Proliferative signals mediated by CD28 superagonists require the exchange factor Vav1 but not phosphoinositide 3-kinase in primary peripheral T cells†
Abbreviation
CD28SACD28 superagonist
Abstract
Almost all responses of naive T cells require co-stimulation, i.e. engagement of the clonotypic TCR with relevant antigen/MHC and the co-stimulatory molecule CD28. How CD28 contributes to T-cell proliferation remains poorly understood, with widely conflicting reports existing which may reflect different methods of co-ligating receptors. Some CD28 mAb, however, can stimulate T-cell proliferation without the need for TCR co-ligation, and thus provide unique tools to dissect proliferative signals mediated through CD28 alone. Using primary peripheral T cells from CD28-transgenic mice, we show that both the YMNM and Lck-binding motifs, but not the Itk-binding motif, in CD28 are required for proliferation. Given that the YMNM motif recruits both phosphoinositide 3-kinase (PI3K) and the exchange factor Vav1, we investigated the role of these two molecules in CD28-mediated proliferation. In p110δD910A/D910A transgenic T cells, which are defective in PI3K activation following CD28 ligation, proliferation was comparable to that in wild-type cells. By contrast, T-cell proliferation was abolished in Vav1−/− cells. Although we did not address the role of Grb2 in CD28 signalling, these results indicate that CD28 can mediate Lck- and Vav1-dependent proliferative signals independently of PI3K.
Introduction
Optimal T-cell responses require co-stimulation, i.e. engagement of the clonotypic TCR with relevant antigen/MHC as well as the co-stimulatory molecule CD28 1, 2. How CD28 contributes to T-cell proliferation remains poorly understood, with widely conflicting reports existing in the literature. The cytoplasmic domain of CD28 contains a number of signalling motifs including two proline-rich motifs that bind Lck and Itk, respectively 3, 4. Additionally, the YMNM motif in CD28 binds both the PI3K p85/p110 heterodimer and Vav1 in association with a Grb2-like adaptor 5, 6. The relative importance of these motifs in co-stimulatory proliferative responses is controversial. For example, Burr et al. 7 demonstrated that the Lck-binding motif is required for proliferation in primary cells, and yet using the same methodology, Andres et al. 8 showed that no particular motif is required for proliferation. Similarly, Okkenhaug et al. 9 used CD28-transgenic mice to show that the tyrosine in the YMNM motif is not required for co-stimulatory proliferation, and yet Harada et al. 10 demonstrated, using an independently derived transgenic mouse, that this tyrosine residue contributed to early T-cell proliferative responses.
One reason for such conflicting results may lie in differences between methods of receptor co-ligation and resultant subtle differences in the strength of each signalling pathway, leading to differences in compensation between the complementary pathways. Many studies crosslink the TCR with CD3 antibodies, high-affinity ligands that can induce co-stimulation-independent proliferative responses for which only one physiological equivalent has been described 2. Titrating down CD3 antibody concentrations such that CD28 ligation apparently drives proliferation may overcome this problem. However, it is unlikely that ligation of few TCR with high-affinity ligands reflects the physiological situation in which low-affinity ligands ligate TCR. One way by which to circumvent these problems is to address the CD28 signalling pathway directly using CD28 superagonistic antibodies (CD28 superagonists, CD28SA) 11, 12.
We have previously generated two functionally distinct types of CD28 antibodies 13-15. Conventional CD28 antibodies recognise an epitope adjacent to the CD80/86-binding site, and like natural ligands, induce proliferative responses only when combined with TCR stimulation. By contrast, CD28SA recognise a membrane proximal epitope and activate T cells without the need for TCR ligation 13, 14. Mitogenic CD28 signals can also be induced after stimulation with the recombinant natural ligand CD80 13. However, activation by recombinant CD80 only occurs if the physiologically monovalent CD28 extracellular region is replaced with a bivalent receptor such as the extracellular domains of CTLA-4. Ligation of such bivalent CD28 chimeras leads to an artificially stable complex in a receptor lattice structure.
Crystallographic evidence and studies of binding stoichiometry suggest that CD28SA similarly induce the formation of a receptor lattice structure 13, 16. These results suggest that CD28SA induces a physiologically relevant, although dysregulated signalling pathway at the extremity of CD28 co-stimulatory signalling, in the same way through which ligation with CD3 antibodies or high-avidity lymphocytic choriomeningitis virus antigens induces co-stimulation-independent signalling at the extremity of the TCR pathway 2, 17. Stimulation with superagonistic antibodies thus provides an exception to the co-stimulatory requirement of T-cell responses, and a unique tool to define CD28-specific events leading to proliferative responses.
Using primary peripheral T cells from CD28-transgenic mice, we show here that both the YMNM and Lck-binding motifs, but not the Itk-binding motif, in CD28 are required for proliferation. We further demonstrate that PI3K signalling is not required for CD28-mediated proliferation, but that the exchange factor Vav1 is essential.
Results and discussion
CD28-mediated proliferation requires integrity of the YMNM and Lck-binding motifs
In order to determine how CD28 contributes to proliferative T-cell responses, we utilised CD28−/− mice that express wild-type or mutated CD28 transgenes at comparable levels (Fig. 1A) 18. Purified T cells were stimulated with CD28SA, which induces proliferation in the absence of TCR ligation, to determine which motifs in CD28 are required to induce proliferation. Cells expressing CD28 mutated in the N-terminal proline-rich motif, which binds to the kinase Itk, proliferated comparably well to cells expressing wild-type CD28 (Fig. 1B). Binding of Itk to CD28 is thus not required to induce proliferation, which is consistent with previous results demonstrating that Itk−/−cells are not defective in proliferation induced through CD28 co-stimulation 19. By contrast, cells expressing CD28 mutated in the C-terminal proline-rich motif, which binds the kinase Lck, did not proliferate following stimulation through CD28, but proliferated comparably to wild-type cells following PMA+ionomycin stimulation (Fig. 1B and C). These results complement previous data demonstrating that the Lck-binding motif is also required during co-stimulation, and that TCR signals cannot compensate for defective signalling through CD28 that cannot bind Lck 4, 7, 20, 21.
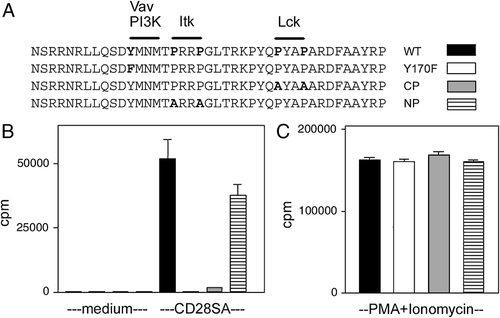
CD28-mediated proliferation requires integrity of both the YMNM and Lck-binding motif. (A) CD28 transgenes expressed in CD28−/− mice. Binding sites of Vav, PI3K, Itk and Lck are indicated, and amino acids that have been mutated are shown in bold. (B) CD28-mediated proliferation requires the YMNM and Lck-binding motifs but not the Itk-binding motif. (C) Comparable proliferation of transgenic T cells stimulated with PMA+ionomycin. Data shown are means±SEM and are representative of three independent experiments.
Cells expressing CD28 with a tyrosine-to-phenylalanine mutation in the YMNM motif, which recruits both PI3K and Vav1, did not proliferate following stimulation through CD28, but proliferated comparably to wild-type cells following PMA+ionomycin stimulation (Fig. 1B and C). The YMNM motif is thus required for CD28-mediated proliferation in our stimulation system, complementing two previous studies that showed a partial requirement for this motif to induce proliferation during co-stimulation 7, 10.
CD28-mediated proliferation does not require phosphoinositide 3-kinase but does require the exchange factor Vav1
Given that the YMNM motif recruits both PI3K and Vav1, we investigated which of these two signalling molecules is required for proliferation. To investigate the role of PI3K, we utilised p110δD910A/D910A mice, which express catalytically inactive p110δ, the major PI3K isoform required for signalling through antigen receptors (Fig. 2A) 22, 23. Activation of PI3K following ligation of CD28, measured by phosphorylation of its downstream substrates protein kinase B/Akt and Foxo1/3a transcription factors, is severely defective in p110δD910A/D910A transgenic T cells 24. However, p110δD910D/D910A transgenic cells proliferated comparably to wild-type cells following stimulation through CD28. By contrast, CD28−/− cells and cells transgenically expressing CD28Y170F did not proliferate (Fig. 2A). Proliferative responses induced by CD28SA or co-stimulation through the TCR and CD28 were also markedly less sensitive to pharmacological PI3K inhibition than following TCR stimulation alone (Fig. 2B). These results indicate that PI3K is dispensable for CD28-mediated proliferative responses, and that another molecule that binds to the YMNM motif, apart from PI3K, is required for CD28-mediated proliferation.
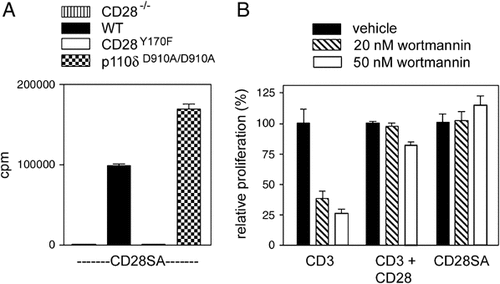
CD28-mediated proliferation does not require PI3K. (A) CD28-mediated proliferation is induced in wild-type and p110δD910A/D910A T cells, but not CD28−/− and CD28Y170F T cells. Data shown are means±SEM and are representative of three independent experiments. (B) Proliferation of wild-type T cells following stimulation through the TCR alone, TCR and CD28, or CD28 alone using CD28SA, in the presence of vehicle or PI3K inhibitor wortmannin. Proliferation is relative to that of cells from each stimulation setting without wortmannin; medium: 127±56 cpm, CD3: 31 125±6168 cpm, CD3+CD28: 113 214±3177 cpm, CD28SA: 119 601±15 472 cpm. Proliferative responses of cells in the presence of 20 or 50 nM wortmannin after CD3 stimulation were significantly less than after stimulation with CD3+CD28 or CD28SA, as determined by the one-way ANOVA and Bonferroni's multiple-comparison test (p<0.01). Data shown are means±SEM and are representative of three independent experiments.
One candidate is the exchange factor Vav1, which is recruited to the YMNM motif in a complex with a Grb2-like adaptor 6. We, therefore, compared proliferative responses of Vav1−/− and wild-type cells, which express comparable levels of CD28 25 (Fig. 3). Vav1−/− cells did not proliferate following stimulation through CD28, but proliferated comparably well to wild-type cells following stimulation with PMA+ionomycin. Similarly, stimulation with PMA+CD28SA partially rescued the proliferative defect of Vav1−/− cells (Fig. 3). These results complement previous data using rat CD28-transgenic mice to demonstrate the importance of Vav1 for CD28-mediated proliferation 11. Therefore, the exchange factor Vav1, but not PI3K, is required for CD28-mediated proliferation in our stimulation system. (Fig. 3)
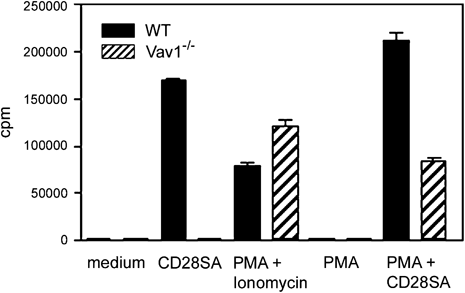
CD28-mediated proliferation requires the exchange factor Vav1. CD28-mediated proliferation is induced in wild-type but not Vav1−/− T cells, whereas stimulation with PMA+ionomycin or PMA+CD28SA induces proliferation of both wild-type and Vav1−/− T cells. Data shown are means±SEM and are representative of two independent experiments.
Concluding remarks
How do these data relate to CD28 signalling during co-stimulation? In our experimental system there is no apparent role for PI3K in CD28-mediated proliferative responses. Our results are consistent with data showing that TCR-transgenic cells expressing CD28Y170F, which cannot recruit PI3K to CD28, proliferate comparably to cells expressing wild-type CD28 9. Thus, CD28 signalling through PI3K is not required for proliferative responses. Nevertheless, TCR-mediated proliferation is dependent on PI3K, and this dependence can be overcome following co-ligation of CD28 with mAb (Fig. 2B) 23, 24. There must be alternative mechanisms, apart from coupling to PI3K, by which CD28 contributes to co-stimulation and overcomes the dependence of the TCR on PI3K.
We show here that CD28 contributes signals dependent on both Vav1 and the Lck-binding motif. It has previously been suggested that Vav1 is dispensable for CD28 signalling 25. However, this study equated responses following co-stimulation and stimulation with PMA and CD28 mAb, a questionable assumption given that Vav1 is upstream of phospholipase Cγ1 activation and production of 1,2-diacylglycerol, the physiological equivalent PMA 26. Given that stimulation with PMA in addition to CD28SA can partially rescue the proliferative defect of Vav1−/− cells (Fig. 3), and therefore compensate for Vav1 deficiency, we argue that Vav1 is upstream of 1,2-diacylglycerol production in CD28-mediated proliferative responses. Under conditions where the CD28 YMNM motif is required for proliferation 10, it is likely that Vav1 contributes to the co-stimulatory response. Similarly, other molecules that signal through Y×N motifs, including natural killer group 2 member D-DAP10, CD19 and non-T cell activation linker 27-30, may also recruit the Grb2–Vav1 complex to induce proliferative signals. With the currently available tools we were not able to address the role of Grb2 in CD28 signalling in primary cells, which will require generation of mice expressing CD28 with an asparagine-to-alanine mutation in the YMNM motif.
Lastly, it has previously been suggested that sustained activation of Lck mediated by CD28 is required for co-stimulatory proliferation 20. CD28SA similarly induce sustained activation of Lck 31, but do not induce proliferation in the absence of Vav1 11. In the context of co-stimulation, signalling through the YMNM and Lck-binding motifs differ in that co-ligation of the TCR can largely compensate for defects in the former, but not the latter motif 7, 9. It is well established that TCR signalling can activate Vav1 32, and therefore it is not surprising that defective activation of Vav1 through the YMNM motif could be compensated for by TCR signalling. By contrast, the sustained activation of Lck mediated by CD28 appears to be a unique feature of CD28, and therefore the major mechanism by which CD28 contributes to co-stimulatory proliferation.
Materials and methods
Animal experiments were performed with the permission of the relevant authorities: Government of Unterfranken, Genehmigung von Versuchsvorhaben 621-2531.01-6/04, and UK Home Office, licence 80/1809. C57BL/6 Vav1−/− and CD28−/− mice expressing wild-type or mutated CD28 transgenes were generously provided by Klaus-Dieter Fischer and Alfred Singer, respectively, and PI3K p110δD910A/D910A mice have been described before 9, 18, 23, 33. CD28SA (clone D665) to mouse CD28 has been described 13.
Lymph node CD4+ T cells were purified by negative selection using the MACS separation system (Miltenyi Biotech, Bergisch Gladbach, Germany), and were stimulated with 10 μg/mL D665 antibody, with or without 25 ng/mL PMA as indicated, on plates for non-adherent cells (Greiner, Frickenhausen, Germany) pre-coated with goat anti-mouse IgG (Sigma, Deisenhofen, Germany) in 0.1 M bicarbonate buffer pH 9.5. For stimulation through the TCR and co-stimulation, cells were cultured on flat-bottom 96-well plates coated with 2 μg/mL anti-mouse CD3 (clone 145-2C11; Becton Dickinson, CA) with or without 5 μg/mL soluble anti-mouse CD28 (clone 37.51; Becton Dickinson). Proliferation was assayed using incorporation of [3H]thymidine, after 3 days of stimulation with [3H]thymidine added for the last 16 h. For experiments requiring PI3K inhibition, cells were stimulated in the presence of vehicle or 20–50 nM wortmannin, as indicated.
Acknowledgements
We thank Klaus-Dieter Fischer for providing Vav1−/− mice, and Alfred Singer for providing CD28-transgenic mice. This work was funded by the Deutsche Forschungsgemeinschaft through HU-295/8 and the Network of the Rudolf-Virchow-Centre, Würzburg, Germany. J. L. E. and K. O. were supported by a Biotechnology and Biological Sciences Research Council David Phillips Fellowship.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




