Reduced Cbl phosphorylation and degradation of the ζ-chain of the T-cell receptor/CD3 complex in T cells with low Lck levels†
Abbreviations
kdknockdown
siRNAshort interfering RNA
Abstract
T cells with short interfering RNA-mediated Lck-knockdown (kd) display paradoxical hyper-responsiveness upon TCR ligation. We have previously reported a possible mechanism for T-cell activation in cells with low levels of Lck depending on Grb2-SOS1 recruitment to the zeta-chain of TCR/CD3 (Methi et al., Eur. J. Immunol. 2007, 37: 2539–2548). Here, we show that short interfering RNA-mediated targeting of Lck caused a dramatic reduction in c-Cbl phosphorylation and a general reduction in protein ubiquitination after TCR stimulation. Specifically, this resulted in reduced ubiquitination of the zeta-chain, yet internalization of TCR/CD3 appeared to be normal after receptor engagement. However, zeta-chain levels were elevated in Lck-kd cells, and confocal microscopy revealed reduced colocalization of CD3-containing vesicles with endosomal and lysosomal compartments. We hypothesize that prolonged stability of internalized T-cell receptor complex may result in extended signaling in T cells with low Lck levels.
Introduction
Engagement of the TCR leads not only to activation of T cells, but also to internalization and lysosomal degradation of the receptor complex 1. The latter process serves to terminate signaling, and has been shown to be dependent on the tyrosine kinase activity of Lck 2, 3 and ubiquitination by c-Cbl 4-6. The E3 ubiquitin ligase c-Cbl functions as a negative regulator of many signaling pathways and ubiquitination marks active enzymes and receptors for degradation [reviewed in 7 and 8]. Notable targets for c-Cbl-mediated ubiquitination are Lck 9, Vav 10, Fyn 11-13, ZAP-70 14, as well as the already mentioned TCR/CD3-complex. c-Cbl itself is activated by tyrosine phosphorylation on several residues, most importantly Y700, Y731 and Y774 13, 15-17. These residues are not, however, substrates of Lck or ZAP-70, but of Fyn and Syk 13, 16, 18, 19, which are activated directly or indirectly by Lck 20.
Cbl exists in two main isoforms, c-Cbl and Cbl-b, with a high level of sequence conservation. Consistent with the negative regulation assigned to the Cbl family of proteins, T cells from c-Cbl−/− and Cbl-b−/− mice were hyperactive upon TCR engagement, although some biochemical distinctions between the phenotypes exist 21-25. T cells from double-knockout mice lacking both c-Cbl and Cbl-b failed to modulate surface TCR after ligand engagement, resulting in sustained TCR signaling and ERK1/2 phosphorylation. However, signaling through the major TCR pathways were not increased 26. These observations are comparable to what we found in T cells with low levels of Lck, which also display prolonged ERK1/2 activation while expression levels of other proteins (Fyn, Csk, PKCα, PLCγ1, LAT, FAK, and Pyk2) remained unaffected 27, 28. We therefore investigated the impact of short interfering RNA (siRNA)-mediated Lck-knockdown (kd) on c-Cbl activity and TCR/CD3 turnover in T cells. As expected, c-Cbl phosphorylation and ubiquitination of the ζ-chain of TCR/CD3 was strongly suppressed in cells with Lck-kd. Despite this, internalization of the TCR/CD3-complex was unaffected, and surface levels of TCR/CD3 were similar in control and Lck-kd cells. After prolonged stimulation, however, ζ-chain levels were reduced in control cells but remained high in Lck-kd, indicating a resistance to lysosomal degradation in the latter case. Confocal microscopy showed formation of CD3-containing vesicles after stimulation of Lck-kd cells, but these vesicles did not merge with endosomes or lysosomes to the same degree as in normal cells.
Results and discussion
Hyper-responsiveness of Lck-kd cells
We have previously reported hyper-responsiveness in Jurkat TAg and primary human T cells with siRNA-mediated Lck-kd 27, 28. Here, we wanted to explore to what extent various levels of Lck would contribute to this phenotype, and the implications of Lck-kd on TCR/CD3 turnover.
Lck levels correlate inversely with transcriptional activation of NFAT-AP-1 in Jurkat T cells
As shown in Fig. 1A and B, optimal kd of Lck was obtained 48 h post transfection. Lck levels then gradually returned to normal as siRNAs became diluted and degraded upon cell growth. Paradoxically, low Lck levels corresponded to high NFAT-AP-1 activation, and the response decreased in a linear fashion when Lck levels returned to normal. Caspase-3 activation and viability of control and Lck-kd cells upon challenge with OKT3, anti-Fas treatment, and serum starvation were examined; however, surprisingly, we could not detect any differences between control or Lck-kd cells for any of these treatments, and increased caspase-3 activity and reduced viability were observed only after anti-Fas treatment and serum starvation (data not shown). We have previously validated the effects of the Lck-specific siRNAs (Lck232) by reproducing the results using another Lck-specific siRNA (Lck377) thus controlling for off-target effects 27, 28.
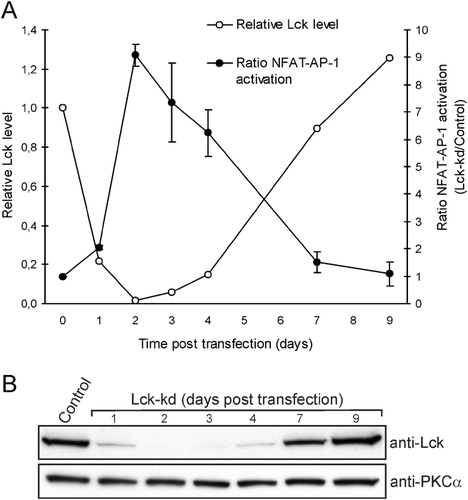
Lck levels correlate inversely with hyper-responsiveness in Jurkat T cells. (A) Jurkat TAg T cells were co-transfected with either a control or a Lck-specific siRNA (Lck-kd), NFAT-AP-1-luciferase and TK-renilla. At the indicated time points (days post-transfection), cells were unstimulated (day 0) or stimulated with OKT3 (1500 ng/mL) for an additional 6 h, and subjected to dual luciferase assay to assess NFAT-AP-1 activation as described in the Materials and methods. (B) Cell lysates from A were subjected to immunoblotting to verify Lck levels (top panel) at the time points investigated. Anti-PKCα served as control (bottom panel) and Lck levels quantified by densitometric scanning were normalized to PKCα and plotted in (A). The data are representative of two experiments and mean±half range is shown in (A).
Reduced c-Cbl phosphorylation and ubiquitination of the ζ-chain of TCR/CD3 in Lck-kd cells
Here, we wanted to investigate in more detail how sustained signaling could be reconciled with reduced levels of Lck. As shown in Fig. 2A and B, the phosphorylation of c-Cbl was strongly inhibited in Jurkat TAg T cells and primary T cells with Lck-kd, both in the basal and stimulated situation. In fact, stimulated Jurkat TAg Lck-kd cells displayed approximately the same degree of c-Cbl phosphorylation as unstimulated control cells. c-Cbl is activated by phosphorylation, and the ubiquitination of ζ-chain of TCR/CD3 and possibly other proteins immunoprecipitated with ζ-chain was strongly reduced in Lck-kd cells (Fig. 2C). This also applied to overall ubiquitination in whole cell lysates (Fig. 2D). To substantiate whether c-Cbl is involved in the process of hyper-responsiveness, we co-transfected Jurkat TAg cells with siRNAs and c-Cbl, or a mutant of c-Cbl where three tyrosine residues (Y700, Y731 and Y774) had been substituted by phenylalanines (c-Cbl 3Y-F). As seen in Fig. 2E, co-transfection with c-Cbl normalized NFAT-AP-1 activation in Lck-kd cells (51±14% reduction, mean±SD, n=3, p≤0.01), indicating that more input from c-Cbl may compensate for the reduced activity of endogenous c-Cbl in these cells. Transfection with control siRNAs and c-Cbl 3Y-F rendered Jurkat T cells significantly more active (1.9±0.2-fold, mean±SD, n=3, p≤0.01, Fig. 2E), indicating that obstruction of c-Cbl phosphorylation augments T-cell signaling and suggesting a role for one or more of the mutated phosphorylation sites. However, overexpression of c-Cbl did not lead to augmented total phosphorylation of c-Cbl in Lck-kd cells as opposed to control cells (Fig. 2F). This is consistent with the observation that Lck is required for phosphorylation of c-Cbl, but also indicates that c-Cbl may act as a negative regulator of TCR signaling in the absence of phosphorylation, although some effects of c-Cbl 3Y-F were observed.
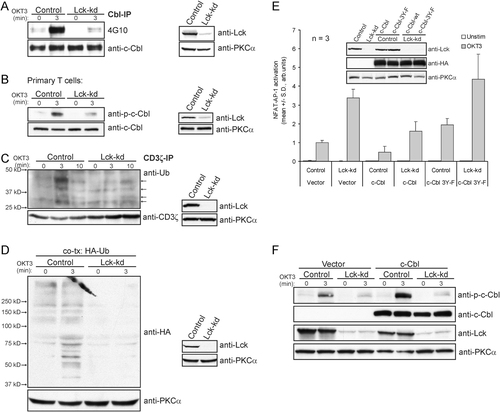
Reduced Cbl phosphorylation and ubiquitination in Lck-kd cells. Jurkat TAg T cells (A, C–F; representative blots of three experiments) or in one experiment primary T cells (B) were transfected with either a control or an Lck-specific siRNA (Lck-kd), in the presence or absence of either HA-Ubiquitin (HA-Ub), c-Cbl, c-Cbl 3 Y-F or empty vector as indicated and incubated for 48 h prior to stimulation. (A) Stimulation with OKT3 (1500 ng/mL) for 3 min before c-Cbl IP (Cbl-IP) and detection of Cbl phosphorylation by immunoblotting with 4G10 mAb. (B) Stimulation with OKT3 (1500 ng/mL and cross linked with F(ab′)2 fragment (20 μg/mL)) for 3 min before immunoblotting of cell lysates with a phospho-specific antibody to P-c-Cbl and an pan-c-Cbl antibody. (C) Stimulation with OKT3 (1500 ng/mL) for 3 or 10 min before ζ-chain IP and detection of ζ-chain ubiquitination by immunoblotting with anti-Ub. Arrows denote possible species of mono or poly-ubiquitinated ζ-chain and/or other co-immunoprecipitated proteins. (D) Stimulation with OKT3 (1500 ng/mL) for 3 min. The lysates were immunoblotted with anti-HA for assessment of ubiquitinated proteins. (E) Stimulation for 6 h (1500 ng/mL OKT3) and assessment of NFAT-AP-1 activity by the dual luciferase assay using NFAT-AP-1 luciferase and TK renilla as reporters. Data represent mean±SD of n=3 experiments. Inset: Cell lysates were subjected to immunoblot analysis with indicated antibodies to verify expression of Lck-kd (anti-Lck blot), transfected, HA-tagged c-Cbl or c-Cbl 3 Y-F (anti-HA blot) and expression (anti-PKCα blot). (F) Stimulation with OKT3 (1500 ng/mL) before immunoblotting of cell lysates with the indicated antibodies.
Normal TCR/CD3 internalization, but reduced ζ-chain degradation in Lck-kd cells
Since ζ-chain ubiquitination was reduced in T cells with low levels of Lck, we hypothesized that receptor turnover could be interrupted in these cells. Surprisingly, the clearance of CD3ε and TCR-αβ from the plasma membrane after OKT3 stimulation was unaffected by Lck-kd (Fig. 3A, and data not shown) consistent with our earlier data 27. However, when we monitored protein levels in whole cell lysates we found higher levels of ζ-chain in Lck-kd than control cells after prolonged stimulation (6 h) (Fig. 3B–D), an effect that was reversible upon c-Cbl overexpression (Fig. 3E). This indicated that degradation of internalized TCR/CD3 was disturbed in Lck-kd cells, and we therefore compared the subcellular localization of CD3 in control and Lck-kd cells +/- c-Cbl overexpression by confocal microscopy. CD3ε was visualized by fluorescently coupled antibodies (green), early and recycling compartments were stained with transferrin (red), and lysosomal compartments were stained with Lysotracker (red) prior to stimulation. CD3-containing vesicles was formed in both control and Lck-kd cells, but internalized CD3 colocalized significantly less with endosomes and lysosomes in Lck-kd compared to control cells (e.g. 52±20% reduced colocalization with endosomes in Lck-kd versus control after 1 h stimulation, mean±SD, n=26 Lck-kd and n=28 control cells quantified from two independent experiments, p≤0.001). Overexpression of c-Cbl was shown to reverse the effect of reduced colocalization, and no significant differences were found in cells stained with transferrin (data not shown) or Lysotracker (Fig. 3F and G).
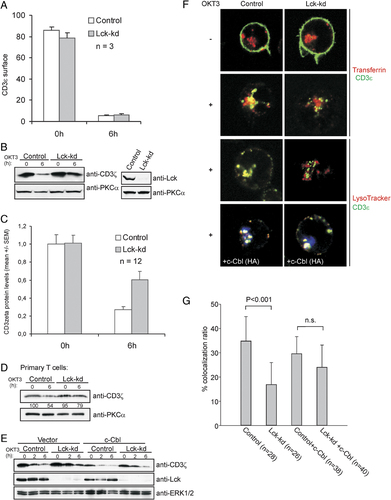
Normal TCR/CD3 endocytosis but reduced ζ-chain degradation in Jurkat T cells with Lck-kd. (A) Jurkat TAg T cells were transfected with either a control or an Lck-specific siRNA (Lck-kd). Forty-eight hours post transfection the cells were stimulated with OKT3 (1500 ng/mL) for 6 h or not, and stained with anti-CD3ε-FITC (SK7) for assessment of surface CD3 on flow cytometry (mean ±SD n=3). (B) Experimental setup is as in A, but the cells were lysed and subjected to immunoblotting for assessment of total cell ζ-chain levels. One representative experiment is shown. (C) The pooled quantified data from n=12 experiments as in (B). (D) The results were confirmed in one experiment in primary T cells which were stimulated for 6 h (1500 ng/mL OKT3 cross linked with 20 μg/mL F(ab')2 fragment), lysed and subjected to immunoblotting with the indicated antibodies. (E) Jurkat TAg cells were transfected with control or Lck-specific siRNA (Lck-kd), and empty vector or c-Cbl (HA-tagged). Forty-eight hours post transfection the cells were stimulated with OKT3 (1500 ng/mL) for the indicated periods of time, and the lysates subjected to immunoblotting with the indicated antibodies. The data are representative of two independent experiments. (F) Jurkat TAg cells were transfected with either a control or an Lck-specific siRNA (Lck-kd), and empty vector (three top rows) or c-Cbl (HA-tagged, bottom row). Forty-eight hours post transfection the cells were washed and stained with anti-CD3ε-Alexa Fluor 488 (green) and transferrin Alexa Fluor 546 conjugate or LysoTracker (red) for 30 min, after which they were stimulated for 1 h with OKT3 (1500 ng/mL). c-Cbl-HA was visualized via secondary antibody staining using an Alexa 647 conjugate (blue). All cells were mounted and visualized by confocal microscopy. Lck knockdown was confirmed by immunoblotting (data not shown). Representative of two independent experiments. (G) Colocalization of internalized CD3 with endosomes and lysosomal compartments in cells with Lck-kd or with Lck-kd and expressing c-Cbl, respectively. The degree of colocalization in individual cells (percent, mean ±SD n=28–40) was determined as total yellow/total green pixels expressed.
Concluding remarks
In a previous paper we proposed a possible mechanism of hyper-responsiveness in T cells with low levels of Lck involving ERK1/2 activation. Grb2-SOS1 was shown to be recruited to the ζ-chain of TCR/CD3 after prolonged stimulation, and signaled through the Ras-Raf-1-ERK1/2 pathway for several hours 28. We now show that Cbl phosphorylation is strongly suppressed in Lck-kd cells, resulting in reduced ubiquitination of the ζ-chain. Surprisingly, our results indicate that neither full Lck activity nor full c-Cbl phosphorylation is necessary for TCR/CD3 internalization, but that they are important for lysosomal targeting and degradation of ζ-chain. Data along these lines have been reported for the EGF receptor 29, 30. However, this is not in complete agreement with previous reports on Cbl knockout mice, where TCR internalization was reduced in both c-Cbl−/−, Cbl-b−/− and Cbl double knockout T cells 26, suggesting that altered activity may result in a different phenotype than complete removal of a protein. In conclusion, we report that reduced signaling from Lck and c-Cbl may lead to prolonged survival and sustained signaling from internalized TCR/CD3-complexes, which may contribute to hyper-responsiveness in cells with Lck kd.
Materials and methods
Antibodies and reagents
Antibodies for Lck, c-Cbl, ζ-chain of TCR/CD3 and anti-ζ-chain AC (antibodies coated on beads) were from Santa Cruz (cat. numbers sc-433, sc-1651, sc-1239 and sc-1239AC), while the antibody for c-Cbl (pTyr731) was from Cell Signaling Technology (cat. number 3554). Antibodies for PKCα, anti-TCR-αβ-PE, anti-CD3-Alexa Fluor 488 and anti-CD3-FITC (SK7) were from BD Biosciences (cat. numbers 610108, 555548, 557694 and 349201). Alexa Fluor 647-conjugated goat anti-mouse IgG1, transferrin from human serum (Alexa Fluor 546 conjugate), and LysoTracker Red was from Invitrogen (cat. numbers A21240, T23364 and L7528). The mouse monoclonal anti-HA was from Nordic BioSite (cat. number HA-11). Anti-phosphotyrosine (pY) mAbs (4G10) were from Upstate (cat. number 05-777). Monoclonal anti-CD3ε (OKT3) for cell stimulation was affinity-purified from supernatants of hybridoma cell lines from ATCC. Peroxidase-conjugated secondary antibodies were obtained from Jackson Immuno-Research Lab.
siRNA duplexes
21-nt siRNA duplexes targeting human Lck mRNA (Lck232; 5′-cugcaagacaaccugguuauc-3′; 5′-uaaccagguugucuugcagug-3′) and a triple G/C mutated control (Lck232M3; 5′-gugcaacacaacgugguuauc-3′; 5′-uaaccacguuguguugcacug-3′) have been described previously 27 and were synthesized in-house. The oligos are named according to the position of the 5′ nucleotide of the sense strand relative to the reference sequences of Lck mRNA (NM_005356).
Cell culture, T-cell purification and transfections
The Jurkat TAg T-cell line, a derivate of Jurkat cells stably transfected with SV40 large T antigen, was cultured in RPMI (Invitrogen) supplemented with 10% FCS, 1:100 penicillin, 1:100 pyruvate and 1:100 non-essential amino acids. For transfections, 20×106 cells were washed and resuspended in 400 μL OptiMEM (Invitrogen), mixed with siRNA and/or cDNA and electroporated at 250V/975 μF in 4 mm cuvettes. Based on previous studies for Lck-kd, 100 nM siRNA was used and the cells were incubated for 48 h before harvesting 27. Transfections with c-Cbl (HA-tagged) were performed as indicated in the figures using 20 μg DNA. Primary CD3+ T cells were purified by subjecting peripheral blood mononuclear cells from healthy Norwegian blood donors to density gradient centrifugation (Lymfoprep, Nycomed Pharma AS) followed by negative selection using paramagnetic beads coated with anti-CD14 and anti-CD19 (111.12 and 111.04, Dynal Biotech). Purified T cells were transfected with siRNA in accordance with the manufacturer's instructions using the Amaxa nucleofector and kit (cat. number VPA-1002). The cells were then incubated for 48 h in complete medium before experiments.
Cell stimulation, lysis and immunoprecipitations
A total of 5×107 cells/mL in RPMI were pre-warmed to 37°C and stimulated with anti-CD3ε antibodies (OKT3 at 1500 ng/mL) for the indicated periods of time at 37°C. Cells were lysed in ice-cold lysis buffer (20 mM Tris pH 7.4, 137 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 5 mM NaF and protease inhibitor cocktail; Complete, cat. number 11697498001 Roche). After 30 min incubation on ice followed by vortexing, the lysates were centrifuged at 13 000×g for 10 min and subjected to either SDS-PAGE/immunoblotting or immunoprecipitation (IP). For IP, antibody-coated beads or antibodies were added and incubations continued at 4°C overnight. Then protein A/G Sepharose beads (Santa Cruz Technology) were added if necessary, followed by additional 60 min incubation. Thereafter, the immune complexes were washed three times in lysis buffer and subjected to SDS-PAGE/immunoblotting.
NFAT-AP-1/TK-Renilla-luciferase assay
The NFAT and AP-1 elements of the proximal interleukin-2 (IL-2) promoter were inserted into a firefly luciferase reporter construct. Cells were co-transfected with this construct and TK-renilla-luciferase (Promega) at a ratio of 10:1 in the presence of siRNA. Forty-eight hours post-transfection, the cells were stimulated for 6 h with OKT3 (1500 ng/mL). Thereafter, the cells were lysed and assayed for dual luciferase activity according to the manufacturers instructions (Promega, cat. number E1960). NFAT-AP-1 activation in each sample was normalized for renilla luciferase activity.
TCR internalization assay
Forty-eight hours post-transfection, Jurkat T cells left unstimulated or stimulated for 6 h with OKT3 (1500 ng/mL) were washed twice in ice-cold PBS, fixed in 4% paraformaldehyde (Sigma-Aldrich) for 5 min at 37°C, washed twice in PBS, and resuspended in PBS w/1% BSA and anti-CD3-FITC or anti-TCR-αβ-PE for 30 min at 4°C. Thereafter the cells were washed three times in PBS w/1% BSA and subjected to flow cytometric analysis for detection of viable CD3ε- or TCR-αβ-positive cells (analyzed using FlowJo software; Three Star).
Confocal microscopy
Cells were surface-labeled with anti-CD3ε Alexa 488 (UCHT1) and incubated with transferrin or Lysotracker Red at 37°C for 30 min prior to stimulation for 30 min with OKT3 (1500 ng/mL). The cells were subsequently attached to poly-L-lysine-coated coverslips and fixed with 3% paraformaldehyde prior to mounting. HA-tagged c-Cbl was stained after cell permebilization with 0.1% TX-100 using anti-HA antibodies and secondary Alexa Fluor-647 antibody conjugates. Imaging was performed using a Zeiss LSM 510-Meta confocal microscope and a 63×/1.4 numerical aperture oil-immersion objective. The ratio of colocalization was determined on randomly selected images (n=26–28) as total yellow signals over total green signals using NIH ImageJ software with the colocalizationRGB plug-in. Colocalization ratios were expressed as mean±SD. Statistical significance was performed using the Student's t-test for normally distributed data.
Acknowledgements
We are grateful to Gladys Tjørhom for the technical assistance. c-Cbl and c-Cbl 3Y-F were gifts from Dr. Alexander Tsygankov (Temple University, USA). This work was supported by The National Programme for Research in Functional Genomics (FUGE), The Research Council of Norway, The Norwegian Cancer Society, Novo Nordic Foundation Committee and the European Union (grant no 037189, thera-cAMP).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




