Phenotypic analysis of human CD4+ T cells specific for immediate early 63 protein of varicella-zoster virus
Abstract
Open reading frame 63 of varicella-zoster Virus (VZV) encodes an immediate early (IE) phosphoprotein (IE63) that is believed to be important for viral infectivity and establishing latency. Evidence suggests that VZV-specific T cells are crucial in the control of viral replication; however, data addressing the existence of IE63 protein-specific CD4+ T cells are limited. Using IFN-γ immunosorbent assays, we identified high frequencies of responses to overlapping peptides spanning the IE63 protein both ex vivo and after in vitro restimulation in healthy VZV-seropositive individuals. We identified a commonly recognised epitope, restricted by HLA-DRB1*1501, which was naturally processed and presented by keratinocytes. We proceeded to investigate the frequency and phenotype of the epitope-specific CD4+ T cells using HLA class II tetrameric complexes. Epitope-specific CD4+ T cells were detectable ex vivo and showed a mixed central and effector-memory differentiation phenotype, with a significant proportion showing evidence of recent activation and rapid effector function. In summary these data implicate persistent low-level or recurrent VZV antigen exposure in healthy immune donors and are compatible with a role for IE63-specific CD4+ T cells in the control of viral reactivation.
Abbreviations:
-
- CLA:
-
cutaneous lymphocyte antigen
-
- IE:
-
immediate early
-
- ORF:
-
open reading frame
-
- PD1:
-
programmed death 1
-
- VZV:
-
varicella-zoster virus
Introduction
Varicella-zoster virus (VZV) is a cytopathic human alphaherpesvirus that causes varicella (chickenpox) upon primary infection and herpes zoster (shingles) upon reactivation from latency in the sensory ganglia 1. The VZV genome comprises more than 70 unique open reading frames (ORF), which encode proteins that are potential targets for the host immune system 1–3. ORF63 encodes a 47-kDa immediate early (IE) phosphoprotein (IE63) 4–6. IE63 is a virion tegument protein 6 that is expressed very early in the viral life cycle, initially in the nucleus of infected cells 4. During latency several VZV genes have been shown to be transcribed 7, 8, including IE63 protein, which has been detected in latently infected rat ganglia 4 and human ganglia 9. During latency the IE63 protein is detected exclusively in the cytoplasm of neuronal cells 10. IE63 has also been shown to be abundantly expressed in the skin of patients during acute varicella infections and zoster episodes 4, 11.
The function of IE63 remains unclear. Initial studies showed that IE63 up-regulates the thymidine kinase promoter and down-regulates the IE62 promoter in transient transfection assays 12; however, more recently Kost et al. 13 showed minimal activity of IE63 on the activity of IE and E promoters. Recent analysis of IE63 has revealed functional domains that are required for replication in vitro and for T cell and skin tropism in the SCIDhu mouse model 14; the regions important in replication were also shown to be critical for establishment of latency 15.
VZV-specific T lymphocytes are thought to be important in the control of viral replication during both primary infection, where the detection of T lymphocyte proliferation correlates with milder disease, and in the maintenance of latency, where the frequency of viral reactivation correlates with waning cellular immunity 16. In one study, VZV lysate-specific T cells circulating in healthy immune donors were found to be largely of the CD4+ T cell subset and CD8+ T cells were not detectable 17, a finding mirrored in our previous studies 18, 19. A number of studies have investigated T cell antigen specificity and have documented reactivity to several VZV proteins including regulatory and structural proteins encoded by ORF10, ORF62 and ORF63 2, 20, and glycoproteins gB, gC, gE and gI 16, 21, 22. We have also previously described several T cell epitopes in ORF4 and gI 18, 19. Potential CD8+ T cell epitopes have been documented within IE62, but reactive cells were only detectable after in vitro expansion 23.
One study has documented the existence of IE63-specific human memory cytotoxic T lymphocytes which were again only detectable following expansion in vitro 20. Therefore, the IE63 protein, which is present during different phases of the viral life cycle, would be a reasonable candidate for a T cell antigenic target for both control of viral replication and induction of responses through vaccination. However, despite the previous identification of immunogenic proteins of VZV and specific CD4+ T cell epitopes 18, 19, none of these responses have been restricted through HLA class II molecules for which HLA class II tetrameric complexes have been available. Therefore, the use of HLA class II tetrameric complexes has not been applied to the field of VZV-specific immunity and existing studies have been based on functional responses which can significantly underestimate the frequency of antigen-specific T cells 24.
Although there is a live viral vaccine available for VZV, it is now recognised that in the setting of good population immunity a single dose in children is associated with relatively rapid waning immunity 25. Furthermore the vaccine is contraindicated in immunosuppressed individuals, who arguably have the most to gain from protection. Therefore a need exists for the development of safer and more efficacious vaccination strategies. Understanding the VZV-specific immune response is likely to be an important pre-requisite for such developments.
Naive CD4+ T cells, like CD8+ T cells, undergo antigen-induced activation, expansion and differentiation into memory and effector cells. Current understanding of the differentiation of CD4+ T cells from naive to terminally differentiated memory cells is restricted, but models have been based on those proposed for CD8+ T cells, with transition through early to intermediate to late differentiation 26, 27. The models predict maturation from naive cells expressing a phenotype of CD27+CD28+CCR7+CD45RA+perforin– through to late differentiated CD27–CD28–CCR7–CD45RA+/–perforin+ cells 26. Furthermore, the antigen-experienced intermediates are generally understood to progress through central memory T cells which are believed to be CD45RO+ cells that express CD62L and CCR7, allowing them to home to secondary lymphoid organs. Central memory T cells have high replicative capacity and relatively low effector function, but can differentiate into effector cells that produce large amounts of IFN-γ or IL-4. Effector-memory T cells, in contrast, are heterogeneous for CD62L expression, have lost the constitutive expression of CCR7, and display receptors required for homing to inflamed tissues. These cells have lower replicative capacity and have rapid effector functions, producing high levels of IFN-γ, IL-4 and IL-5 within hours of antigenic stimulation 27, 28.
We sought to investigate the central and effector memory status of VZV-specific T cells, using an approach that was not dependent on a functional activation step that can potentially introduce phenotypic changes. We observed high frequencies of IE63-specific CD4+ T cells in the peripheral blood of healthy immune donors and proceeded to map a novel DRB1*1501-restricted epitope. Using IE63/DRB1*1501 HLA class II tetrameric complexes, we characterised the ex vivo frequency and phenotype of IE63 tetramer-binding cells and showed a mixed central and effector-memory phenotype with frequent evidence of recent activation. These findings would support the occurrence of regular VZV re-exposure or reactivation within healthy immune donors, and would be consistent with a role for IE63-specific CD4+ T cells in the control of viral replication.
Results
Responses to overlapping IE63 peptides
The overall ex vivo T cell responses to VZV IE63 protein were determined using PBMC derived from healthy seropositive individuals with a history of primary infection. PBMC were tested by IFN-γ ELISPOT for responses to 27 overlapping 20-mer peptides spanning the IE63 protein. Responses to overlapping IE63 peptides ranged in both breadth and magnitude, as seen in Fig. 1A. Four donors failed to respond above the positive cut-off (mean + 3 SD of the nil) to any peptide, but the remainder (85%) responded to at least one peptide. There was also a range in the magnitude of responses to individual peptides with the maximum reaching 1085 IFN-γ-producing cells per million PBMC for an individual peptide. In contrast, we did not observe responses to any peptides in the 12 seronegative controls (data not shown).
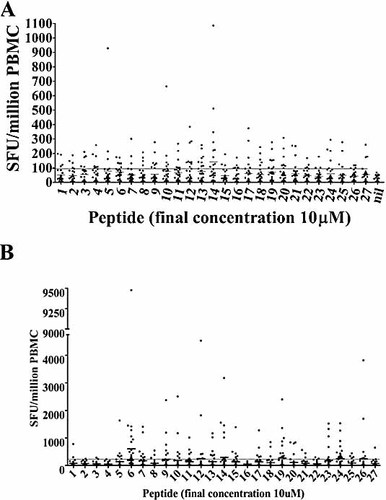
(A) Ex vivo IFN-γ ELISPOT responses to overlapping IE63 peptides in 26 healthy immune donors with a history of primary VZV infection (each dot represents the mean peptide response in one individual). The long horizontal bar shows the mean + 3 SD of the irrelevant peptide. For each peptide the median is shown as a small horizontal bar. (B) Cultured IFN-γ ELISPOT responses to overlapping IE63 peptides in 26 healthy immune donors with a history of primary VZV infection (each dot represents the mean peptide response in one individual). The long horizontal bar shows the mean + 3 SD of the irrelevant peptide. For each peptide the median is shown as a small horizontal bar.
The average age of the donors was 34.4 years (range 26–63 years) and the average age at primary infection was 6.4 years (range 3–15 years). The median time since primary infection was 21.7 years (range 17–52 years), and none of the donors had a history of reactivation. There was no significant correlation between individual peptide-specific responses or total IE63 peptide responses with age or time from primary infection. Overall these data suggest that IE63 protein-specific T cells circulate at persistently high levels in the blood of healthy immune donors with a history of primary infection.
We then proceeded to investigate the proliferative potential of the IE63-specific T cells. We observed high levels of IE63-specific T cells after 14 days of culture, reaching a maximum of 9475 IFN-γ-producing cells per million PBMC (379/40 000) (Fig. 1B). These data showed that the IE63-specific T cells within the peripheral blood show good proliferative capacity.
Responses to IE63 are dominated by CD4+ T cells
To further characterise the responding populations, we undertook intracellular cytokine analysis, and observed that the majority of ex vivo and cultured IE63 protein-specific responses were mediated by CD4+ T cells (Table 1). We used a number of different VZV antigens both ex vivo and also to stimulate VZV-specific T cell expansion in vitro. Table 1 shows that when using IE63-based peptides, VZV-infected cell lysate or the live viral vaccine to stimulate PBMC, the majority of IE63 protein-specific responses were mediated by CD4+ T cells.
|
Initial stimuli |
Testing stimuli |
CD3+ CD8+ IFN-γ+mean % |
CD3+ CD4+ IFN-γ+mean % |
|---|---|---|---|
|
Peptide 24 |
Nil |
0.007 |
0.030 |
|
|
Peptide 24 |
0.010 |
0.367 |
|
|
ORF63 pooled |
0.007 |
0.437 |
|
|
Lysate |
0.003 |
0.030 |
|
|
Vaccine |
0.007 |
0.263 |
|
All ORF63 peptides |
Nil |
0.000 |
0.007 |
|
|
Peptide 24 |
0.007 |
0.697 |
|
|
ORF63 pooled |
0.033 |
0.743 |
|
|
Lysate |
0.003 |
0.043 |
|
|
Vaccine |
0.000 |
0.030 |
|
Lysate |
Nil |
0.017 |
0.237 |
|
|
Peptide 24 |
0.047 |
0.563 |
|
|
ORF63 pooled |
0.033 |
1.040 |
|
Vaccine |
Nil |
0.010 |
0.107 |
|
|
Peptide 24 |
0.033 |
0.247 |
|
|
ORF63 pooled |
0.027 |
0.403 |
- a) Table shows mean responses in three individuals. PBMC were incubated with an initial stimulus, as shown, for 14 days, then tested for IFN-γ production in response to a second stimulus (as shown).
These data showed that despite using a number of different forms of antigenic stimulation, IE63 protein-specific T cells were strongly dominated by CD4+ T cells, which was similar to our findings for T cell responses to IE4 and gI 18, 19. Such relative paucity of VZV-specific CD8+ T cells may be in part related to the known HLA class I down-regulation induced by VZV. Having characterised the overall pattern of responses, we proceeded to investigate one of the more commonly recognised regions in more detail.
Characterisation of responses to peptide 24 HLA-DRB1*1501 epitope
Several 20-amino acid peptides gave strong responses, but on further analysis these were typically comprised of more than one epitope. In contrast, we noted that peptide 24 induced strong responses in all DRB1*1501-positive individuals, raising the possibility that a single strong DRB1*1501-restricted epitope lay within the 20-amino acid peptide (AIERYAGAETAEYTAAKALT). To determine the optimal epitope and anchor residues, truncations were made by sequentially removing residues from each end. Responses were assessed to these truncated peptides by cultured IFN-γ ELISPOT.
Fig. 2A documents the responses observed and shows that residues 2 and 12 are critical for responses to this peptide. To determine the HLA restriction of the response to peptide 24, we used blocking antibodies or partially matched keratinocyte lines or transfected murine fibroblasts. IFN-γ ELISPOT set up with peptide-specific lines tested against relevant peptide (63-24) alone, or after pre-treatment with HLA-DR-, HLA-DP- or HAL-DQ-blocking antibodies, showed that the response is restricted through HLA-DR (Fig. 2B). Titration of the response showed detectable IFN-γ to nM and pM concentrations of peptide and that although the minimal epitope required residues 2 and 12, the optimal epitope comprised residues –2–13 (QRAIERYAGAETAEY) (Fig. 2C).
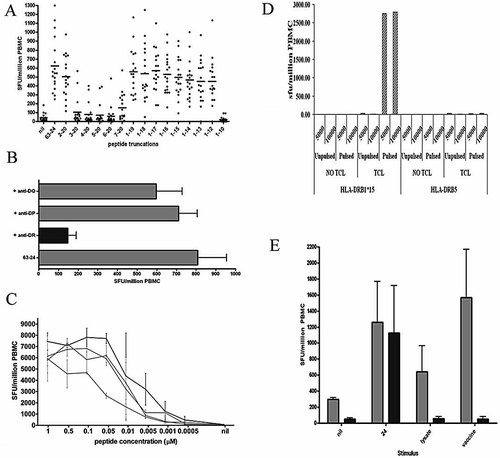
(A) IFN-γ ELISPOT responses to peptide truncations from peptide 24 of IE63 (20-mer AIERYAGAETAEYTAAKALT), n=12. (B) IFN-γ ELISPOT responses using a peptide-specific line incubated with the specific peptide (20-mer AIERYAGAETAEYTAAKALT) directly added to T cells or peptide added after prepulsing with HLA class II-blocking antibodies, n=7. (C) Using highly enriched CD4+ T cell populations (n=3), IFN-γ ELISPOT responses were performed with titrated concentrations of peptides, showing that the optimal sequence is –2–13 (QRAIERYAGAETAEY). Black circles: –2–13; grey squares: peptide 24 20-mer (AIERYAGAETAEYTAAKALT, 0–20); grey triangles: –1–14; grey diamonds: 1–14. (D) IFN-γ ELISPOT responses showing that HLA-DRB1*15 and not HLA-DRB5 murine fibroblast cell lines pulsed with peptide 24 are recognised by specific CD4+ T cells. Shown is a representative example of three CD4+ T cell lines. (E) Peptide 24 is efficiently processed and presented by human keratinocytes. Light grey bars represent keratinocytes pulsed with peptide 24 (24), viral lysate or live Oka vaccine showing efficient presentation. In contrast, the dark bars show that DRB1*1501-transfected murine fibroblasts were only able to present peptide. Shown is a representative example of three CD4+ T cell lines.
Tranfected murine fibroblast lines were pulsed for 1 h with either peptide or control, washed and then used as presenting cells in IFN-γ ELISPOT. Fig. 2D shows that the T cell line responds to the HLA-DRB1*1501-transfected line and not HLA-DRB5-transfected presenting cells (all unpulsed presenting cells elicited no response). Therefore, the response to peptide 24 is restricted via HLA-DRB1*1501. We proceeded to investigate whether the epitope would be processed and presented by keratinocytes for presentation to the T cells. Fig. 2E shows that peptide 24-specific T cells were able to efficiently recognise keratinocytes infected with live viral vaccine or pulsed with viral lysate.
To determine the relative contribution that the responses to this peptide and to IE63 make to the overall viral-specific response, we compared responses in HLA-DRB1*1501-positive individuals to the epitope, a pool of all 27 overlapping IE63 peptides, infected cell lysate and vaccine. Fig. 3 shows examples of such intracellular cytokine staining from one individual and confirms that peptide 24 IFN-γ responses were mediated by CD4+ T cells. The data from three DRB1*1501 donors are shown in Table 1, which illustrates that responses to peptide 24 comprise a large proportion of the overall response to the IE63 protein.
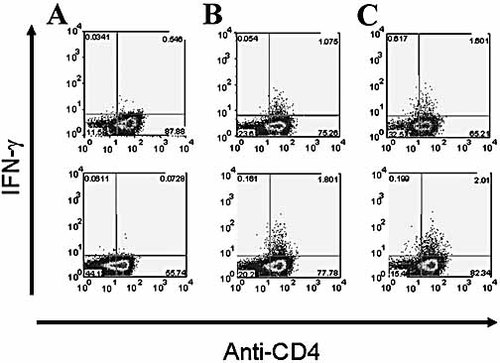
Intracellular cytokine staining showing that the response to peptide 24 is mediated by CD4+ T cells. IFN-γ production of cells initially generated using either peptide 24 (top row) or a pool of overlapping peptides encompassing the whole of IE63 (bottom row) and then stimulated with no stimulus (A), peptide 24 (B) or a pool of overlapping peptides encompassing the whole of IE63 (C). Shown is a representative example of five T cell lines.
These data showed that it was possible to map the peptide 24 response in detail and to characterise the HLA restriction to be HLA-DRB1*1501. Furthermore the epitope is naturally processed and presented by keratinocytes and comprises a large proportion of the overall IE63 response in DRB1*1501-positive individuals.
Ex vivo phenotypic analysis of responses to IE63 HLA-DRB1*1501 epitope
Having mapped an IE63 epitope presented by DRB1*1501, we proceeded to use an HLA-DRB1*1501 tetrameric complex to investigate IE63-specific CD4+ T cells further. Firstly we sought to determine the ex vivo frequency of tetramer+ cells in healthy VZV-seropositive HLA-DRB1*1501-positve individuals with a history of primary VZV infection but no clinical reactivation. Although tetramer+ CD4+ T cells were found to be at relatively low frequencies in seropositive HLA-DRB1*1501 individuals (mean = 0.006% CD4+ T cells, range 0.001–0.016), they were significantly higher than the frequencies seen in non-HLA-DRB1*1501 individuals (p=0.0003; Fig. 4A, B). The frequency of tetramer+ cells detected to this epitope was comparable to those seen in individuals infected with HIV 29, CMV 30, hepatitis C 31 and influenza 32 and in those with severe atopic disease 33.
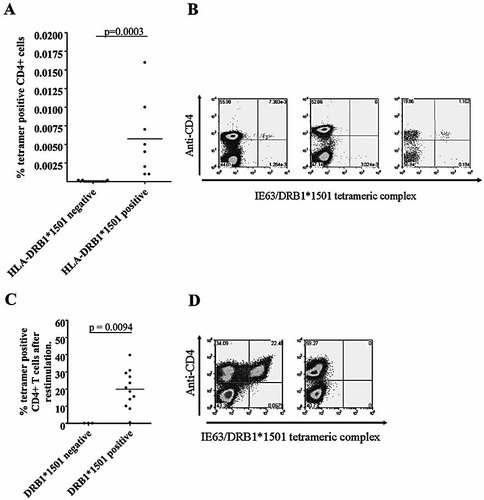
(A) Frequency of CD4+ tetramer+ cells ex vivo in HLA-DRB1*1501-positive (n=8) and -negative (n=10) individuals (p=0.0003). (B) Examples of CD4+ tetramer+ staining in HLA-DRB1*1501-positive (left panel) and -negative (middle panel) individuals ex vivo. Staining of HLA-DRB1*1501-positive PBMC after anti-PE bead enrichment (right panel). (C) Frequency of CD4+ tetramer+ cells in HLA-DRB1*1501-positive (n=12) and -negative (n=3) individuals after short-term (14-day) culture (p=0.0295). (D) Examples of CD4+ tetramer+ staining in HLA-DRB1*1501-positive (left panel) and -negative (right panel) individuals after short-term culture.
We were able to generate large numbers of tetramer+ cells after short-term incubation of PBMC from healthy HLA-DRB1*1501-positive VZV-seropositive individuals with peptide 24 (or VZV vaccine or lysate) (mean = 19.85% CD4+ T cells, range 0.41–39.74). Again these values were significantly higher than those observed for HLA-DRB1*1501-negative individuals (mean = 0.0016% CD4+ T cells, range 0.0008–0.0027) (p=0.0094, Fig. 4C, D).
To characterise the activation and differentiation status of the tetramer+ CD4+ T cell population, we performed ex vivo phenotypic analysis using a large panel of markers relevant to activation, differentiation, homing and memory. The level of expression of each marker on tetramer+ CD4+ T cells is shown in Fig. 5. As the frequency of tetramer+ cells was low ex vivo, phenotypic analyses were repeated with tetramer enrichment using anti-PE beads 30. This confirmed the ex vivo phenotypic analysis described below.
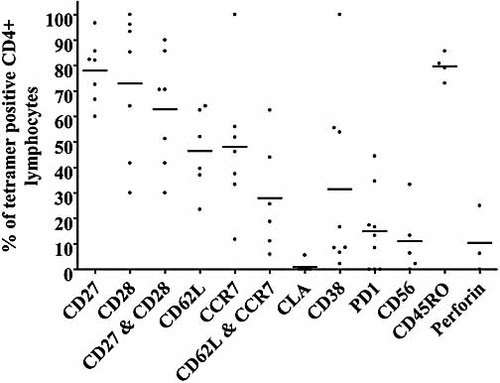
Ex vivo phenotypic analysis of IE63-specific tetramer+ CD4+ T cells (n=7).
Expression of the co-stimulatory molecules CD27 and CD28 was high on the tetramer+ CD4+ T cells, with expression at 78 ± 4.69% and 72.93 ± 10.62%, respectively, and 62.83 ± 8.51% having co-expression. However, these levels were lower than those observed in the whole CD4+ T cell population (CD27+: 88.41 ± 1.84%; CD28+: 78 ± 7.76%; and CD27+CD28+: 69.22 ± 7.33%). The IE63-tetramer-specific cells were found to have a mixed expression of the lymph node homing markers CD62L and CCR7 (CD62L: 46.46 ± 6.483%; CCR7: 48.08 ± 10.26%; and CD62L+CCR7+: 27.98 ± 2.838%). Indeed these levels were significantly lower than those observed for the overall CD4+ population (CD62L: 63.2 ± 3.8% (p=0.0244); CCR7: 75.2 ± 2.8% (p=0.0278); and CD62L+CCR7+: 53.6 ± 3.2% (p=0.0123)). The tetramer+ cells also exhibited lower expression of the skin homing marker cutaneous lymphocyte antigen (CLA; 0.93 ± 0.93% compared to 1.43 ± 0.34% on the total CD4+ population).
The tetramer+ cells showed a significantly higher expression of CD45RO than the total CD4+ population (79.66 ± 2.6% for the tetramer+ cells compared to 38.21 ± 5.16% for the overall CD4+ cells). The IE63 tetramer+ cells had lower expression of CD38 (31.5 ± 12.34%) than the total CD4+ population (42.23 ± 6.8%) and higher expression of both perforin (10.42 ± 7.5% compared to 2.84 ± 0.48%) and programmed death 1 (PD1, 15 ± 5.2% compared to 7.19 ± 1.2%).
Overall these data show that the tetramer+ cells displayed a distinct cell surface phenotype: largely positive for the co-receptors CD27 and CD28, mixed for the lymph node homing markers CCR7 and CD62L, low for the skin homing marker CLA, and high for the memory marker CD45RO (Fig. 5). Therefore in VZV-seropositive individuals these IE63-specific DRB1*1501-restricted CD4+ T cells show a mixed central and effector-memory differentiation phenotype.
Discussion
IE63 is believed to be important for viral infectivity and establishing latency. The protein is expressed very early during viral replication and is the most abundantly expressed transcript during latency. Therefore, IE63 is a reasonable candidate for understanding immune control of viral replication and for potential future vaccination developments.
Using IFN-γ ELISPOT assays, we have shown high levels of ex vivo rapid effector functional responses to IE63 peptides in healthy immune donors with a history of primary infection. We have shown that the responses to IE63 peptides are dominated by CD4+ T cells and that such T cells recognise naturally processed and presented antigen. We proceeded to characterise one of the strongest responses in detail and showed that residues 2 and 12 of the 20-mer (AIERYAGAETAEYTAAKALT) were critical for presentation and that the sequence QRAIERYAGAETAEY comprised the optimal epitope. Using partially matched presenting cells we have determined that the responses seen to this peptide were restricted by HLA-DRB1*1501.
There was no correlation between IE63 responses and age of donor or time from primary infection which suggests that there is persistence of the IE63-specific T cells in our cohort. It is possible that re-exposure to varicella maintains such high levels of reactive cells 34, 35 or periodic subclinical reactivation may boost the VZV-specific T cell frequency. It will clearly be important to extend these investigations to very elderly individuals to determine whether late falls in the frequency of IE63-specific T cells do occur as predicted by other studies. The presence of IE63 protein-specific T cells in healthy immune donors with a history of primary infection implicates such cells as being involved in the control of viral reactivation.
In this study we have utilized the first VZV-based tetrameric complex to enable the identification and characterisation of HLA-DRB1*1501 IE63-specific T cells ex vivo in healthy VZV-seropositive individuals. Although we observed a low frequency of IE63-specific tetramer+ cells ex vivo, they were comparable to the frequency of HLA class II tetramer+ cells in individuals infected with HIV 29, CMV 30, hepatitis C 31 and influenza 32, and in those with severe atopic disease 33. The cells showed rapid effector function and good proliferative capacity.
Approximately 40% of the VZV-specific tetramer+ cells expressed CD62L and CCR7, suggesting that less than half of them have the capacity to home to secondary lymphoid organs. These cells were also predominantly negative for the skin homing marker CLA, implying that future homing may not preferentially be towards the skin. However it will be important to investigate the expression of other markers that have been putatively linked to cutaneous homing, including CCR4 and CCR10.
The majority of the VZV tetramer+ cells were CD45RO+, therefore suggesting that they were of an antigen-experienced phenotype but had not yet matured to late differentiation as has been observed for CMV-specific CD4+ T cells (Fig. 6) 26, 30, 36. Although the tetramer+ cells had lower expression of CD38 than the overall CD4+ T cell population, approximately 30% of the tetramer+ cells did express CD38, suggesting that a subpopulation of the specific cells may have had recent exposure to VZV antigens through either re-exposure or subclinical reactivation. A proportion of the tetramer+ cells (15%) express the marker PD1, a negative regulator of activated T cells, which is markedly up-regulated on the surface of exhausted T cells as well as activated T cells. The VZV IE63-specific CD4+ T cells detected in these experiments were predominantly CD28+, CD27+, CD45RO+/–, CCR7+/–, CD62L+/– and perforin–. The combination of surface and intracellular proteins observed in our VZV IE63-specific CD4+ T cells suggests that these cells would comprise a mixed population of early/intermediate differentiated CD4+ T cells with a mixed central and effector-memory phenotype.
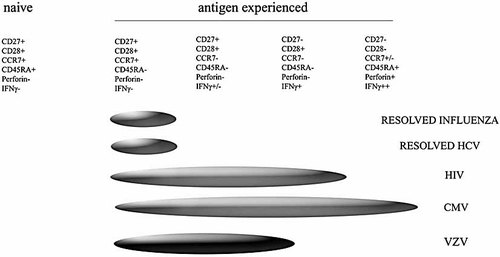
Phenotype of VZV-specific CD4+ T cells within proposed models of CD4+ T cell differentiation.
Although the frequencies of tetramer-binding cells were similar to those in contrasting disease settings, there were some interesting differences in the phenotype of viral-specific CD4+ T cells. For example, in HIV-infected individuals, the majority of the HIV-specific CD4+ T cells were CCR7– 29, 30, suggesting that the HIV-specific T cells were more mature than the VZV-specific T cells documented herein. The HIV studies showed an inverse correlation between CCR7 expression by tetramer-binding cells and plasma load of viral RNA, implying either that antigenic drive is a crucial determinant of CCR7 expression or that CCR7+ cells contribute to the reduction in load. It is likely that VZV-specific T cells are exposed to far less antigenic load in healthy immune donors as VZV load is typically undetectable in such individuals. However the current data suggest that the VZV-specific T cells are encountering antigen, albeit at lower levels than that observed in HIV infection.
A significant proportion of the VZV-specific CD4+ T cells were CD38+, CCR7– and PD1+, implying that there is some degree of persistent or recurrent viral replication. This would also fit with findings in individuals with resolved influenza 32 and hepatitis C 31, in whom the viral-specific CD4+ T cells largely sustain CCR7 expression, a pattern more in keeping with early differentiation.
CMV is also a member of the herpes virus family and provides some interesting comparisons. CMV-specific CD4+ T cells are thought to express a late differentiation phenotype characterised by relative loss of CD27 30. The majority of the VZV-specific CD4+ T cells maintained expression of CD27, again implying that VZV antigenic drive is lower than that for CMV as well as for HIV. Taken together these data suggest that VZV-specific CD4+ T cells express a mixed central and effector-memory state of differentiation (Fig. 6) compatible with persistent low-level or recurrent antigen exposure. In due course, it will be important to increase the number of concurrent markers to further characterise the phenotypic subgroups of antigen-specific T cells. However on the basis of these and other data, we would predict that IE63-specific CD4+ T cells are frequently encountering antigen either through reactivation or re-exposure, providing enough stimulation to drive a significant proportion of the cells towards a phenotype of CCR7–CD62L–CD38+PD1+. By understanding the phenotype of T cells associated with protective immunity, it may be possible to identify those at risk of reactivation and further optimise vaccination strategies.
Clearly it will now be important to build on these data by temporal analysis in different groups of individuals (including primary infection, reactivation, elderly donors and following vaccination) in order to more closely define their potential role in control of disease. It will also be important to investigate how long IE63-specific T cells are stable through larger cohort studies, particularly including elderly volunteers. Comparisons with our previously defined epitopes, assessed by intracellular cytokine analysis, are limited as many meaningful markers are down-regulated upon activation; therefore it will be crucial to identify epitopes in other proteins that are restricted through HLA class II molecules for which tetrameric complexes can be used. Nevertheless it is hoped that the current data will be helpful for such future comparisons.
Overall we have shown that IE63 protein is a commonly recognised T cell antigen within VZV and that responses are dominated by CD4+ T cells. We have shown that these responding T cells recognise naturally processed and presented antigen. We have identified an IE63-specific CD4+ T cell epitope restricted via HLA-DRB1*1501, and using the first VZV-specific tetrameric complex, have begun to elucidate the phenotype of the responding cells. The initial phenotypic analysis shows that the IE63-specific CD4+ T cells circulating in the peripheral blood have a mixed central and effector-memory differentiation phenotype, characterised by the expression of high levels of CD27 and CD28, with mixed expression of CD62L and CCR7, and low expression of CLA. These data implicate IE63-specific CD4+ T cells as being important in the control of viral reactivation and we hope the findings will inform future studies of viral pathogenesis and novel vaccine development.
Materials and methods
Study population
The study participants consisted of 26 healthy seropositive adult individuals with a history of primary VZV infection but no clinical reactivation, and 12 seronegative individuals. Mean age of the donors was 34.4 years, with a mean age at primary infection of 6.4 years. Twelve of the 26 individuals were HLA-DRB1*1501-positive. Ethical approval was granted by the Oxfordshire ethics committee and informed consent was gained from all donors.
PBMC were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation. Cells were then resuspended in RPMI 1640 plus 10% human AB serum for ex vivo ELISPOT assays, ex vivo intracellular cytokine assays and cell cultures.
Peptide generation
A panel of 27 synthetic 20-mer peptides overlapping by ten amino acids were synthesized using standard 9-fluorenylmethoxycarbonyl chemistry. These peptides span the entire protein encoded by ORF63 of VZV 3. Purity was established by HPLC and the individual peptides were dissolved at 10 mg/mL in DMSO and stored at –20°C. They were diluted to 40 μg/mL in RPMI for use in ELISPOT.
IFN-γ ELISPOT and cultured ELISPOT
Assays were performed using IP lined 96-well multi-screen plates (Millipore UK Ltd). Wells were coated with 50 μL of IFN-γ catcher antibody (mAb 1.D1K; Mabtech) diluted to 15 μg/mL in sterile PBS pH 7.4 and incubated 16 h at 4°C. Plates were washed six times and blocked with 200 μL R10 per well for 1 h at 37°C. PBMC were diluted to 1×106/mL in hR10 and 100 μL was added to each well. Peptides were added to a final concentration of 10 μM and the plates were incubated overnight (16 h) at 37°C. When using the pool of 27 IE63 peptides, each peptide was at a final concentration of 4 μM. PHA was used as the positive control at 2 μg/mL per well and RPMI alone as a negative control. An irrelevant peptide was used to assess non-specific IFN-γ responses. The plates were developed by addition of 1 μg/mL of biotin-linked anti-IFN-γ mAb (mAb 7-B6-1-Biotin; Mabtech) as a detection antibody, which is subsequently conjugated to streptavidin-alkaline phosphatase (Mabtech), and visualised using an alkaline phosphatase conjugate substrate kit (170-643, as instructions, Bio-Rad). Spots were enumerated using an automated AID ELISPOT reader. Background (cells plus irrelevant peptide) was subtracted and data expressed as number of spot-forming units per 106 PBMC. Significant positive responses were defined as those greater than the mean + 3 SD of irrelevant control peptides.
For cultured ELISPOT assays, T cell lines were set up as described below and incubated at 37°C and 5% CO2 for 10 days. On day 13 the cells were washed twice in PBS, then returned to a 24-well culture plate in hR10. On day 14, cells were counted and diluted to 0.4×106/mL in hR10 and then ELISPOT were carried out as described above.
Partially matched presenting cells (B cell lines and keratinocytes) were incubated with 100 μL of 0.1 mM peptide, or lysate or vaccine for 1 h at 37°C, then washed in triplicate. Presenting cells (1000–5000) were added to wells with PBMC/T cell line concentrations as above. ELISPOT were then carried out as described above.
Intracellular cytokine staining
Fresh PBMC or T cell lines were stimulated with either culture medium alone, PMA (50 ng/mL) and ionomycin (1 mM), peptide (5 μM), or pooled peptides (each peptide 1 μM) for 16 h at 37°C and 5% CO2 with the addition of GolgiStop (Becton Dickinson) after 2 h of incubation. On day 2, cells were stained for the surface markers CD3, CD8 and CD4 (BD Pharmingen), and after permeabilization using intracellular cytokine staining kit (Becton Dickinson) for cytokine IFN-γ, and analysed by flow cytometry 37.
Serology and HLA typing
Serum was analysed for VZV IgG antibodies using the VIDAS Varicella-Zoster IgG Assay (bioMérieux). All PBMC, keratinocyte and B cell lines were HLA-typed by PCR utilizing sequence-specific primers phototyping 38.
Establishment of T cell, B cell and keratinocyte lines
Freshly separated PBMC were diluted to 2×106 cells/mL and plated onto a 24-well plate, then incubated with peptides at a final concentration of 4 μM, at 37°C and 5% CO2. At day 3, IL-2 was added at a concentration of 100 U/mL. Cells were maintained in RPMI containing IL-2 and 10% human AB serum.
VZV-infected cell extract (10-514-001; ABI) was used at 5 μg/mL, and Vero uninfected cell extract (10-508-001; ABI) was used at 5.4 μg/mL. VZV Oka vaccine Varilrix (GlaxoSmithKline) was added to a final concentration of 104 PFU/mL.
Three established keratinocyte lines were used: HaCat cells (a gift from Dr. N. Fusenig) were spontaneously developed from adult epidermal keratinocytes; NK and NFK are HPV-16 immortalised keratinocytes (a gift from Dr. E. O'Toole). All three lines were HLA-typed as described above.
Murine fibroblast cell lines transfected with either HLA-DRB1*15 or HLA-DRB5 (kindly supplied by Prof. Lars Fugger) were maintained in DMEM/10% FCS at 37°C with 5% CO2.
Blocking antibodies
Cells from short-term cultures were incubated with 10 µL of mAb at 0.2 mg/mL specific for HLA-DR (L243), HLA-DQ (SPV-L3) (kindly supplied by Prof. Lars Fugger) or HLA-DP (B7.21; Leinco Technologies) at 37ºC for 1 h before addition of peptides for ELISPOT.
Tetramer and phenotypic staining and flow cytometry
DRB1*1501 iTAg MHCII tetramer was purchased from Beckman Coulter. DRB1*1501 tetramer was complexed to VZV IE63 peptide 24 (amino acids 229–243, QRAIERYAGAETAEY). Unless stated otherwise, cell lines and PBMC were incubated with 2 μg/mL HLA class II tetramer for 60 min at 37°C in hR10. We analysed the tetramer expression within the CD4+ T cell subset by gating on the lymphocytes and excluding B cells, monocytes and dead cells (ViaProbe-positive population). This permitted two colours for phenotypic analysis of the tetramer+ cells.
The cell surface marker antibodies CD4-Pacific blue (Biolegend), CD14-PerCP, CD19-PerCP and 7-aminoactinomycin D (all BD Pharmingen) were added for 20 min at room temperature. For phenotypic analysis of tetramer+ CD4+ cells, antibodies to CD27 (FITC), CD28 (allophycocyanin), CD38 (allophycocyanin), CD45RO (allophycocyanin), CD62L (allophycocyanin), CD56 (PE-Cy7), CCR4 (PE-Cy7), CCR7 (PE-Cy7), CLA (FITC), PD1 (FITC) and perforin (FITC) were added with the other surface antibodies. Stained cells were washed with PBS, and fixed in 0.5% PBS/formaldehyde. Cells were acquired on a CyAn™ (DakoCytomation) and analysed using FlowJo software.
Tetramer enrichment
PBMC were incubated with 2 μg/mL HLA class II tetramer for 60 min at 37°C in hR10 and surface markers as above. PBMC were then incubated with anti-PE beads (Miltenyi Biotech) for 20 min at 4°C, before being passed through a MACS MS separation column (Miltenyi Biotech). Cells were then analysed as described above.
Acknowledgements
We are most grateful to the Medical Research Council and Commonwealth Commission for their generous support of the studies and to all of the donors who gave blood samples for analysis.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no conflict of interest.




