Continuous generation of colitogenic CD4+ T cells in persistent colitis
Abstract
Inflammatory bowel diseases take chronic courses due to the expansion of colitogenic CD4+ cells. However, it is unclear whether the persistent disease is driven by continuous reactivation of colitogenic memory CD4+ cells to generate effector CD4+ cells or by continuous generation of effector CD4+ cells from naïve cells. To clarify this issue, we performed a series of sequential adoptive transfers of Ly5.2+ and Ly5.1+ CD4+CD45RBhigh cells into RAG-2–/– mice at different time points. We show here that the secondarily transferred CD4+CD45RBhigh cells can be converted to CD4+CD44highCD62L–IL-7Rαhigh effector-memory T cells even in the presence of pre-existing effector-memory CD4+ cells. Although the total cell numbers of CD4+ cells in established colitic mice were consistently equivalent irrespective of the number of primarily transferred cells, the ratio of primarily and secondarily transferred cells was dependent on the ratio of the transferred cell numbers, but not on the order of the transfer. Of note, we found that primarily transferred CD4+ cells produced significantly lower amounts of IFN-γ and IL-17 than CD4+ cells arising from secondary transfer. In conclusion, the continuous generation of colitogenic CD4+ cells that compensate for exhausted CD4+ cells may be one of the mechanisms involved in the persistence of colitis.
Abbreviations:
-
- IBD:
-
inflammatory bowel disease
-
- LP:
-
lamina propria
-
- MLN:
-
mesenteric LN
-
- PB:
-
peripheral blood
-
- SP:
-
spleen
-
- TCM:
-
central-memory T
-
- TEM:
-
effector-memory T
Introduction
Intestinal mucosal surfaces are continuously exposed to antigens of the intestinal flora 1. However, the gut-associated immune system defends against systemic circulation of harmful intestinal antigens and induces systemic tolerance toward intestinal commensal antigens by various mechanisms including suppression by regulatory CD4+ T cells 2–7. In contrast, inflammatory bowel disease (IBD) is associated with activation of the local and systemic immune responses due to a lack of tolerance to intestinal bacterial antigens 6, 7. Although the etiology of IBD is uncertain, there is much evidence suggesting that the pathogenesis of IBD involves dysregulated recognition of the intestinal bacterial antigens, resulting in the generation of colitogenic CD4+ effector and memory T cells. Nevertheless, the nature of the colitogenic CD4+ T cells over time is not fully understood especially in terms of the perpetuation of chronic colitis.
In general, IBD progresses steadily or relapses after remission throughout life 8, 9. Although it is likely that the persistent disease is caused by the activation and expansion of colitogenic CD4+ effector T cells, several possible mechanisms may be involved. One possibility is that the persistency is driven by the initial colitogenic CD4+ memory T clones acting like memory stem cells 10 throughout the entire course of disease. In this case, colitogenic CD4+ effector T cells would be generated from colitogenic CD4+ memory T cells that are established at the initial onset, but are presumably suppressed by regulatory CD4+ T cells in remission 5, 7. However, this scenario has one obstacle, namely that memory T cells are believed to be generated for the first time after antigen clearance, but not in the persistent presence of antigens. Certainly, such is the case in models of chronic viral infections, such as lymphocytic choriomeningitis virus (LCMV) and influenza A virus infections 11. Since the possible antigens for colitogenic CD4+ T cells are derived from intestinal bacteria and/or self antigens in the intestine that are never eliminated from the body, it is doubtful whether colitogenic CD4+ memory T cells could actually be established in such a situation. A second possibility is that the same or different epitope-specific colitogenic CD4+ effector T cells are generated and expand after priming from newly recruited naïve CD4+ T cells from the thymus. In the case of multiple sclerosis, for example, it is thought that recruitment and activation of new autoimmune T cells evoke repeated disease episodes 12. Newly recruited T cells could recognize distinct autoantigenic epitopes on the same antigen, or even be specific for different autoantigens through the epitope-spreading cascade. In the case of persistent colitis, it is also unclear whether pre-existing colitogenic CD4+ effector or memory T cells prevent or ignore the priming, expansion, and phenotypic and functional conversion of newly recruited naïve CD4+ T cells to effector or memory CD4+ T cells. To evaluate these unsolved, but critical, issues, we performed sequential adoptive transfers of Ly5.1+ and Ly5.2+ CD4+CD45RBhigh T cells into immunodeficient RAG-2–/– mice at different time points to induce chronic colitis.
Results
Newly transferred CD4+CD45RBhigh cells are converted into effector-memory T cells in colitic mice
To assess the possibility that continuous generation of new colitogenic CD4+ T cells from naïve CD4+ T cells occurs routinely in colitic mice, we performed sequential adoptive transfers of Ly5.2+ and Ly5.1+ CD4+CD45RBhigh T cells into RAG-2–/– mice at different time points. To do this, we divided RAG-2–/– recipient mice into three groups (Fig. 1A): Group 1, RAG-2–/– mice transferred with Ly5.2+CD4+CD45RBhigh T cells at 0 wk; Group 2, RAG-2–/– mice transferred with Ly5.2+CD4+CD45RBhigh T cells at 0 wk and with Ly5.1+CD4+CD45RBhigh T cells at 5 wk; and Group 3, RAG-2–/– mice transferred with Ly5.1+CD4+CD45RBhigh T cells at 5 wk. Mice were observed for 10 wk after the first transfer of Ly5.2+CD4+CD45RBhigh T cells. In Group 1 and Group 2, mice developed colitis over time after the first transfer of Ly5.2+CD4+CD45RBhigh T cells, and ongoing clinical scores estimated by diarrhea with increased mucus in the stool, anorectal prolapse, hunched posture, and weight loss gradually increased after transfer, reaching to the maximum score 10 wk after the first transfer regardless of whether the second transfer was made (Fig. 1B). Similarly, Group 3 mice gradually developed colitis with time after the transfer of Ly5.1+CD4+CD45RBhigh T cells, and the ongoing clinical score reached a similar level to that of Groups 1 and 2 mice at 10 wk (Fig. 1B). Histological examination showed prominent epithelial hyperplasia with glandular elongation with a massive infiltration of mononuclear cells in the lamina propria (LP) of the colon from all groups of mice at 10 wk (Fig. 1C). Histological scorings revealed no significant differences between the three groups (Fig. 1D).
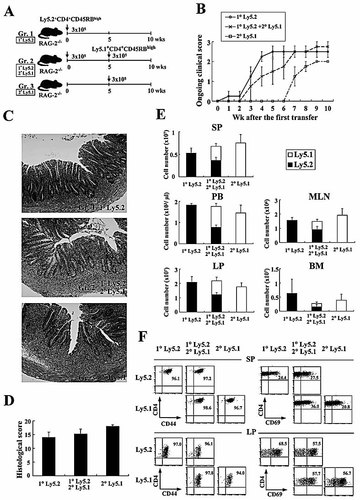
Newly recruited CD4+CD45RBhigh T cells are primed and converted to CD4+CD44high T cells. (A) Experimental design. C57BL/6-Ly5.2-RAG-2–/– mice were divided into three groups as described in the Materials and methods. (B) Ongoing clinical scores for the three groups were determined at the indicated times. (C) Histopathological findings of colon. Original magnification, ×100. (D) Histological scores were determined at 10 wk after the first transfer. (E) Recovered cell numbers of CD3+CD4+ T cells from SP, PB, LP, MLN, and BM. (F) Phenotypic characterization of SP and LP CD4+ T cells after the transfer of CD4+CD45RBhigh T cells. Representative results shown are from six mice per group.
A further quantitative evaluation of CD4+ T cell accumulation was made by isolating CD4+ T cells from various sites, such as spleen (SP), peripheral blood (PB), LP, mesenteric lymph nodes (MLN), and bone marrow (BM). Consistent with the similar clinical and histological severity of colitis between the three groups, the total recovered cell numbers in all sites were equivalent among the groups at 10 wk after the first transfer (Fig. 1E). Interestingly, in Group 2 mice, the ratio of Ly5.2+ and Ly5.1+ CD4+ T cells in all sites was almost 1:1, in accordance with the 1:1 ratio of the transferred cell numbers (Fig. 1E). Furthermore, we found that all the transferred CD4+CD45RBhigh T cells, whether Ly5.1+ or Ly5.2+, were converted to CD4+CD44high effector or memory cells both in SP and LP (Fig. 1F). In addition, substantial numbers of CD4+ T cells both in SP and LP, especially in LP, expressed the activation marker CD69 (Fig. 1F). These data indicated two findings. First, secondarily transferred Ly5.1+CD4+CD45RBhigh naïve T cells could be converted to CD4+CD44high effector or memory cells even in the presence of pre-existing Ly5.2+ effector or memory cells that had previously expanded in colitic mice. Second, newly (secondarily transferred) and previously (primarily transferred) established CD4+CD44high effector or memory cells compete with each other and the already present CD4+ T cells to occupy the space available to a constant number of CD4+ T cells in established chronic colitis at 10 wk.
Competition between colitic CD4+ T cells is dependent on the transferred cell numbers
To further assess the mechanism of the competition between the first (old) and the second (new) transferred CD4+ T cells in colitic mice, we next divided RAG-2–/– mice into three groups according to the number of Ly5.2+CD4+CD45RBhigh T cells transferred at the first transfer (Fig. 2A): Group 1, 3 × 104 cells (named “10^4”), Group 2, 3 × 105 cells (“10^5”), and Group 3, 3 × 106 cells (“10^6”). All groups of mice were secondarily transferred with the same number (3 × 105) of Ly5.1+CD4+CD45RBhigh T cells at 5 wk after the first transfer and killed at 10 wk after the first transfer. At 5 wk, the assessment of ongoing clinical scores revealed that Group 2 (“10^5”) and Group 3 (“10^6”) mice started to develop wasting disease and colitis, but the clinical severities of all groups were not significantly different at 5 wk after the first transfer (Fig. 2B), although the severity assessed by ongoing clinical scores of Group 1 (“10^4”) tended to be low. After the second transfer, all groups of mice progressively developed wasting disease and colitis to a similar extent until 10 wk after the first transfer (Fig. 2B). Like ongoing clinical scores, histological findings at 10 wk revealed that all groups of mice developed severe colitis with a massive infiltration of mononuclear cells in the LP (Fig. 2C), and the histological scores confirmed this finding in multiple colon sections (Fig. 2D).
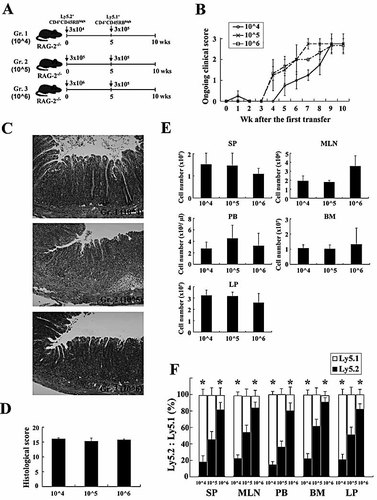
Newly generated CD4+CD44high T cells compete with pre-existing CD4+CD44high T cells depending on the transferred cell numbers. (A) Experimental design. C57BL/6-Ly5.2-RAG-2–/– mice were divided into three groups as described in the Materials and methods. (B) Ongoing clinical scores for the three groups were determined at the indicated times. (C) Histopathological findings of colon. Original magnification, ×100. (D) Histological scores were determined at 10 wk after the first transfer. (E) Recovered cell numbers of CD3+CD4+ T cells from SP, PB, LP, MLN, and BM. Cell numbers of Ly5.1+ or Ly5.2+ CD3+CD4+ T cells were determined by four-color flow cytometry. (F) Phenotypic characterization of SP, MLN, PB, BM and LP CD4+ T cells after the transfer of CD4+CD45RBhigh T cells. Results shown are from six mice per group. *p<0.05 vs. Ly5.2 ratio in same group.
Interestingly, the total cell numbers recovered from various sites at 10 wk were not significantly different between the groups in spite of the different numbers of the transferred cells in the first transfer (Fig. 2E), indicating that the space occupied by colitic CD4+ T cells in each tissue of established colitic mice is equivalent. Furthermore, the ratio of Ly5.2+ cells to Ly5.1+ cells in colitic mice at 10 wk was dependent on the ratio of the transferred cell numbers: it was less than unity in Group 1 (“10^4”) mice and greater than unity in Group 3 (“10^6”), whereas in Group 2 (“10^5”) mice transferred with the same numbers of Ly5.2+ cells and Ly5.1+ cells at different times, the ratio of these cell types was almost 1:1 at various sites (Fig. 2F).
We next examined whether the transferred CD4+CD45RBhigh T cells could be differentiated into effector or memory CD4+ T cells in this sequential transfer experiment. As shown in Fig. 3, almost all CD4+ T cells in any tissues, whether Ly5.1+ or Ly5.2+ cells, had a phenotype of CD4+CD44highCD62L–IL-7Rα+ effector-memory T (TEM)-like cells, in contrast to the originally transferred CD4+ CD45RBhighCD44low T cells. Interestingly, in Group 3 (“10^6”), which received a high number (3 × 106 cells/mouse) of CD4+ CD45RBhigh T cells at the first transfer, higher numbers of central-memory CD4+ T (TCM)-like cells were generated in the SP and the MLN both in Ly5.2+ cells and Ly5.1+ populations. This agrees with a recent report that precursor numbers can impact the differentiation and the homeostasis of the resultant memory cells 13.
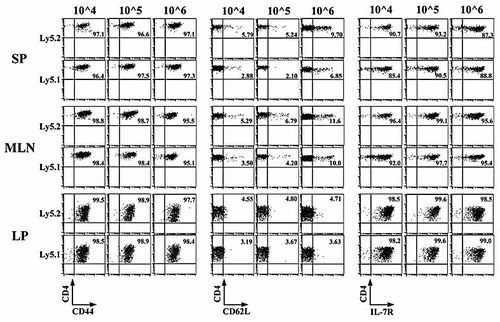
Phenotypic characterization of SP, MLN and LP CD4+ T cells after a sequential adoptive transfer as described in Fig. 2. Flow cytometric analysis shows that most of the transferred CD4+CD45RBhigh T cells in Group 1 (10^4), Group 2 (10^5), and Group 3 (10^6) mice have the characteristics of CD4+CD44highCD62L–IL-7Rαhigh TEM cells. Results shown are from six mice per group.
TCR Vβ repertoire is constant between first and second transferred cells
Although we found that the secondarily transferred CD4+CD45RBhigh T cells could differentiate into CD4+ TEM-like cells in vivo, even in the persistent presence of previously developed CD4+ TEM-like cells from the first transfer (Fig. 1–3), it was unclear whether newly recruited CD4+ T cells recognize the same antigenic epitopes as the previously recruited CD4+ T cells at the first transfer. To clarify this issue, splenic Ly5.1+ and Ly5.2+ CD4+ T cells from three groups were analyzed for their TCR Vβ repertoire by three-color flow cytometry. It is reasonable to use SP CD4+ T cells in place of LP CD4+ T cells for this assay, since we previously demonstrated that colitic SP CD4+ T cells had similar characteristics in that they were CD4+CD44highCD62L–IL-7Rαhigh TEM-like cells 14, and were also colitogenic cells by which colitis can be transferred to new recipient mice 15. As shown in Fig. 4, a polyclonal dominant TCR Vβ repertoire with dominant Vβ2, Vβ4, Vβ8.1/8.2, and Vβ14 was almost constant between previously and newly transferred cells, regardless of the different numbers of the first transfer, indicating that colitogenic CD4+ T cells recognizing the same or similar antigenic epitopes developed in accordance with the frequency or number of colitogenic antigen-specific naïve CD4+ T cells. In addition, the pattern of TCR Vβ repertoire in colitic mice, whether with older Ly5.2+ and newer Ly5.1+ CD4+ T cells, was similar to that of originally transferred CD4+CD45RBhigh T cells (Fig. 4).
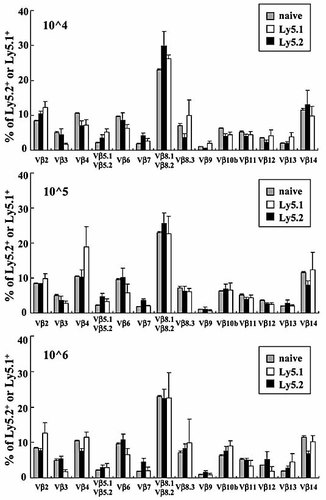
Flow cytometric analysis of Vβ families on the surface of the splenic CD4+ T cells in the Group 1 (10^4), Group 2 (10^5), and Group 3 (10^6) mice described in Fig. 2. To analyze the TCR Vβ family repertoire, splenic cells were four-color-stained with PerCP®-conjugated anti-CD3mAb, allophycocyanin-conjugated anti-CD4 mAb, PE-conjugate anti-Ly5.1 or Ly5.2 mAb, and the indicated mAb of a panel of 14 FITC-conjugated Vβ mAb. Each percentage value indicates the frequency of each Vβ (n=6). Naïve, CD4+CD45RBhigh T cells serves as a control.
Previously generated, older CD4+ T cells produce less IFN-γ and IL-17
It has recently been demonstrated that, during chronic viral infection, the functions of virus-specific CD8+ T cells often become impaired and exhausted in the persistent presence of viral antigens, in contrast to the highly functional effector and memory CD8+ T cells generated after virus clearance in acute infection 11, 16. Since colitogenic CD4+ T cells in colitic RAG-2–/– mice induced by an adoptive transfer of CD4+CD45RBhigh T cells are likely to expand by responding to resident enteric bacterial antigens that are persistently resident in the colonic lumen, it was possible that colitogenic CD4+ TEM-like cells gradually become exhausted over time after the transfer. To assess whether newly recruited TEM-like cells that are differentiated from the secondarily transferred CD4+CD45RBhigh T cells can compete and prevail against the previously established and possibly exhausted TEM-like cells in colitic mice, we next divided RAG-2–/– mice into two groups (Fig. 5A): Group 1, RAG-2–/– mice transferred with Ly5.2+CD4+CD45RBhigh T cells at 0 wk, and Group 2, RAG-2–/– mice transferred with Ly5.2+CD4+CD45RBhigh T cells at 0 wk, and secondarily transferred with Ly5.1+CD4+CD45RBhigh T cells at 13 wk after fully establishing colitis by the first transfer. Mice were observed for 17 wk after the first transfer.
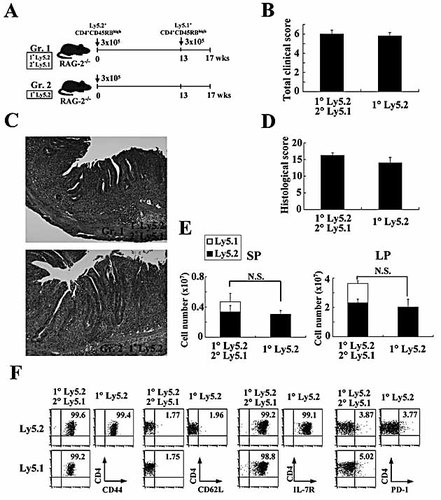
Pre-existing CD4+CD44high T cells are gradually exhausted. (A) Experimental design. C57BL/6-Ly5.2-RAG-2–/– mice were divided into two groups: Group 1, RAG-2–/– mice transferred with Ly5.2-derived CD4+CD45RBhigh T cells at 0 wk (n=6), and Group 2, RAG-2–/– mice transferred with Ly5.2-derived CD4+CD45RBhigh T cells at 0 wk, and again transferred with Ly5.1-derived CD4+CD45RBhigh T cells at 13 wk after the first transfer (n=6). Mice were observed for 17 wk after the first transfer. (B) Total clinical scores were determined at 17 wk after the first transfer as described in Materials and methods. Data are indicated as the mean ± SEM of six mice per group. (C) Histopathological findings of colon. Original magnification, ×100. (D) Histological scores were determined at 17 wk after the first transfer. Data are indicated as the mean + SEM of six mice in each group. (E) Recovered cell numbers of CD3+CD4+ T cells from SP and LP. The cell number of Ly5.1+ or Ly5.2+ CD3+CD4+ T cells was determined by four-color flow cytometry. Data are indicated as the mean + SEM of six mice per group. N.S., not significant. (F) Phenotypic characterization of LP CD4+ T cells after the transfer of CD4+CD45RBhigh T cells. Representative results shown are from one mice per group (six mice per group were analysed).
The assessment of clinical scores revealed that Group 1 and 2 mice developed wasting disease and severe colitis at the plateau level from approximately 6 wk after the first transfer and remained at this level until 13 wk after the first transfer without dropout by death (data not shown). At autopsy at 17 wk, the total clinical scores of both groups were not significantly different (Fig. 5B). Histological findings revealed that both groups of mice developed severe colitis with a massive infiltration of mononuclear cells in the LP (Fig. 5C), and histological scores also confirmed no differences between two groups in multiple colon sections (Fig. 5D). Interestingly, the cell numbers recovered from the SP and LP were not significantly different between the groups regardless of the second transfer (Fig. 5E), indicating again that the space occupied by colitogenic CD4+ T cells in each colitic mouse is equivalent. As shown in Fig. 5F, almost all LP CD4+ T cells in any tissues, whether old Ly5.2+ cells or new Ly5.1+ cells, had a phenotype of CD4+CD44highCD62L–IL-7Rα+ TEM-like cells.
Of note, we found that the old LP Ly5.2+ cells produced significantly less IFN-γ (Th1) and IL-17 (Th17) cytokines than the new LP Ly5.1+ cells (Fig. 6A and B), indicating that the old LP Ly5.2+ cells long after the first transfer had fallen into exhaustion. In addition, the ratios of IL-17+, but not IFN-γ+, cells in the old MLN and SP Ly5.2+CD4+ cells were significantly decreased as compared with those of new Ly5.1+ cells (Fig. 6A and B), suggesting that colitogenic CD4+ T cells in various sites became exhausted over time. In particular, the LP shows the greatest level of cell exhaustion, at least in the expression of IFN-γ. Since it has been reported that PD-1 is one of markers for exhausted CD8+ T cells in chronic LMCV infection in mice and HIV infection in human 17–19, we also checked this molecule in old Ly5.2+ cells and new Ly5.1+ cells. Contrary to the above data of cytokine production and previous reports of exhausted PD-1-expressing CD8+ T cells 17–19, no difference in PD-1 expression was found between old Ly5.2+ cells and new Ly5.1+ cells at 17 wk (Fig. 5F), indicating that PD-1 is not an appropriate marker for the exhaustion for murine CD4+ T cells, at least in the present time course of the sequential adoptive transfer protocol.
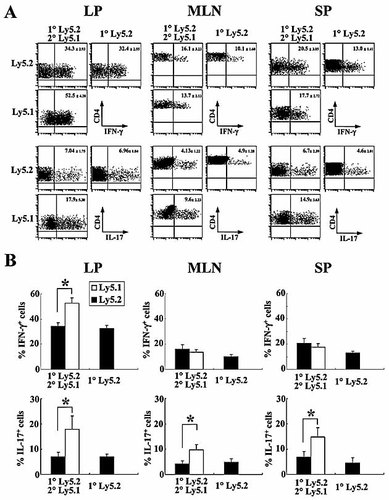
Preexisting CD4+CD44high T cells are gradually exhausted. (A) Expression of IFN-γ and IL-17 on freshly isolated cells from LP, MLN, and SP in the Group 1 and Group 2 mice described in Fig. 5. Cells were labeled for Ly5.1, Ly5.2, CD4, and intracellular IFN-γ or IL-17. Ly5.2+ and Ly5.1+ CD4+ cells were gated and analyzed for the presence of CD4+IFN-γ+ cells or CD4+IL-17+. Number in upper quadrant represents the percentage of IFN-γ+ or IL-17+ cells among CD4+ cells. (B) The ratios of IFN-γ+ or IL-17+ cells among Ly5.1+ or Ly5.2+ cells were analyzed by gating Ly5.1 or Ly5.2 on CD4+ cells. Results shown are from six mice per group. *p<0.05.
Discussion
In the present study, we demonstrated that newly recruited naïve CD4+CD45RBhigh T cells can be primed, expand and differentiate into CD4+CD44highCD62L–IL-7Rαhigh TEM-like cells in colitic RAG-2–/– mice induced by adoptive transfer of CD4+CD45RBhigh T cells in competition with previously established colitogenic TEM-like CD4+ T cells. Of note, the patterns of TCR Vβ repertoire were constantly similar between the first and the second transfers, indicating that a set of colitogenic polyclonal CD4+ T cells compete for each clone-specific survival signal. This is most easily explained by TCR recognition of a specific and limiting antigen epitope-MHC class II ligand and homeostatic cytokines, such as IL-7 20, 21. Furthermore, we found that old LP CD4+ TEM-like cells produced less IFN-γ and IL-17 than newly developed LP CD4+ TEM-like cells, suggesting that old colitic LP CD4+ T cells showed immunological exhaustion over time. These findings suggest that continuous generation of colitogenic CD4+ T cells from newly recruited naïve CD4+ T cells to compensate for the older exhausted CD4+ T cells is one of the mechanisms sustaining chronic colitis.
Although IBD is thought to be caused by colitogenic effector CD4+ T cells, which probably respond to intestinal bacterial antigens and damage the target intestine, the nature of the colitogenic CD4+ T cells over time remains largely unclear. How are these colitogenic CD4+ T cells generated and maintained in the body of patients with IBD? Are these colitogenic CD4+ T cells just effector CD4+ T cells, which are generated from naïve CD4+ T cells or from colitogenic memory CD4+ T cells acting like memory stem cells 10, and which are established at the initial attack and reside in the body throughout life? This question arises basically from the concept that ‘true’ memory T cells are established for the first time after antigen clearance from the body as often assessed in memory CD8+ T cells in animal models of acute viral infection and vaccination 22–25. According to this scenario, it seems that colitogenic ‘true’ memory CD4+ T cells cannot be built up in our colitis model, since the possible target intestinal bacteria are never eliminated, but persist throughout life in chronic colitis both in animal models and human IBD. However, recent evidence suggests that maintenance of the CD8+ and CD4+ T cells in chronic infection is dependent on antigens 26, 27, although it seems inappropriate to call such cells memory cells. We here showed that colitic CD4+ T cells of this transfer model strongly expressed CD44 and IL-7Rα, which is a reliable marker for memory, but not effector, CD4+ T cells; and also we recently demonstrated that IL-7, which is an important factor for survival of memory CD4+ T cells 21, is essential for the persistence of colitis by showing that IL-7–/– × RAG-1–/– mice transferred with colitogenic LP CD4+CD44highIL-7Rαhigh cells did not develop colitis 28. Since the survival of memory, but not effector, CD4+ T cells is believed to be dependent on IL-7 21, our results suggest that colitogenic CD4+CD44highCD62L–IL-7Rαhigh TEM cells are sustained at least in part in colitic mice even in the persistent presence of intestinal bacteria. Consistent with the present findings, we recently demonstrated that substantial numbers of colitogenic CD4+CD44highCD62L–IL-7Rαhigh TEM-like cells reside in colitic BM, which is believed to lack intestinal bacterial antigens but produce IL-7 28.
Naïve CD4+ T cells are known to proliferate extensively, probably in response to foreign or self antigens, and are converted to memory-like CD4+CD44high T cells in lymphopenic immunodeficient mice, such as SCID and RAG-2–/– mice, in the process of ‘lymphopenia-driven proliferation’ 29. Thus, it is likely that CD4+CD44highIL-7Rαhigh cells in CD4+CD45RBhigh T cell-transferred colitic SCID mice represent memory-like cells, but not ‘true’ memory cells. Otherwise, it is also possible that the IL-7-dependent colitogenic CD4+ T cells in the presence of intestinal bacterial antigens in colitic mice have unique characteristics that differentiate them from conventional effector or ‘true’ memory CD8+ and CD4+ T cells defined mainly by studies of models of acute viral infection. Further studies will be needed to address this issue.
Colitogenic CD4+ T cells may also be continuously generated from naïve CD4+ T cells that reside in the periphery or are newly generated from the thymus, because it is still unknown whether memory CD4+ T cells, either ‘true’ memory or memory-like (TEM-like) cells, are long-lived. Thus, it was of interest to determine whether the generation of new colitogenic CD4+ T cells from naïve CD4+ T cells is inhibited by the pre-existence of colitogenic CD4+ T cells, and also whether it is involved in the persistence of disease, although it is known that adoptively transferred naïve CD4+ T cells extensively proliferate and are converted to TEM-like CD4+ T cells presumably in response to intestinal bacteria under lymphopenic conditions like those in immunodeficient SCID and RAG-2–/– mice 25. In a setting in the absence of antigens, it has recently been demonstrated that pre-existing ‘true’ memory CD4+ T cells in RAG-2–/– mice after adoptive transfer prevent the proliferation and conversion of newly transferred naïve CD4+ T cells to the memory-like phenotype 30. Furthermore, the same group previously demonstrated that RAG–/– mice transferred with a large number of naïve CD4+CD45RBhigh T cells (12 × 106 cell/mouse) do not develop colitis 31. This finding indicates that two mechanisms, cytokine (IL-7) competition and clonal competition, may restrain the activation and overgrowth of a small number of transferred naïve T cells in the lymphopenic condition. Nevertheless, the fact that newly recruited naïve CD4+CD45RBhigh T cells can be activated and expand in established colitic mice, in which space available to them should already be occupied by a large number of colitogenic CD4+ T cells, may suggest the older colitogenic effector or memory CD4+ T cells are exhausted and permit the expansion of newly recruited colitogenic CD4+ T cells in our model. By contrast, surprisingly, we found that newly recruited CD4+CD45RBhigh T cells can be primed and expand extensively even in established colitic mice in which pre-existing colitogenic CD4+ T cells were fully expanded and occupied the space of CD4+ T cells. Thus, our present results may link the frequent recurrence in natural history of IBD to the continuous generation of colitogenic CD4+ effector and memory T cells, because the frequency of recurrence gradually decreases in accordance with immunosenescence in patients with a decreased supply of naïve T cells by thymic involution. Interestingly, in addition, the total cell number of CD4+ T cells was constant, and the ratio of pre-existing and newly recruited CD4+CD44high T cells in the body was dependent on the ratio of the transferred cell numbers, indicating that both old and new CD4+ T cells compete for space and a possibly constant amount of IL-7 depending on the frequency of transferred colitogenic naïve CD4+ T cells.
Of note, we also demonstrated that production of IFN-γ and IL-17 by older LP CD4+CD44highT cells was significantly less as compared with newly developed LP CD4+CD44highT cells (Fig. 5), indicating that these LP CD4+CD44highIL-7Rαhigh cells become exhausted over time, as seen in CD8+ T cells in persistent virus infection 17, 18. Thus, it appears that colitogenic LP CD4+ T cells in colitic mice are a mixture of IL-7-dependent CD4+ TEM-like cells and exhaustion-facing effector cells derived from these CD4+ TEM-like cells after encountering intestinal bacterial antigens. Consistent with this, the most affected site of cell exhaustion as indicated by cytokine production seemed to be the LP (Fig. 6), which is thought to be the effector site. Nevertheless, it remains unknown why mice transferred secondarily with CD4+CD45RBhigh T cells did not show exacerbation of the disease as compared with mice without the second transfer in clinical and histological evaluations (Fig. 5B and D). As a clue, we recently performed over seven sequential transfers of colitic LP CD4+ cells obtained from colitic CD4+CD45RBhigh cell-transferred SCID mice into new SCID mice. Although SCID mice transferred with colitic LP CD4+ cells stably developed colitis over several transfers, the severity of colitis declined with the increasing number of transfers [32]. Thus, it is likely that the exhaustion of CD4+ T cells requires longer to become clinically and histologically evident than does the decline in cytokine production. Further study will be needed to address this issue.
In summary, we propose that continuous generation of colitogenic CD4+ T cells from naïve CD4+ T cells is critically involved in the persistence of chronic colitis, suggesting that it is important not only to target the pre-existing colitogenic CD4+ T cells but also to suppress and control the new generation of colitogenic CD4+ T cells in developing a strategy for the treatment of IBD.
Materials and methods
Animals
C57BL/6N-Ly5.2 mice were purchased from Japan Clea (Tokyo, Japan). C57BL/6N -Ly5.1 and C57BL/6N -Ly5.2-RAG-2 deficient (RAG-2–/–) mice were obtained from Taconic Laboratory (Hudson, NY) and Central Laboratories for Experimental Animals (Kawasaki, Japan). Mice were maintained under specific pathogen-free (SPF) conditions in the Animal Care Facility of Tokyo Medical and Dental University. All donors and recipients were used for adoptive transfer experiments at 6–10 wk of age. All experiments were approved by the regional animal study committees (permission number: 2006–049) and were done according to institutional guidelines and Home Office regulations.
Antibodies
The mAb other than biotin-conjugated anti-mouse IL-7Rα (A7R34; eBioscience, San Diego) were obtained from BD PharMingen (San Diego, CA) and used for purification of cell populations and flow cytometry analysis; 145–2C11, FITC-, PE- and PerCP®-conjugated anti-mouse CD3; RM4–5, PE- and allophycocyanin-conjugated anti-mouse CD4; 16A, FITC-conjugated anti-mouse CD45RB; IM7, allophycocyanin-conjugated anti-mouse CD44; MEL-14, PE-conjugated anti-mouse CD62L; H1.2F3, FITC- and PE-conjugated anti-mouse CD69; A20, FITC- and PE-conjugated anti-mouse Ly5.1 (CD45.1); 104, FITC-conjugated anti-mouse Ly5.2 (CD45.2); J43, PE-conjugated anti-mouse PD-1; XMG1.2, PE-conjugated anti-mouse IFN-γ; TC11–18H10, PE-conjugated anti-mouse IL-17; B20.6, FITC-conjugated anti-mouse Vβ2; KJ25, FITC-conjugated anti-mouse Vβ3; KT4, FITC-conjugated anti-mouse Vβ4; MR9–4, FITC-conjugated anti-mouse Vβ5.1/2; RR4–7, FITC-conjugated anti-mouse Vβ6; TR310, FITC-conjugated anti-mouse Vβ7; MR5–2, FITC-conjugated anti-mouse Vβ8.1/2; B21.14, FITC-conjugated anti-mouse Vβ8.3; MR10–2, FITC-conjugated anti-mouse Vβ9; B21.5, FITC-conjugated anti-mouse Vβ10b; RR3–15, FITC-conjugated anti-mouse Vβ11; MR11–1, FITC-conjugated anti-mouse Vβ12; IN12.3, FITC-conjugated anti-mouse Vβ13; 14.2, FITC-conjugated anti-mouse Vβ14. Biotinylated antibodies were detected with PE-streptavidin (BD PharMingen).
T cell preparation
For isolation of peripheral lymphocytes, 600 µL PB was collected from each mouse and diluted 1:1 with PBS. The diluted blood was layered over Lymphosepar II (IBL, Gunma, Japan) and centrifuged at 400 × g for 30 min at room temperature. The lymphocytes were then isolated from the plasma-Ficoll interface. SP and MLN were mechanically disrupted into single cell suspensions. BM was collected from the femur by flushing with sterile PBS. For the preparation of colonic LP cells 32, colon was first flushed extensively to eliminate the lumen content, then longitudinally opened and cut into small pieces. The dissected mucosa was incubated with Ca2+ Mg2+-free Hanks’ BSS containing 1 mM DTT (Sigma-Aldrich, St. Louis, MO) for 30 min to remove mucus, then treated with 3 mg/mL collagenase (Roche Diagnostics GmbH, Germany) and 0.01% DNase (Worthington Biomedical Co., Freehold, NJ) for 2 h. After filtering through gauze, cells were pelleted two times through a 40% isotonic Percoll solution, and then subjected to Ficoll-Hypaque density gradient centrifugation (40%/75%). Enriched CD4+ T cells were obtained by positive selection using anti-CD4 (L3T4) MACS magnetic beads. The resultant cells contained >94% CD4+ cells when analyzed by FACSCalibur.
Adoptive transfer protocols
In the first set of adoptive transfer experiments, we divided C57BL/6-Ly5.2-RAG-2–/– mice into three groups: Group 1, RAG-2–/– mice transferred with 3 × 105 Ly5.2-derived CD4+CD45RBhigh T cells at 0 wk of the starting point (n=6); Group 2, RAG-2–/– mice transferred with 3 × 105 Ly5.2-derived CD4+CD45RBhigh T cells at 0 wk and with 3 × 105 Ly5.1-derived CD4+CD45RBhigh T cells at 5 wk after the first transfer (n=6); Group 3, RAG-2–/– mice transferred with 3 × 105 Ly5.1-derived CD4+CD45RBhigh T cells at 5 wk after the starting point (n=6). Briefly, CD4+ T cells were isolated from splenocytes using the anti-CD4 (L3T4) MACS magnetic separation system (Miltenyi Biotec, Auburn, CA). Enriched CD4+ T cells were labeled with PE-conjugated anti-mouse CD4 mAb and FITC-conjugated anti-CD45RB mAb, then sorted to yield the CD45RBhigh (highest staining 30%) fraction on a FACS Aria (Becton Dickinson, Sunnyvale, CA). Each mouse was injected intraperitoneally with 3 × 105 CD4+CD45RBhigh T cells once. Mice were observed and killed at 10 wk after the starting point.
In the second set of adoptive transfer experiments, we divided C57BL/6-Ly5.2-RAG-2–/– mice into three groups: Group 1, RAG-2–/– mice transferred with 3 × 104 Ly5.2+CD4+CD45RBhigh T cells at 0 wk (“10^4”, n=6); Group 2, RAG-2–/– mice transferred with 3 × 105 Ly5.2+CD4+CD45RBhigh T cells at 0 wk (“10^5”, n=6); and Group 3, RAG-2–/– mice transferred with 3 × 106 Ly5.2+CD4+CD45RBhigh T cells at 0 wk (“10^6”, n=6). All groups of mice were transferred with 3 × 105 Ly5.1+CD4+CD45RBhigh T cells at 5 wk after the first transfer. Mice were killed at 10 wk after the starting point.
In the third set of adoptive transfer experiments, we divided C57BL/6-Ly5.2-RAG-2–/– mice into two groups: Group 1, RAG-2–/– mice transferred with 3 × 105 Ly5.2+CD4+CD45RBhigh T cells at 0 wk of the starting point (n=6); and Group 2, RAG-2–/– mice transferred with 3 × 105 Ly5.2+CD4+CD45RBhigh T cells at 0 wk and with 3 × 105 Ly5.1+CD4+CD45RBhigh T cells at 13 wk after the first transfer (n=6). Mice were skilled at 17 wk after the starting point.
Disease monitoring and clinical scoring
The recipient mice after T cell transfer were weighed initially, then three times per week thereafter. They were observed for clinical signs of illness: hunched appearance, piloerection of the coat, diarrhea, and blood in the stool. Mice were killed at the indicated times and assessed for a total clinical score as the sum (0–6 points) of three parameters: hunching and wasting, 0 or 1; colon thickening, 0–3 (0, no colon thickening; 1, mild thickening; 2, moderate thickening; 3, extensive thickening); and stool consistency, 0–2 (0, normal beaded stool; 1, soft stool; 2, diarrhea). To monitor clinical signs during the observation period, the ongoing clinical score is defined as the sum (0–3 points) of the two parameters other than colon thickening 33.
Histological examination
Tissue samples were fixed in PBS containing 10% neutral-buffered formalin. Paraffin-embedded sections (5 μm) were stained with H&E. The sections were analyzed without prior knowledge of the type of T cell reconstitution and recipients. The area most affected was graded by the number and severity of lesions. The mean degree of inflammation in the colon was calculated using a modification of a previously described scoring system 33.
Flow cytometry
To detect the surface expression of a variety of molecules, isolated cells were preincubated with an FcγR-blocking mAb (CD16/32; 2.4G2, BD PharMingen) for 20 min followed by incubation with specific FITC-, PE-, PerCP-, allophycocyanin-, or biotin-labeled antibodies for 30 min on ice. Biotinylated antibodies were detected with PE-streptavidin. For intracellular staining for IFN-γ and IL-17 34, cells were stimulated with 50 ng/mL phorbol-12-myristate-13 acetate (PMA; Calbiochem, CA) and 500 ng/mL ionomycin (Sigma-Aldrich) for 10 h, then 5 μg/mL brefeldin A (GolgiPlug; BD PharMingen) was added. Cells were first preincubated with FcγR-blocking mAb for 20 min, and then stained with PerCP-anti-CD3 mAb, allophycocyanin-anti-CD4 mAb, and FITC-anti-CD45.1 or anti-CD45.2 mAb. The stimulated cells were fixed and permeabilized with Cytofix/CytopermTM (BD PharMingen) at 4°C for 30 min. Staining and washing were performed in Perm/Wash BufferTM (BD PharMingen), and cells were stained with PE-conjugated anti-IFN-γ or anti-IL-17 mAb.
Statistical analysis
The results were expressed as the mean ± SEM. Groups of data were compared by Mann-Whitney U test. Differences were considered to be statistically significant when p<0.05.
Acknowledgements
This study was supported in part by grants-in-aid for Scientific Research, Scientific Research on Priority Areas, Exploratory Research and Creative Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology; the Japanese Ministry of Health, Labor and Welfare; the Japan Medical Association; Foundation for Advancement of International Science; Terumo Life Science Foundation; Ohyama Health Foundation; Yakult Bio-Science Foundation; Research Fund of Mitsukoshi Health and Welfare Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
The authors declare no financial of commercial conflict of interest.