. Genomic DNA from purified B cells of control (lane 1, IκBα+/+; lane 2, IκBαf/+ x Cre) and IκBα cKO mice (lane 3, IκBαf/f x Cre). (B) Loss of IκBα in B cells of IκBα cKO mice. Splenic B cells of control (lane 1, IκBαf/f; lane 2, IκBαf/+ x Cre) and IκBα cKO mice (lane 3, IκBαf/f x Cre) were purified and protein extracts were blotted to filters and detected with an anti-IκBα antibody. α-Tubulin served as a loading control. (C) Electromobility shift assays of protein extracts from splenic B cells of control (IκBαf/f) and IκBα cKO (IκBαf/f x Cre) mice. Supershift analysis was performed with antibodies indicated. p(50)2 denotes p50 homodimers.
MZB cells and their precursors are reduced in the absence of IκBα
NF-κB family members play important roles in B cell maturation and function. We therefore asked whether inactivation of the main NF-κB inhibitor IκBα affects B cell differentiation. We analyzed splenic B cells with antibodies against the cell surface markers CD21, CD23, IgM and IgD. We provided evidence that the MZB cell subset (CD21hi, CD23lo, IgMhi, IgDlo) was markedly reduced in IκBα cKO mice (Fig. 2A). To analyze whether the residual MZB cells in cKO mice represent relatively rare cells that have not undergone ablation of the IκBα gene, we sorted MZB cell fractions in cKO and control mice and performed semi-quantitative RT-PCR analysis for IκBα mRNA expression. We detected small amounts of IκBα mRNA in residual MZB cells of cKO mice underlining the prominent role for IκBα in sustaining MZB cell development.
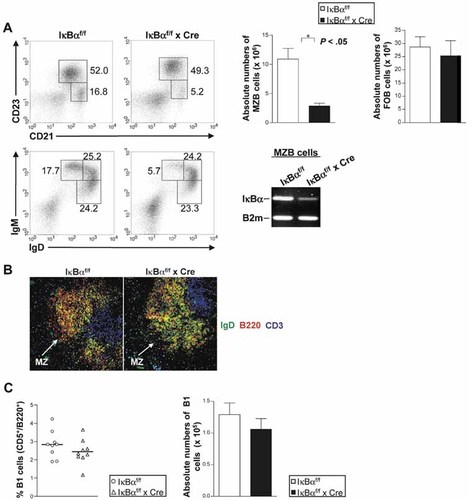
Decreased MZB cell numbers in IκBα cKO mice. (A) Left panel: flow cytometric analysis of MZB and FOB cells in control and IκBα cKO mice. Splenic B cells were analyzed by flow cytometry for the expression of CD21 and CD23. MZB cells and FOB cells were defined as CD21hiCD23loIgMhiIgDlo and CD21hiCD23hiIgMloIgDhi, respectively. The numbers denote the percentages of cells in the indicated squares. Upper right panels: absolute numbers of MZB cells. Numbers were calculated by multiplying the fraction of each B cell subset analyzed by the total number of CD19+ cells. Data are mean + SD from four independent experiments. *p<0.05. Lower right panel: MZB cells were isolated from IκBα cKO and control mice and RT-PCR analysis of IκBα was performed. MZB cells were defined as CD21hiCD23lo. (B) Immunofluorescent histochemistry performed on non-immunized splenic cryosections with anti-IgD-FITC, anti-CD3-Cy5 or anti-B220-Cy3. Arrows indicate the marginal zone (MZ). (C) Flow cytometric analysis of B1 B cells in control and IκBα cKO mice for B220 and CD5. Short horizontal bars in the left panel indicate the mean. Absolute numbers in the right panel were calculated by multiplying the fraction of the B1 B cell subset analyzed by the total number of cells. Data are mean + SD from six independent experiments.
In agreement with flow cytometric data, histological examination of the spleen revealed that in cKO mice MZB cells (B220+IgDlo) were diminished compared to control mice (Fig. 2B). Analysis of B lineage cells in the bone marrow, peripheral blood and lymph nodes did not show any differences between control and IκBα cKO mice (data not shown). No significant difference could be detected in the number of B1 B cells in the peritoneal cavity of control and IκBα cKO mice as demonstrated by flow cytometric analysis with the cell surface markers B220 and CD5 (Fig. 2C).
Next, we analyzed MZB precursors (pre-MZB) using the markers IgD and CD1d since it has been reported that pre-MZB and MZB cells express high levels of CD1d. As expected, the CD1dhi population was reduced in number in IκBα cKO mice (Fig. 3A, upper panel). The CD1dhi population was further distinguished in MZB (IgMhiCD23lo, lower panel) and their precursors (IgMhiCD23hi, lower panel). Both pre-MZB and MZB cells were reduced in cKO mice (Fig. 3A and B).
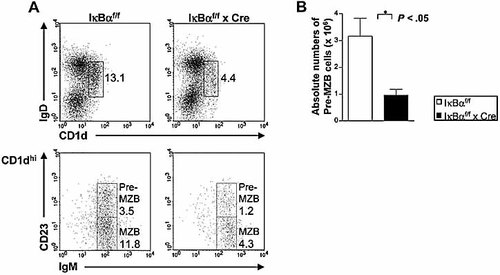
Decreased MZB precursor numbers in IκBα cKO mice. (A) Flow cytometric analysis of pre-MZB cells in control and IκBα cKO mice. Splenic B cells were analyzed by flow cytometry for the expression of CD1d, IgD, IgM and CD23. Pre-MZB and MZB cells were defined as CD1dhiCD23hiIgMhi and CD1dhiCD23loIgMhi, respectively. The numbers denote the percentages of cells in the indicated squares. (B) Absolute numbers of pre-MZB cells. Numbers were calculated by multiplying the fraction of each B cell subset analyzed by the total number of CD1dhi cells. Data are mean + SD from four independent experiments. *p<0.05.
Immune responses of IκBα cKO mice
Although MZB cells secrete more IgM and IgG3 than other Ig isotypes, the expression of all Ig isotypes was unaltered in IκBα cKO with decreased MZB cell numbers as compared to control mice (Fig. 4A). To test whether the loss of MZB cells in cKO mice affected immune responses, mice were immunized with the di-nitrophenol (DNP)-conjugated T cell-dependent keyhole limpet hemocyanin (KLH) and the T cell-independent type 1 lipopolysaccharide (LPS) antigens. When analyzing the primary immune response 10 and 20 days after the first injection of DNP-KLH, control mice produced more DNP-specific antibodies of the IgG1 isotype, compared to IκBα cKO mice (Fig. 4B and data not shown). Ten days after the booster immunization, which was done at day 21, control mice persistently produced higher IgG1. Analyzing serum at day 87, we still observed an immune response in both groups with differences in IgG1 and IgG3 (higher levels in control mice, **p<=0.001). Levels of all other Ig subtypes were unaltered. Taken together, these data show that the MZB cell defect conferred only minor changes in the immune response to antigens, which are typically used in immunological studies.
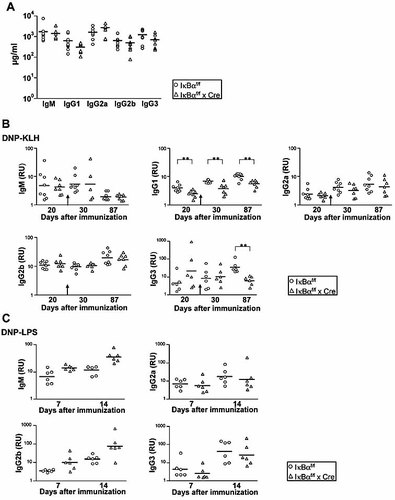
Humoral immune responses in the IκBα cKO mice. (A) Serum Ig concentrations in 3-month-old non-immunized control and IκBαf/f x Cre mice. Seven to ten littermates were examined for each phenotype. (B) Humoral responses to T cell-dependent antigens. Concentrations of DNP-specific antibodies on day 20 after the initial immunization with 50 µg of DNP-KLH are shown for seven (IκBαf/f × Cre) and eight (IκBαf/f) littermates. Secondary responses against booster immunization with 50 µg DNP-KLH (indicated by the arrow) were measured on days 30 and 87. (C) Humoral responses to T cell-independent antigens. Concentrations of DNP-specific antibodies on days 7 and 14 after the initial immunization with 100 µg of DNP-LPS are shown for six (IκBαf/f x Cre) and six (IκBαf/f) littermates. Short horizontal bars indicate the mean. RU: random units. **p<0.001.
We next examined the effects of blood-borne bacteria in IκBα cKO mice. To that end, we used the Staphylococcus aureus infection model, which was recently described in MZB cell-deficient Rbpj cKO mice 12. Lack of the key mediator of Notch signaling Rbpj results in a severe loss of MZB cells and in increased mortality rates after blood-borne bacterial infection with S. aureus 12. We intravenously administered 1 × 107 CFU S. aureus 26. IκBα deficiency conferred high sensitivity to lethal effects of blood-borne bacteria (Fig. 5), indicating a defect of MZB cells, which are involved in clearance of bacteria from blood in our model.
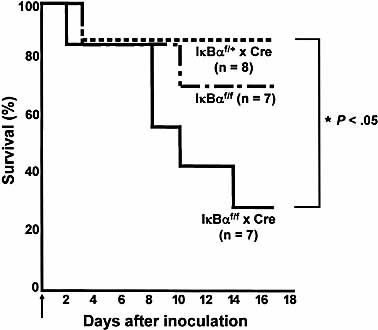
IκBα cKO mice are susceptible to blood-borne S. aureus infection. Control and IκBαf/f x Cre mice were intravenously injected with 1 × 107 CFU S. aureus. The arrow shows the point of injection. Survival over time is shown.
Decreased Notch2 signaling activity in IκBα-deficient B cells
Notch2 signaling has been recognized as a major driving force of MZB development 1, 13, 14. We therefore examined Notch2 signaling activity in splenic B cells of control and IκBα cKO mice by RT-PCR analysis (Fig. 6A). Our study showed that Notch2 levels in IκBα-deficient B220+ splenic B cells (Fig. 6A) as well as in the MZB, follicular B (FOB, defined as CD21hiCD23hi) and non-follicular B (NFB, defined as CD21-CD23-) fractions were markedly decreased compared to controls (Fig. 6B). In addition, expression of the Notch2 target genes Deltex1 (Dtx) and Hes1 and the key mediator of Notch signaling, Rbpj, was down-regulated in IκBα-deficient B cells (Fig. 6A). In contrast, expression levels of the B cell-specific transcription factors Ebf1 and Pax5 remained unaltered (Fig. 6A). It has been reported that Notch2 signaling induces the expression of CD21 14. Our analysis revealed that splenic B cells of IκBα cKO mice showed reduced expression levels of CD21, while the expression of CD23 was not altered (Fig. 6C).
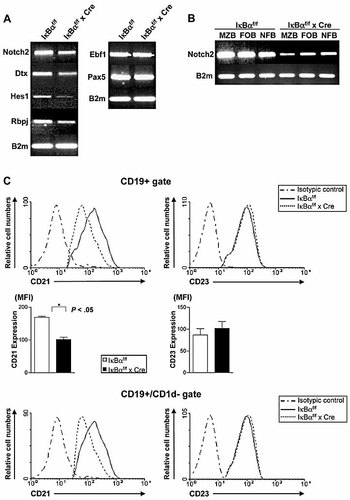
Down-regulated Notch2 activity in IκBα-deficient B cells. (A) Splenic B cells from IκBαf/f x Cre and IκBαf/f mice were isolated and RT-PCR of Notch2, Dtx, Hes1, Rbpj, Ebf1, Pax5 and B2m was performed. Data are representative of three independent experiments. (B) MZB, FOB and NFB cells were isolated from IκBαf/f x Cre and IκBαf/f mice and RT-PCR of Notch2 was performed. NFB, FOB and MZB cells were defined as CD21-CD23-, CD21hiCD23hi, and CD21hiCD23lo, respectively. (C) Reduced expression of CD21 on all CD19+ B cells and on non-MZ/preMZ cells (CD19+CD1d-) from IκBαf/f and IκBαf/f x Cre mice, respectively. Data are mean + SD from three independent experiments. MFI, mean fluorescence intensity. *p<0.05.
Discussion
Although gene targeting experiments demonstrated that NF-κB family members are implicated in the development of MZB cells 11, 15, the role of IκB in MZB cell development is not clear. Here we demonstrate that the NF-κB inhibitor IκBα is required for MZB cell development, since B cell-specific IκBα cKO mice showed markedly reduced numbers of MZB cells and their precursors. Furthermore, IκBα deficiency conferred high sensitivity to lethal effects of blood-borne bacterial infection with S. aureus.
Knockout studies in mice demonstrated that the generation of MZB cells requires NF-κB p50, p52, p65, RelB and c-Rel 11, 15–18. In our model inactivation of IκBα results in constitutive activation of NF-κB in B cells as it has been described in conventional IκBα knockout mice that die within 5–7 days after birth 27–29. Constitutive NF-κB activity mainly consisted of p50 homodimers in our model. It has been shown that p50 homodimers can mediate inhibition of NF-κB dependent target genes 30.
Although we can not rule out that in mature B cells inactivation of IκBα caused the MZB cell loss, we suggest from our data that mainly constitutive NF-κB activation mediated through inhibitory p50 homodimers 30 contributes to deregulated MZB cell development. It has been described that p50 homodimers in unstimulated cells are associated with the histone deacetylase HDAC-1, bind to DNA and repress NF-κB-dependent gene expression 31. Following stimulation, p50/p65 heterodimers containing phosphorylated p65 enter the nucleus and displace DNA-bound p50:HDAC-1, thereby providing a mechanistic explanation for repression by nuclear p50 homodimers in unstimulated cells 31. In this context, it seems contradictory that lack of p50 also results in a defect in MZB cell development and reduced MZB cell numbers 11, 16, 32. We hypothesize that a very fine-tuning of p50-containing complexes is necessary to allow appropriate MZB cell development and differentiation of MZB cell precursors. In addition to p50 homodimers, we further showed an increase in p50/p65 containing heterodimers. As a result of this imbalanced NF-κB regulation, the expression of putative NF-κB-induced inhibitors of MZB cell differentiation could be increased and thus block MZB cell development.
Phenotypes of mice lacking subunits of the NF-κB family are assumed to be relevant to those from Notch2-deficient mice with respect to defective MZB cell development, since NF-κB is closely linked to the Notch signaling pathway 33–40. Recently, one study provided in vivo evidence for the genetic interaction between Notch2 and NF-κB pathways in MZB cell development 16. Here, we show that inactivation of IκBα in B cells results in decreased expression levels of Notch2 and its target genes Dtx, Hes1 and CD21, and the key mediator of Notch signaling Rbpj. Data from Notch2 cKO mice showed that Dtx is expressed and regulated in MZB cells through Notch2, suggesting that decreased Notch2 activity in IκBα-deficient B cells results in down-regulation of Dtx 14. It remains to be elucidated by which mechanisms IκBα deficiency regulates Notch2 activity in our model.
Two models have been proposed to explain defects in MZB cell development of gene-disrupted mice 1. Several groups argued that MZB cell differentiation is inhibited by excess of BCR signaling and therefore inactivation of genes, which are involved in BCR signaling, disturbs normal MZB cell development 3–9. These genes include BCR signaling related proteins such as Aiolos, Igα mutant, Lyn, CD19, CD22, Rac2. This theory, however, is controversial 41, 42 and probably specificity of the BCR is the primary determinant for the developing B cell to choose between FOB or MZB cell fate 1. The second driving force seems to be signaling through Notch2 and p50 13, 14. B cells selected to mature into the MZB cell lineage can only do so if they receive additional signals from Notch2 and p50 1. Our data suggest that both pathways are linked through IκBα, since IκBα inactivation and constitutive NF-κB activation results in deregulated Notch2 activity in mature B cells. Identification of the specific mechanisms needs further investigations.
Materials and methods
Generation of IκBα cKO mice
IκBα cKO mice were generated as previously described 25. Mice were genotyped by PCR using the following primers: lox1 5′-gtggagtcagatgtagcac-3′ and lox2 5′-agaaagggataagccatgga-3′. The expected sizes of the PCR products are 363 bp for the floxed allele and 275 bp for the wild-type allele. For site-specific recombination in B cells, IκBαf/f mice were crossed with CD19-Cre mice 43 and genotyped by PCR using the following primers: CD19.9 5′-gtctgaaagcattccaccggaa-3′ (common primer), CD19.8 5′-ctgcgtgcaatccatcttgtt-3′ and Neo4117 5′-aatgttgtgctgccatgcctc-3′. The expected sizes are 950 bp for the recombined and 550 bp for the wild-type allele. All mouse experiments were approved by the local committee of the Landesamt für Arbeitsschutz, Gesundheitsschutz und technische Sicherheit Berlin (Berlin, Germany).
Southern Blot
For detection of the floxed and deleted Ikba allele, DNA isolated from the indicated tissues was digested with KpnI and hybridized with a 32P-labeled 3′-flanking probe as described previously 25. The expected sizes were 16.00 kb for the wild-type allele and 2.75 kb for the deleted allele, respectively.
Immunoblotting and electrophoretic mobility shift assay
Whole-cell extracts were prepared and quantitated by Bradford protein assay 44. Proteins (30 μg) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Protein load was normalized by Ponceau red staining. Membranes were incubated with rabbit polyclonal anti-IκBα (C21, Santa Cruz) or mouse anti-tubulin mAb (B-5-1-2, Sigma), followed by HRP-conjugated secondary antibodies (Santa Cruz) and detected by enhanced chemiluminescence (Amersham Pharmacia Biotech). EMSA was performed as described previously 45. Equivalent amounts of cell extract (5 µg) were applied in each case. Protein amount was determined by Bradford protein assay. The polyclonal antibodies used for supershift analysis were anti-p50 (NLS), anti-p52 (447), anti-p65 (A), anti-c-Rel (C) and anti-RelB (C-19, all Santa Cruz).
Flow cytometry analysis and antibodies
The following mAb were used: FITC-conjugated anti-CD1d (1B1) and anti-CD5.2 (MM3201, Caltag), PE-conjugated anti-CD21 (7G6), anti-IgD (11–26) and anti-B220 (RM2604, Caltag), biotin-conjugated anti-CD19 (1D3), anti-IgM (II/41, all Becton Dickinson), allophycocyanin (APC)-conjugated anti-CD23 (MCD2305, Caltag). All analyses were performed by FACSCalibur (Becton Dickinson). Data were obtained by the analysis of 1 × 105 viable cells as determined by forward and side scatter profiles and analyzed with CellQuest software (Becton Dickinson).
Immunohistochemistry
Sections were rehydrated in TBS (0.1 M Tris pH 7.5, 0.15 M NaCl) supplemented with 0.1% Tween 20 (TBST) and transferred into a vertical flow staining chamber (Thermo Life Sciences). Sections were pre-incubated twice with TBST containing 5% mouse serum for 10 min each. Sections were incubated with a mixture of appropriately diluted fluorescent dye-coupled antibodies in 2.5% serum/TBST for 1.5 h and washed three times with TBST. Sections were washed three times with TBST and stained twice for 2 min with 1 μg/mL DAPI/TBST to visualize nuclei. Sections were washed three times with TBST and mounted with MOWIOL. Images were acquired using an Olympus BX61 microscope. The following antibodies were used: anti-B220-Cy3 (TIB146), anti-CD3-Cy5 (17A2), anti-IgD-FITC (TIB176) (provided by Elisabeth Kremmer, GSF Munich, Munich, Germany). Cy3 and Cy5 conjugates (Amersham) were prepared as recommended by the manufacturer.
Immunization of mice
Ten-to-twelve-week-old mice were immunized by intraperitoneal injection of 100 µg of di-nitrophenol (DNP)-LPS, or 50 µg DNP-keyhole limpet hemocyanin (KLH; Biosearch Technologies). Animals were bled at the indicated time points and serum was obtained and stored at –80°C. For bacterial infection, the S. aureus strain ATCC 25923 was used. Mice were intravenously injected with 0.2 mL of bacterial solution, which contained 1 × 107 CFU of viable S. aureus. The survival of the animals was analyzed over a period of 16 days.
ELISA
ELISA were conducted using affinity-purified anti-mouse IgM, IgG1, IgG2a, IgG2b and IgG3 (all Becton Dickinson) to generate standard curves. To determine concentration of Ig and hapten-specific (DNP) antibodies, 2 µg of rat anti-mouse isotype-specific antibodies (Becton Dickinson) or hapten-conjugated BSA (Biosearch Technologies) was used as capture agents, respectively. Appropriate dilution of serum samples were loaded for 1 h, followed by addition of biotin-conjugated anti-mouse isotype-specific antibodies and streptavidin-peroxidase-conjugate (Sigma). o-Phenylenediamine (Sigma) was used as a substrate. Enzyme activities were measured at 450 nm in a microplate spectrophotometer (Bio-Rad).
Cell sorting
Each indicated subset was isolated on FACSAria (Becton Dickinson). For magnetic cell sorting of resting splenic B cells an isolation kit (Miltenyi Biotec) was used. Mouse B cells were isolated by depletion of non-B cells according to manufacturer‘s instructions. For experiments examining mRNA expression in sorted cells, 5 × 105–1 × 106 cells from each fraction were subjected to RT-PCR analysis.
RNA analysis
Total RNA from splenic B cells was extracted from 1 × 107 cells using RNeasy® Mini Kit (Qiagen) and converted into cDNA by the SuperscriptTM® II RNase H Reverse Transcriptase Kit (Invitrogen) according to manufacturer's instructions. RT-PCR analysis was performed using mouse IκBα (sense 5′-cagccccgcacagccatgtttcag-3′ and antisense 5′-catggagtccaggccgctgtcgtg-3′), Notch2 (sense 5′-acatcatcacagacttggtc-3′ and antisense 5′-cattattgacagcagctgcc-3′), Hes1 (sense 5′-agcacagaaagtcatcaaagcc-3′ and antisense 5′-ttcatgcactcgctgaagcc-3′), Dtx (sense 5′-gagtggctgaatgagcacag-3′ and antisense 5′-ctgcgacatgctgttgaagt-3′), Rbpj (sense 5′-cgaccctgtatcacaactcc-3′ and antisense 5′-ccattccctcatagaacgtg-3′), Pax5 (sense 5′-cagcaaaattcttggcaggt-3′ and antisense 5′-tgctgtgtgaacaggtctcc-3′) and Ebf1 (sense 5′-ccaactcaccctatgccatt-3′ and antisense 5′-ccgttcttcagcatttggat-3′) specific primers. To serve as an internal control, mouse B2m (sense 5′-ttctggtgcttgtctcactg-3′ and antisense 5′-ccgttcttcagcatttggat-3′) was amplified from all cDNA templates generated. For PCR reaction, 1–2 µL cDNA was amplified using Amplitaq DNA Polymerase (Roche). PCR amplifications were performed under standard conditions. Each sample was amplified in triplicate.
Statistical analysis
All data are shown as mean ± SD. Statistics were performed using the Mann-Whitney U test.
Acknowledgements
We thank K. Rajewsky for the CD19-Cre and Mx-Cre mice and Katharina Pardon, Antje Wollny, Birgit Eckelt and Ursula Rüschendorf for excellent technical assistance. This work was supported by grants of the Deutsche Krebshilfe (10–1973-Ju I).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
The authors declare no financial or commercial conflict of interests.