Activation-induced cytidine deaminase induces DNA break repair events more frequently in the Ig switch region than other sites in the mammalian genome
Abstract
Activation-induced cytidine deaminase (AID) produces DNA breaks in immunoglobulin genes during antibody diversification. Double-stranded breaks (DSB) in the switch region mediate class switch recombination, and contribute to gene conversion and somatic hypermutation in the variable regions. However, the relative extent to which AID induces DSB in these regions or between these and other actively expressed sequences is unknown. Here, we exploited an enhancer-trap plasmid that identifies DSB in actively expressed loci to investigate the frequency and position of AID-induced vector integration events in mouse hybridoma cells. Compared to control cells, wild-type AID stimulates plasmid integration into the genome by as much as 29-fold. Southern and digestion-circularization PCR analysis revealed non-uniformity in the integration sites, with biases of 30- and 116-fold for the immunoglobulin κ light chain and μ heavy chain genes, respectively. Further, within the immunoglobulin μ gene, 73% of vector integrations map to the μ switch region, an enhancement of five- and 12-fold compared to the adjacent heavy chain variable and μ gene constant regions, respectively. Thus, among potential highly transcribed genes in mouse hybridoma cells, the immunoglobulin heavy and light chain genes are important AID targets, with the immunoglobulin μ switch region being preferred compared to other genomic sites.
Abbreviations:
-
- AID:
-
activation-induced cytidine deaminase
-
- Cμ region:
-
μ gene constant region
-
- CSR:
-
class switch recombination
-
- DC-PCR:
-
digestion-circularization PCR
-
- DSB:
-
double-stranded break
-
- neo:
-
neomycin phosphotransferase gene
-
- SHM:
-
somatic hypermutation
-
- Sμ region:
-
μ gene switch region
-
- SSB:
-
single-stranded break
-
- VH:
-
heavy chain variable region
Introduction
Activation-induced cytidine deaminase (AID) is essential for three distinct processes crucial to the generation of immunoglobulin (Ig) diversity: somatic hypermutation (SHM), gene conversion and class switch recombination (CSR) 1. SHM and gene conversion increase antibody-binding affinity by introducing sequence changes into the antibody variable region in stimulated B cells (reviewed in 2, 3). CSR produces antibodies with new biological effector functions by targeting antibody switch regions, and through a recombination-mediated event, pairs the expressed heavy chain variable (VH) region with downstream constant regions (reviewed in 4). While these processes normally depend on AID expression in activated B cells 1, 5, 6, they can be induced to occur in cultured cell lines upon expression of AID 5, 7, 8.
The processes of SHM, CSR and gene conversion are thought to depend on the generation of specific DNA lesions that can include nucleotide mismatches, abasic sites, single-stranded breaks (SSB), double-stranded breaks (DSB) and the intervention of translesional (error-prone) DNA polymerases 9–12. DSB in the switch region appear critical for CSR, but they also occur in the variable region, contributing to gene conversion and possibly SHM 13–17. In fact, two recent studies show that DSB in the variable region are necessary intermediates in gene conversion 18, 19. DNA DSB are thought to arise from cellular mismatch repair activities acting on U:G mismatches formed when AID deaminates closely spaced cytidines on opposite chains in single-stranded DNA (reviewed in 20).
AID is considered to mutate actively expressed genes 21–23, in particular the Ig variable and switch region sequences as discussed above. A previous study 24 showed that AID-induced DNA repair foci co-localized with the Ig switch region. However, cells may utilize common DNA repair foci 25, 26, suggesting that other sites in addition to the switch region may be damaged by AID. Other studies have shown that AID induces DSB in Ig sequences 13, 15, 17, but did not address whether other genomic sites were prone to AID-induced DNA breaks.
Sequencing studies of a small number of genes selected on the basis of their requirement for B cell development and/or their involvement in oncogenic translocations suggested that Bcl-6 27, 28, CD95 29, c-myc, PIM1, RhoH, PAX5 30, CD79a and CD79b 31 are also targets of AID mutation. Although these studies suggest that AID induces DNA breaks in Ig and non-Ig sequences, the frequency with which AID generates breaks within different Ig gene sub-regions and how this compares to other genomic loci is unknown. This information has important implications for the oncogenic potential of AID, given that the failure to repair DNA breaks can lead to cell death, and aberrant DNA break repair can result in translocations, which are hallmarks of B cell neoplasia.
Because previous approaches are not feasible in a genome-wide screen, we adopted an alternate procedure to identify genes targeted by AID. The approach exploits an enhancer-trap pSV2neo 32-based vector system that generates stable G418-resistant (G418R) transformants following integration into actively expressed genes 33. Based on the knowledge that vector integration is stimulated by DSB 34, 35, and as reviewed above, on the preference for AID to introduce breaks in actively expressed sequences, we tested the hypothesis that AID expression would increase the frequency of stable transformants with the enhancer-trap plasmid, and further, that analysis of vector integration would provide information on genomic sites targeted by AID. Our studies support this hypothesis and reveal that among potential highly expressed genomic sites, the Ig heavy (IgH) and light chain genes are important AID targets, with the IgH µ gene switch (Sμ) region being preferred over the adjacent VH and µ constant (Cµ) regions, and other sites in the genome.
Results
Experimental system and rationale
This study examines the capacity of AID-expressing hybridoma cells to stably incorporate transfected enhancer-trap plasmid DNA, with the additional objective of mapping AID-induced vector integration sites within the genome. The enhancer-trap plasmids used in this study are derivatives of pSV2neo 32 in which the SV40 early region enhancer facilitating neomycin phosphotransferase (neo) gene expression is deleted 33. The enhancer-trap feature enriches for genomic integration sites that are active in promoting neo gene expression 33. Since DSB stimulate integration of transfected plasmid DNA 34, 35 and AID is thought to target DSB to actively expressed genes 21, 22, 36, we reasoned that this vector system would yield an increased frequency of stable transformants in AID-expressing cells, and that analysis of vector integration events would identify genomic sites that are subject to AID cleavage.
Increased stable transformation frequency in hybridoma cells expressing wild-type AID
Recipient Sp6/HL hybridoma cells were transfected with pCEP4hAID 8 or pCEP4hAIDC90A, a construct containing a catalytically inactive mutant of AID 37, and hygromycin-resistant (HYGR) transformants were screened by RT-PCR to identify those expressing AID. Twelve AID-expressing clones were recovered (data not shown). Western analysis (Fig. 1) confirmed the expected AID protein in three cell lines expressing wild-type AID mRNA (Sp6-10, Sp6-12, Sp6-22; lanes 3–5), and in three cell lines expressing catalytically inactive AID C90A mRNA (Sp6-3, Sp6-8, Sp6-31; lanes 6–8), but not in recipient Sp6/HL cells (lane 1), or in a HYGR transformant in which AID mRNA is absent (Sp6-1, lane 2). The levels of wild-type or mutant AID expression were quantified using densitometry and the respective AID/β-actin ratios are shown beneath each lane.
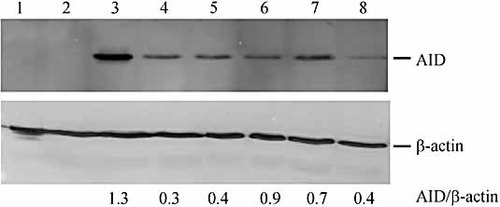
Analysis of AID expression. Western blot analyses for AID and β-actin expression in the parental Sp6/HL hybridoma and transfected clones. The blot presents a representative sample of cell lines: lane 1, wild-type Sp6/HL hybridoma; lane 2, the non-AID expressing HYGR transformant Sp6-1; lanes 3–5, the wild-type AID-expressing HYGR transformants Sp6-10, Sp6-12 and Sp6-22; lanes 6–8, catalytically inactive AID-expressing HYGR transformants Sp6-3, Sp6-8 and Sp6-31. The AID/β-actin ratio is shown beneath each lane.
Cell lines expressing wild-type or mutant AID were transfected with the uncut 9.6-kb enhancer-trap plasmid vector, pTCμEn– (Fig. 2) 38. Initial experiments utilized this vector in order to assess whether AID-induced breaks in the Cμ region stimulate vector integration by homologous recombination. In later studies (described below), transfections were performed with the enhancer-trap pSV2neo vector alone. Following transfection with uncut pTCμEn–, the frequency of stable G418R transformants was determined (Fig. 3A). Wild-type AID-expressing cell lines (Sp6-10, Sp6-12 and Sp6-22) revealed a significant stimulation in the frequency of stable transfection compared to non-AID-expressing Sp6-1 cells or recipient Sp6/HL cells. In contrast, no significant differences in transfection efficiencies were observed between Sp6/HL and cell lines Sp6-3, Sp6-8 and Sp6-31 expressing catalytically inactive AID. In general, the stable incorporation of transfected vector correlates with the level of expression of wild-type AID: cell line Sp6-10, in which densitometric analysis reveals an AID/β-actin ratio of 1.3, features a 29-fold stimulation in transfection efficiency, followed by cell line Sp6-22 (AID/β-actin ratio = 0.4) and cell line Sp6-12 (AID/β-actin ratio = 0.3) with 18-fold and threefold stimulations, respectively.
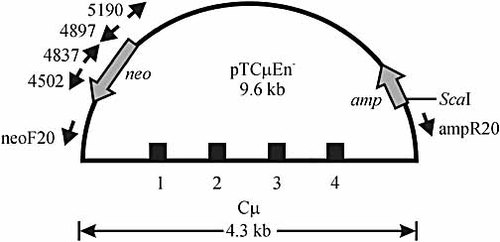
Structure of the enhancer-trap pTCμEn– vector. The 9.6-kb enhancer-trap pTCμEn– plasmid vector contains a 4.3-kb XbaI segment from the mouse IgH Cμ region inserted into a derivative of pSV2neo 32, from which the 372-bp NsiINdeI segment encompassing the SV40 early region enhancer responsible for expression of the neo gene is deleted 33. The ScaI site used for pTCμEn– linearization is shown. PCR primers neoF20, 4897, 5190, 4837, 4502 and ampR20 were used in the analysis of vector integration junctions and bind specifically to pSV2neo backbone sequences beginning at nucleotide positions 268, 2673, 2985, 2632, 2278 and 5643, respectively (refer to Materials and methods and Fig. 5, 6 for further details). Cμ exons 1–4 are indicated; amp, β-lactamase gene.
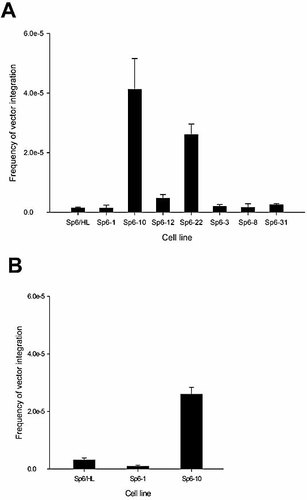
Effect of AID expression on plasmid transfection efficiency. Uncut (A) or ScaI-linearized pTCμEn– (B) vector DNA was transfected into recipient hybridoma cell lines, and the efficiency of generating stable G418R transformants was determined. The mean ± SEM of two to five replicate transfections is presented for each cell line.(A) One-way ANOVA of log-transformed data reveals a significant difference in transfection frequency between cell lines expressing wild-type AID (F=21.92, p<0.0001). To determine the nature of the differences, the means were compared using a least square means contrast, which revealed no significant difference in transfection frequency between Sp6/HL and Sp6-1 (F=0.38, p=0.55) but a significantly higher transfection efficiency in Sp6-10 (F=60.13, p<0.0001), Sp6-12 (F=5.20, p=0.046) and Sp6-22 (F=31.61, p<0.0001) when compared to Sp6/HL. In contrast, one-way ANOVA comparing Sp6/HL to cell lines Sp6-3, Sp6-8 and Sp6-31 expressing catalytically inactive AID showed no significant difference between transfection efficiencies (F=0.72, p=0.57). (B) The difference in transfection efficiencies was significant according to one-way ANOVA (F=60.24, p<0.0001). A comparison of means using least square means contrasts showed that while there is no significant difference between the frequency of transfection in Sp6/HL and Sp6-1 (F=0.56, p=0.48), the frequency was significantly higher in Sp6-10 compared to Sp6/HL (F=93.93, p<0.0001).
While consistent with AID-induced genomic DSB stimulating incorporation of transfected uncut vector, the possibility that AID cleavage of the circular vector might enhance integration into pre-existing genomic breaks was considered. In this case, little or no stimulation in transformation efficiency would be expected following transfection of AID-expressing cells with linearized (ScaI-digested) vector. Although somewhat lower in comparison to uncut DNA, in AID-expressing Sp6-10 cells, a significant 13-fold higher frequency of stable transformants was observed compared to control Sp6/HL or Sp6-1 cells (Fig. 3B). Thus, we conclude that AID-induced cleavage of the genome provides sites for integration of transfected vector DNA.
The chromosomal IgH μ gene switch region is a preferred site of vector integration
To investigate whether genomic integration sites include the chromosomal IgH μ locus, 48 and 25 G418R transformants recovered from the AID-expressing Sp6-10 and Sp6-22 hybridoma cell lines, and 55 G418R transformants recovered following transfection of Sp6/HL and Sp6-1 hybridoma cell lines were examined by Southern analysis 39 according to the mapping strategy in Fig. 4. The enzymes PacI and PaeR7I do not reside within the pTCμEn– vector, and therefore, vector integration into the IgH µ gene is expected to alter the size of the endogenous 14.7-kb PacI/PaeR7I fragment detected with µ-specific probe N.
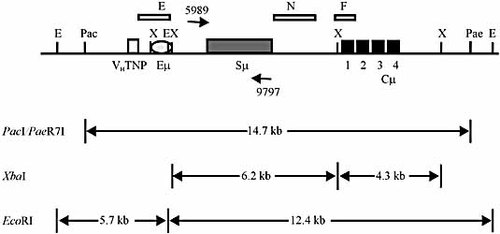
Southern analysis of the Ig chromosomal μ gene. The structure of the endogenous TNP-specific chromosomal IgH μ locus in the Sp6/HL mouse hybridoma cell line 38 is presented. The sizes (in kb) of fragments generated by digestion of genomic DNA with the enzyme combination PacI/PaeR7I, or the individual enzymes XbaI or EcoRI followed by hybridization with probe fragments E, N and F are indicated. Probe E is a 1983-bp BamHI/EcoRI fragment from unrearranged μ genomic DNA; probe fragment N consists of adjacent 475-bp and 495-bp NheI fragments; probe F is an 870-bp XbaI/BamHI fragment specific for the Cμ region. PCR primers designated 5987 and 9797 serve in the analysis of unique Sμ:vector integration junctions and bind specifically 5′ and 3′ of the chromosomal Sμ region, respectively (refer to Materials and methods and Fig. 5 for further details). Cμ exons 1–4 are indicated; E, EcoRI restriction enzyme site; X, XbaI restriction enzyme site. The diagram is not drawn to scale.
Southern analysis revealed disruption of the endogenous IgH μ locus in only 6/55 G418R transformants (11%) recovered from the Sp6/HL or Sp6-1 cell lines not expressing AID. In contrast, in the AID-expressing Sp6-10 and Sp6-22 cell lines, the IgH μ locus is disrupted in 25/48 (52%) and 8/25 (32%) of the G418R transformants, respectively, i.e. an approximately fourfold increase. Thus, when considered with the aforementioned 29-fold stimulation in transformation efficiency, AID-expressing cells display a preference for vector integration into the chromosomal IgH μ locus by as much as 116-fold. In the remaining transformants recovered from either AID-expressing or AID-non-expressing cell lines, the endogenous IgH μ locus is intact, suggesting vector integration in another position in the genome. For a representative Southern blot, refer to Supporting Information Fig. 1.
More detailed analysis of vector integration sites within the IgH μ locus was conducted on the 25 and eight G418R transformants recovered from the AID-expressing Sp6-10 and Sp6-22 hybridoma cell lines, respectively. As shown in Fig. 4, the enzymes XbaI and EcoRI cleave the endogenous IgH μ locus into TNP-specific Ig VH (VHTNP), Sμ and Cμ region segments, whose disruption by pTCμEn– integration can be conveniently detected in Southern analysis with μ-specific probes E, N or F, respectively. Table 1 presents a summary of the mapping information. For representative Southern blots, refer to Supporting Information Fig. 2.
|
Hybridoma cell line |
Number of G418R transformants analyzed |
IgH μ gene sub-region vector integration sitea) |
|||
|---|---|---|---|---|---|
|
|
|
Sμ |
VHTNP |
Cμ |
Complex |
|
Sp6-10 |
25 |
20 |
4 |
1 |
– |
|
Sp6-22 |
8 |
4 |
1 |
1 |
2 |
|
Total |
33 |
24 (73%) |
5 (15%) |
2 (6%) |
2 (6%) |
- a) The percentage of transformants is presented in parentheses.
On average, the Sµ region is disrupted in 73% of transformants recovered from the AID-expressing Sp6-10 and Sp6-22 hybridoma cell lines. In contrast, the adjacent VHTNP and Cμ regions are disrupted in only 15% and 6% of the transformants, respectively. More complex rearrangements involving the Sμ and adjacent VHTNP or Cμ regions are observed in a minor fraction of Sp6-22 transformants (6%). Therefore, we conclude that the IgH Sμ region is a preferred site of vector integration in AID-expressing cells, being stimulated five- and 12-fold compared to the adjacent VHTNP or Cμ regions, respectively. Further, use of the pTCμEn– vector revealed no obvious enhancement of AID-induced homologous recombination between vector-borne and chromosomal Cμ regions.
The IgH Sμ region occupies ∼4.7 kb of the 6.2-kb XbaI fragment (Fig. 4) shown in the mapping studies above to harbor a substantial fraction of IgH μ locus vector integrations. To verify vector integration directly into the Sμ region, 20 G418R Sp6-10 transformants that according to Southern analysis were disrupted for Sµ were subjected to PCR using primer combinations specific for the pSV2neo vector backbone (Fig. 2) and chromosomal Sμ region (Fig. 4). A PCR product was obtained with at least one pair of primers in 15 transformants (refer to Fig. 5A for representative cell lines), and of these, four were sequenced. In each clone, vector sequences adjoin Sμ sequences confirming vector integration directly into the 4.7-kb Sμ region (Fig. 5B).
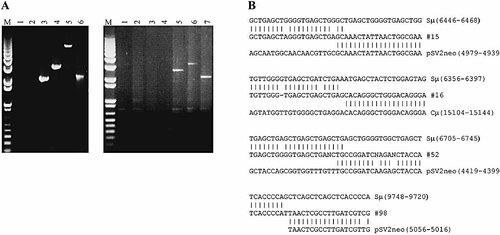
PCR amplification and DNA sequence analysis of vector:Sμ junctions. (A) Representative G418R Sp6-10 transformants from which a vector:Sμ junction fragment was amplified by PCR using vector and chromosome-specific primers (for further details, refer to Materials and methods and Fig. 2, 4). The PCR products visible in transformants shown in the gel on the left were obtained with 5′ chromosomal Sμ primer 5989 and vector primer 5190, while those in the gel on the right were obtained with 3′ chromosomal Sμ primer 9797 and vector primer 5190. Negative control samples include the recipient Sp6/HL hybridoma present in lane 1 of each gel, and a random transformant in which vector integration has occurred outside the chromosomal IgH Sμ region in lane 2 in the gel on the right. The remaining numbered lanes present Sp6-10 transformants containing vector integrations in the Sμ region as identified by Southern analysis. The 1-kb molecular weight marker bands are presented in the lans designated M. The Sμ region PCR products in transformants 15, 16 and 52 visible in lanes 3, 4 and 6, respectively, in the gel on the left, and in transformant 98 in lane 7 in the gel on the right, were selected for DNA sequencing (see below). In some transformants, a specific Sμ PCR product is not visible (lane 2 in the gel on the left, and lanes 3 and 4 in the gel on the right), although usually a product could be obtained with another primer combination. (B) Regions of homology between the mouse IgH Sμ region and the enhancer-trap pTCμEn– vector in transformants 15, 16, 52 and 98 are indicated in the nucleotide numbering to the right of the alignments. In the case of the Sμ region, nucleotide numbering is based on the mouse genomic IgH μ gene sequence compiled by P. Tucker and F. Blattner (personal communication from P. Tucker), which contains a larger segment of the Sμ region than is available either on the corresponding GenBank sequence (accession number AC073553) or the mouse IgH μ gene sequence reported in Koop et al. 62. Sequence alignments with the pSV2neo 28 backbone portion of the transfected pTCμEn– vector follow the GenBank numbering sequence (accession number U02434), while those aligning with the Cμ region portion of the pTCμEn– vector are based on the sequence data of P. Tucker and F. Blattner.
Analysis of vector integration sites by digestion-circularization PCR
As suggested from the Southern analysis, a sizeable fraction of transformants from AID-expressing and AID-non-expressing cells bear vector integrations in unknown sites outside the chromosomal IgH μ locus. Therefore, digestion-circularization PCR (DC-PCR) was adopted as an alternate means of surveying vector integration sites (Fig. 6). For DC-PCR, the enhancer-trap pSV2neoEn– vector was used, which is identical to pTCμEn– (Fig. 2) except that it lacks the IgH Cμ region. AID-expressing Sp6-10 and control Sp6/HL hybridoma cell lines were electroporated with uncut pSV2neoEn– and genomic DNA isolated from independent G418R transformants was digested with either MscI or BglII. The enzymes cut within or near the intact vector-borne neo gene in each transformant and provide a second cut site in genomic DNA surrounding the integrated vector, thus creating a linear DNA fragment containing both vector and chromosomal sequences. The linear products were self-ligated, PCR-amplified using vector-specific primers, and the products sequenced to determine the integration site.
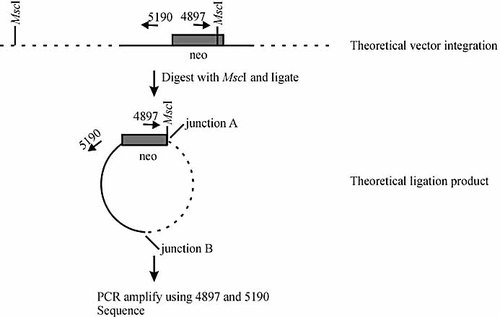
DC-PCR. Uncut pSV2neoEn– was electroporated into AID-expressing Sp6-10 and control Sp6/HL hybridomas and independent G418R transformants selected. Genomic DNA was digested with MscI (illustrated) or BglII (not illustrated), ligated, and in the case of MscI-digested DNA, PCR-amplified using primers 4897 and 5190 (denoted by arrows) (for BglII-digested DNA, ligation products were amplified using primers 4837 and 4502). Genomic DNA:vector junctions were designated as type A if they were formed by ligation of the complimentary MscI (or BglII) half sites, or as type B if they were formed by the vector integration event itself. DNA sequence analysis was performed on the isolated PCR products. For further details, refer to the Materials and methods section and Fig. 2.
Of 94 independent G418R Sp6-10 transformants screened, 29 yielded DNA products that could be sequenced. Likewise, of 58 independent G418R Sp6/HL transformants screened as controls, 16 were successfully amplified and sequenced. The failure to amplify more independent transformants is likely a function of the unknown distance between the vector-borne and chromosomal MscI or BglII restriction enzyme sites and the difficulty of amplifying large ligation products with primers specific to the vector backbone.
As summarized in Table 2, 16 of the 29 Sp6-10 clones were characterized by vector integration into a c-myc/IgH translocation in the recipient Sp6/HL hybridoma. The Sp6/HL hybridoma is derived from a fusion involving the myeloma P3-X63-Ag8 40 in which the c-myc/IgH translocation has been characterized and contains the 3′ portion of c-myc fused end-to-end with the IgH Sμ region (41, reviewed in 42). Interestingly, of the 16 vector insertions that occurred in the translocated chromosome, 13/16 integrations occurred in the IgH Sμ portion, 1/16 occurred between exons 1 and 2 of the translocated c-myc portion, and 2/16 occurred in the Sμ region, but with insertion of additional (probably) filler nucleotides at the vector: DNA junction. Furthermore, in an additional three clones, vector integration occurred in the endogenous IgH Sμ region. Thus, 18/29 vector integrations occurred in Sμ region sequences (Table 2). The remainder of the vector integrations in the Sp6-10 transformants occurred in other IgH genes, the κ light chain variable region, or in other locations in the hybridoma genome (Table 2).
|
Integration siteb) |
Frequency of integrationc) |
|
|---|---|---|
|
|
Sp6-10 |
Sp6-HL |
|
Endogenous Sμ |
3/29 |
– |
|
c-myc/Sμ |
16/29 |
– |
|
IgH κ |
3/29 |
– |
|
Ig κ variable region |
2/29 |
1/15 |
|
Chromosome 10 (Mdm2) |
1/29 |
3/15 |
|
Chromosome 7 (MegF8) |
1/29 |
– |
|
rRNA intragenic spacer |
– |
1/15 |
|
Chromosome 16 |
– |
1/15 |
|
Chromosome X |
– |
1/15 |
|
Repetitive sequence |
3/29 |
8/15 |
- a) Transformants were obtained by transfecting AID-expressing Sp6-10 or non-AID-expressing Sp6/HL hybridomas with pSV2neoEn–.
- b) Determined by DC-PCR cloning and sequencing.
- c) Frequency of integration into this site relative to other sites in the genome.
None of the control Sp6/HL clones revealed vector integrations in Sμ, c-myc or c-myc:Sμ sequences. Instead, the majority showed vector integration into repetitive DNA sequences, and other locations in the hybridoma genome including a single integration in the Ig κ gene (Table 2). Comparison of the data reveals that AID expression elevates vector integration into chromosomal IgH genes (the endogenous Sμ and the IgH γ genes) compared to Ig κ genes by a ratio of 3:1. To a first approximation, the frequency of vector integration into the Ig κ genes in AID-expressing Sp6-10 cells (2/29) appears similar to that in Sp6/HL control cells (1/15). However, when considered in relation to the aforementioned 29-fold stimulation in vector integration in Sp6-10 cells (Fig. 3A), it is clear that AID-induced vector integration into the Ig κ genes is enhanced 30-fold compared to control Sp6/HL cells. The sequences of vector:chromosome junctions obtained by DC-PCR analysis are shown in Supporting Information Fig. 3.
The remaining 66 Sp6-10 and 42 Sp6/HL G418R transformants that could not be amplified by DC-PCR were tested for their capacity to produce normal TNP-specific IgM by lysis of TNP-coupled sheep red cells in agarose plate spot tests 40, 43. The rationale was that if AID-induced vector insertion occurred in the chromosomal IgH μ locus, mainly by disruption of the VHTNP or Sμ regions as suggested from the Southern analysis of AID-induced Sp6-10 and Sp6-22 clones described above, then a significant fraction of the Sp6-10 transformants would fail to make normal TNP-specific IgM and hence would be non-cytolytic in this assay. The analysis revealed that 52% (34/66) and 17% (7/42) of the independent Sp6-10 and Sp6/HL transformants were non-cytolytic, suggesting vector integration into the IgH μ locus, while the rest made normal cytolytic TNP-specific IgM consistent with vector integration outside the IgH μ locus. These results confirm the earlier Southern analysis, which revealed vector integration into the IgH μ locus in 52 and 11% of transformants isolated from the Sp6-10 and Sp6/HL hybridoma cell lines, respectively.
To determine whether the non-cytolytic phenotype is the result of vector integration into the Sμ region, a subset of ten non-cytolytic Sp6-10 transformants was examined by PCR using vector and Sμ-specific primers similar to that described in Fig. 5A. A specific PCR product was observed in 7/10 cell lines (data not shown). Thus, like the previous Southern analysis of transformants with vector insertions in the IgH μ locus, the PCR analysis suggested that the IgH Sμ region is the site of vector integration in a large fraction of the non-cytolytic transformants. Thus, the negative outcome of the initial DC-PCR analysis utilizing vector-specific primers is likely attributable to the length or repetitive nature of the Sμ region, which is known to be problematic for PCR amplification 44.
Discussion
Previous studies have revealed that AID induces SSB and DSB into Ig genes 15–17, 45. Although AID targets DNA breaks to the Ig variable region and switch regions 15, 16, 24, the relative frequency of breaks within these regions, and between these and other genomic sequences is unknown. This knowledge is important given the potential impact of AID-induced DSB on biological function: DSB in the variable region are considered important for gene conversion 19 and possibly SHM 13–17, although in the latter process are also potentially deleterious 46; DSB within the switch region are necessary for CSR and are known to occur at high frequencies in vivo 24 and in hybridomas 47; DSB in other genomic sites may stimulate oncogenic translocations 48. As described here, we exploited an enhancer-trap vector whose stable integration records actively expressed genomic sites, to examine transformation frequencies and integration sites in AID-expressing hybridoma cells. Since DSB stimulate vector integration 34, 35 and AID can generate DSB in actively expressed genes, we reasoned that this vector system would permit comparison of the relative frequencies of AID-induced DSB within the Ig variable and switch regions and between these and other sites in the genome.
We observed that stable incorporation of transfected plasmid DNA is stimulated by as much as 29-fold in murine hybridoma cell lines expressing wild-type AID compared to control hybridoma cell lines expressing no AID or catalytically inactive AID. The results are consistent with ectopically expressed AID generating DSB in the hybridoma genome, which capture transfected enhancer-trap plasmid DNA prior to rejoining. Southern analysis was performed to determine whether plasmid integration events included the haploid, TNP-specific IgH μ gene. In the AID-expressing Sp6-10 and Sp6-22 hybridoma cell lines, plasmid integration into the chromosomal IgH μ gene was enhanced on average by approximately fourfold compared to control cell lines not expressing AID. Therefore, when considered in relation to the above-noted 29-fold enhancement in transformation, the highest level of AID expression in cell line Sp6-10 stimulates vector integration into the chromosomal IgH μ gene by as much as 116-fold compared to other sites in the genome. More detailed mapping of AID-induced vector integration sites within the chromosomal IgH μ gene reveals that the vast majority of vector integrations (73%) have occurred directly into the Sμ region compared to only 15% and 6% for the adjacent VHTNP or Cμ regions, and thus, elevations of approximately five- and 12-fold, respectively, in favor of the Sμ region.
In the remaining G418R transformants, vector integration has occurred outside the chromosomal IgH μ locus and since the genomic location was unknown, the alternate strategy of DC-PCR was adopted for locus identification (Table 2). In AID-expressing Sp6-10 cells, the vast majority of vector integration targets also include chromosomal Ig gene sequences, the c-myc gene, or the Sμ region of a c-myc/Sμ translocation present in the hybridoma cells. Compared to control cells, AID induces vector integration into the Ig κ genes by 30-fold, and compared to Ig κ genes, elevates integration into chromosomal IgH genes by a ratio of 3:1. By far the most frequent integration events involve Sμ region sequences, being observed in 18/29 transformants (62%). Further, in the fraction of transformants that were not amenable to DC-PCR analysis, conventional PCR using vector and Sμ-specific primers confirmed the preference for the IgH Sμ region as the site of vector integration.
In contrast, the vast majority of vector integration sites in G418R transformants isolated from Sp6/HL control cells not expressing AID include simple repetitive sequences, and importantly, no vector integrations were observed in the Sμ region, the c-myc gene or the chromosome harboring the c-myc:Sμ translocation. Thus, these studies support the previous Southern analysis in showing that the IgH Sμ region is preferred as a target for enhancer-trap vector integration compared to the adjacent VHTNP and Cμ regions, and other sites in the genome.
The preference for the IgH Sμ region in vector integration parallels previous work showing AID-induced DSB in this region 13, 17, 24, 49 and provides support for the premise that AID-induced vector integration sites map DSB in actively expressed genes. Therefore, our results suggest that AID preferentially generates DSB in the IgH Sμ region at a significant frequency compared to the adjacent VHTNP and Cμ regions, or the rest of the hybridoma genome. Switch recombination is stimulated by Ig gene transcription 50, and this together with switch-specific DNA binding proteins and/or the presence of RGYW/WRCY target motifs and sequences that may adopt secondary structures such as R-loops, G-quartets and palindromes, which are considered important in AID cleavage 51, may favor AID-induced DSB in the switch region.
Interestingly, in hybridoma cells not expressing AID, the switch region is prone to insertion of transfected DNA 52, 53, although as shown here, this background level can be stimulated several fold in the presence of AID. In the adjacent VH and Cμ regions, AID cleavage motifs and associated structural features may be less frequent, explaining the lower frequency of AID-induced breaks within these adjacent, and also, highly transcribed regions. Nevertheless, the relatively high frequency of vector insertion into the VH region was not expected, and has implications for two recent reports suggesting that DSB are intermediates in variable region gene conversion in chicken DT40 cells. In one report, it was concluded that one-ended DSB (perhaps as a consequence of replication stalling at an SSB) stimulate gene conversion, since two-ended DSB may not be frequent in the variable region 19. However, the findings reported here indeed suggest that two-ended DSB may occur at high frequencies in the VH region, consistent with the notion that two-ended DSB are intermediates in gene conversion 18.
The preference for the IgH Sμ region is noteworthy, especially in view of the many actively expressed genes that are considered to be AID targets (Bcl-6, CD95, PIM1, RhoH, PAX5, CD79a, CD79b and potential several others as indicated in the Introduction) 27–31, 36, 54, 55. However, it is important to emphasize that to identify these genes, researchers first chose tumor suppressors or oncogenes important in B cell development and then tested whether they were targeted by AID 27–31. These approaches differ markedly from the novel genome-wide screen for AID target sites adopted in the present study.
Similarly, as described in the Introduction, although previous studies have shown that the IgH Sμ region is subject to AID-induced DSB 13, 17, 24, 49, this was not determined relative to other sites in the genome. Thus, our study is unique in showing that relative to the IgH Sμ region, other genes may not be targeted to the same degree by AID. Although AID mutates actively expressed genes, a potential limitation is that in this study, vector integration identifies actively expressed genes in hybridoma cells. If the profile of candidate target sites in the hybridoma cells differs significantly from AID-expressing centroblast B cells, it may bias the scope of genes targeted by vector integration.
Finally, our results have important implications for the oncogenic potential of AID, given that the failure to repair DNA breaks can lead to cell death, and aberrant DNA break repair often involving Ig genes can result in chromosome rearrangements, which are hallmarks of B cell neoplasia. Traditionally, chromosome rearrangements involving the IgH and Ig light chain genes have been considered on the basis of errors in V(D)J recombination (48, reviewed in 56). Our finding of a preference for the IgH Sμ region as an AID target, and the observation that IgH and Ig light chain genes are targeted at a high frequency compared to other genomic sites, suggest that while AID-induced DSB may serve an important role in CSR and in antibody diversification processes of gene conversion and SHM, under appropriate circumstances, AID-induced DSB may also be recruited into pathways that result in oncogenic translocations.
Materials and methods
Hybridoma cells and culture conditions
The mouse hybridoma Sp6/HL bears a single copy of the TNP-specific chromosomal IgH μ gene and makes normal, cytolytic TNP-specific IgM (κ-chain) 38, 40. The igm10 hybridoma is an Sp6/HL-derived mutant that has lost the TNP-specific μ gene 38. The conditions for hybridoma growth in Dulbecco's modified Eagle's medium are described elsewhere 38, 40.
Construction of stable transformants expressing human AID
The vector pCEP4hAID contains the 560-bp cDNA segment encoding human AID inserted into the backbone of pCEP4, which contains the HYGR gene 8. To create the vector pCEP4hAIDC90A, the wild-type AID cDNA in pCEP4hAID was replaced with a catalytically inactive form of AID containing the substitution mutation C90A 37. 50 μg of the respective vectors were digested with EcoRV/NruI, and transfected into 2×107 Sp6/HL hybridoma cells by electroporation 57. Selection for HYGR cells was performed in batch culture, and limited dilution cloning (0.1 cell/well) in 96-well tissue culture plates was used to recover independent HYGR transformants, which were saved for analysis of AID expression levels.
Routine screening of HYGR transformants for AID expression was performed by RT-PCR. RNA was prepared by Trizol extraction (Invitrogen). DNaseI-treated RNA (3 mg) were reverse-transcribed using Superscript (Invitrogen) according to manufacturer's specifications. Each sample (5 µL) was amplified with Biotools Taq polymerase (Interscience) using forward and reverse primers (5′-TAGACCCTGGCCGCTGCTACC-3′ and 5′-CAAAAGGATGCGCCGAAGCTGTCTGGAG-3′, respectively), derived from a 316-bp segment specific to the human AID sequence 58. The conditions used for PCR have been described previously 59.
Western blot analysis of AID expression was performed using standard procedures. To detect the 24-kDa protein, the membrane was probed with anti-mouse AID antibody (AID 7E7; Cell Signaling Technology Inc., Danvers, MA), and then with HRP-conjugated secondary antibody (goat anti-mouse IgG, γ chain-specific; SouthernBiotech) as suggested by the manufacturer. Immunoblot signals were detected using ECL reagent (Amersham Biosciences). Quantification of protein bands in Western blots was performed by densitometry using Scion Image software (Scion Corporation, Frederick, MD).
Assay for stable transfection
The pTCμEn– vector contains a 4.3-kb segment from the IgH Cμ region inserted into a derivative of the vector pSV2neo 32 in which the 372-bp Nsi/NdeI SV40 early region enhancer segment responsible for expression of the neo gene was deleted 33. Uncut or linear pTCμEn– vector was transfected into 5×106 wild-type or mutant AID-expressing or -non-expressing Sp6/HL mouse hybridoma cells by electroporation as described 57. Immediately following electroporation, the hybridoma cells were distributed in tissue culture plates at low density (∼3000 viable cells/well) in Dulbecco's modified Eagle's medium supplemented with 1.2 mg/mL G418 to recover G418R transformants 33, 59. The low frequency of transfection with the enhancer-trap vector system (∼10–5/cell) makes it highly likely that each G418R transformant represents the progeny of an independent G418R cell 33, 59.
Digestion-circularization PCR
To investigate AID-induced vector integration sites, a DC-PCR strategy was used. Independent stable G418R transformants were generated as described above using an uncut, SV40 enhancer-deleted version of the vector pSV2neo 32 (GenBank accession number U02434), which we refer to as pSV2neoEn–. Genomic DNA was prepared from independent transformants and 30 μg were digested with either MscI or BglII and joined with T4 DNA ligase using conditions suggested by the manufacturer (Invitrogen). The ligation products were PCR-amplified and sequenced on an ABI Prism automated sequencer (Model 3730, ABI Inc.) using standard procedures (College of Biological Science DNA Facility, University of Guelph) to determine the site(s) of vector integration. Vector-specific primers 5190 (5′-CCAGTAGCTGACATTCATCC-3′) and 4897 (5′-AGAGGCTATTCGGCTATGAC-3′) were used to amplify and sequence ligation products generated by MscI cleavage, whereas vector-specific primers 4502 (5′-GAAGCCGGTCTTGTCGATCA-3′) and 4837 (5′-AACACGGCGGCATCAGAGCA-3′) were used to amplify and sequence products generated by BglII cleavage.
Sequences were compared to mouse genomic sequences using a blastn comparison (http://www.ncbi.nlm.nih.gov). Alignments with the highest bit score and lowest E value were used to identify vector integration sites. Sequences were further characterized using Repeat Masker software to identify repetitive elements (http://www.repeatmasker.org).
IgM analysis
Culture supernatants from individual G418R transformants were examined for secreted TNP-specific IgM production by lysis of TNP-coupled sheep red cells by an agarose plate spot test as described previously 40, 43.
General techniques
Genomic DNA was prepared by SDS-proteinase K treatment as described by Gross-Bellard et al. 60. Plasmid DNA was propagated in Escherichia coli DH5α, and isolated by column purification (Marligen Biosciences). Restriction enzymes were purchased from New England Biolabs and used in accordance with manufacturer's specifications. For Southern analysis, restriction-enzyme-digested genomic DNA was electrophoresed through 0.7% agarose gels, transferred to nitrocellulose membrane, hybridized with 32P-labeled probe, and band sizes visualized by autoradiography according to standard procedures 61. PCR amplification of Sμ:vector junctions exploited various combinations of pSV2neo and chromosomal Sμ-specific primer pairs. Vector primers 5190, 4897 (described above), neoF20 (5′-GCTGACTGTCAACTGTAGCA-3′) and ampR20 (5′-AAGTGCCACCTGACGTCTAA-3′) are specific to the pSV2neo backbone 32 of the transfected enhancer-trap pTCμEn– vector (refer to Fig. 2 for nucleotide binding positions). Chromosome-specific primers 5989 (5′-GACAGAGAAGGCCAGACTCA-3′) and 9797 (5′-CTCAGCTCAGCTCACTCCAG-3′) bind 5′ and 3′ of the mouse chromosomal Sμ region (refer to Fig. 4 for nucleotide binding positions). PCR products were sequenced on an ABI Prism sequencer Model 3730 using standard procedures (CBS DNA Facility, University of Guelph).
Acknowledgements
We thank members of the Baker and Martin laboratories for their helpful comments, and Brian C. Husband for assistance with statistical methods. This work was supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Ph.D. studentship to S.A.L., and operating grants from the Canadian Cancer Society to A.M and the Canadian Institutes of Health Research (CIHR) to M.D.B.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




