Defective Artemis nuclease is characterized by coding joints with microhomology in long palindromic-nucleotide stretches
Abstract
T–B–NK+ severe combined immunodeficiency (SCID) is caused by a defect in V(D)J recombination. A subset of these patients has a mutation in one of the non-homologous end joining (NHEJ) genes, most frequently the Artemis gene. Artemis is involved in opening of hairpin-sealed coding ends. The low levels of residual Dh-Jh junctions that could be amplified from patients’ bone marrow precursor B cells showed high numbers of palindromic (P)-nucleotides. In 25% of junctions, microhomology was observed in the P-nucleotide regions, whereas this phenomenon was never observed in junctions amplified from bone marrow precursor B cells from healthy controls. We utilized this difference between Artemis-deficient cells and normal controls to develop a V(D)J recombination assay to determine hairpin-opening activity. Mutational analysis of the Artemis gene confirmed and extended the mapping of an N-terminal nuclease active site, which contains several indispensable aspartate residues. C-terminal deletion mutants did not show such severe defects in the V(D)J recombination assay using transient overexpression of (mutated) Artemis protein. However, a C-terminal deletion mutation causes T–B–NK+ SCID, indicating that the Artemis C terminus is essential for V(D)J recombination at the normal Artemis expression level. The V(D)J recombination assays used in this study contribute to the diagnostic strategy for T–B–NK+ SCID patients.
Abbreviations:
-
- β-CASP motif:
-
metallo-β-lactamases-associated CPSF Artemis SNM1 PSO2
-
- P:
-
palindromic
-
- RSS:
-
recombination signal sequence
Introduction
T–B–NK+ severe combined immunodeficiency (SCID) is caused by a defect in V(D)J recombination of immunoglobulin (Ig) and TCR genes 1. V(D)J recombination takes place during B- and T-cell differentiation and is responsible for the generation of antigen-specific receptors 2, 3. The first step of this process consists of the induction of a single strand nick by the recombination-activating gene (RAG) 1 and RAG2 proteins at the junction of the coding V, (D) and J elements and the recombination signal sequences (RSS) flanking the coding elements 4, 5. The blunt signal ends of both RSS ligate directly into a signal joint (sj), whereas the covalently sealed hairpins of the coding end require further processing prior to coding joint (cj) formation. Artemis is the nuclease that opens the hairpin-sealed coding ends 6, 7. The ligation step is carried out by the XRCC4/Ligase IV complex in conjunction with the recently identified XLF (Cernunnos) 8–13. In ∼30% of T–B–NK+ SCID patients mutations are found in the RAG1 and RAG2 genes 14, 15. A subset of the remaining patients show hypersensitivity to ionizing radiation and have mutations in Artemis or LIG4 6, 16–21. Mutations in the XLF gene have been found in patients with growth retardation, microcephaly, and immunodeficiency due to profound T and B-cell lymphocytopenia 13.
In the Artemis protein three regions have been defined based on sequence analysis, i.e. the metallo-β-lactamase (β-Lact) homology region (amino acids 1–155), the β-CASP motif (metallo-β-lactamases-associated CPSF Artemis SNM1 PSO2; amino acids 156–385) and the C-terminal region (386–692) 6, 22. The β-Lact/β-CASP domain contains the catalytic core for hairpin opening 23, 24.
We studied DH-JH gene rearrangements in bone marrow (BM) cells from Artemis-deficient SCID patients and found high numbers of palindromic (P)-nucleotides in the junctional regions. This characteristic was used to develop a V(D)J recombination assay to discriminate a possible Artemis defect in T–B–NK+ SCID patients. We used this assay to extend the mapping of the N-terminal nuclease-active site.
Results and discussion
In vivo DH-JH junctions in Artemis-deficient SCID patients
As expected, the frequency of DH-JH junctions in bone marrow mononuclear cells (BMMC) of Artemis-deficient patients was lower than in healthy donors. Therefore, a second round of PCR was performed to obtain sufficient quantities of PCR products for further analysis. The presence of DH-JH junctions in BMMC of all patients, including the patients with large genomic deletions, indicates that DH-JH junctions can be formed even in the complete absence of Artemis protein. Therefore, residual activity of a mutated Artemis protein cannot be the explanation for these junctions.
The majority (90%) of sequences of the DH-JH junctions of the Artemis-deficient patients showed long stretches of P-nucleotides (Table 1). The average length of the P-nucleotide stretches was 6.5 nucleotides per junction, which is significantly more than the 0.4 nucleotides in healthy controls (Table 2). Long P-nucleotide stretches have been identified previously in Artemis-deficient mice and in an in vitro V(D)J assay 23, 25. Long stretches of P-nucleotides result from aberrant hairpin opening. In Artemis-proficient cells, the coding end hairpins are opened preferentially near the tip 7, 26. This results theoretically in the presence of a few P-nucleotides, but exonuclease activity often deletes these P-nucleotides. In the absence of Artemis, hairpin opening apparently occurs more downstream of the tip, giving rise to much longer P-nucleotide stretches.
|
DH |
3′del DH |
P-nucleotidesa) |
N-nucleotides |
P-nucleotides |
5′del JH |
JH |
|---|---|---|---|---|---|---|
|
Artemis-2 |
||||||
|
DH1-26 |
0 |
GTAGT |
|
|
0 |
JH4 |
|
DH1-26 |
0 |
GTAGTAGCTC |
GGGGT |
AGTATTCAGC |
0 |
JH1 |
|
DH1-26 |
0 |
GTAGTA |
|
AT |
0 |
JH6 |
|
DH2-15 |
0 |
GGAGTAGT |
TCTCAAAGTGTACAAA |
AGT |
0 |
JH4 |
|
DH2-21 |
0 |
GGAAT |
TTTAGACCAACAAAA |
|
–1 |
JH1 |
|
DH3-22 |
–2 |
|
TCTCTCA |
|
–1 |
JH1 |
|
DH2-15 |
0 |
GGAGTAG |
|
T |
0 |
JH4 |
|
DH3-9 |
0 |
GTTATAAG |
GT |
CATCA |
0 |
JH3 |
|
DH3-22 |
–3 |
|
CAC |
CATCA |
0 |
JH3 |
|
DH4-23 |
–9 |
|
TAAAGT |
AGT |
0 |
JH4 |
|
DH4-17 |
0 |
|
CAATG |
GTAAT |
0 |
JH6 |
|
DH4-23 |
0 |
GGAGT |
CGAGTAATAGGGC |
TAGTAGTAAT |
0 |
JH6 |
|
DH4-23 |
–5 |
|
GG |
AGTAGT |
0 |
JH4 |
|
DH5-12 |
0 |
GTA |
CCA |
AAAGTAGT |
0 |
JH4 |
|
DH5-5 |
0 |
|
CGTAACC |
T |
0 |
JH4 |
|
DH5-5 |
–3 |
|
CTCC |
GCATCA |
0 |
JH3 |
|
DH5-12 |
–4 |
|
CTT |
AAGTAGT |
0 |
JH4 |
|
DH6-6 |
–1 |
|
|
TTCAGC |
0 |
JH1 |
|
Artemis-4 |
||||||
|
DH5-12 |
0 |
GT |
AATCGAGTAAT |
AT |
0 |
JH6 |
|
DH2-15 |
0 |
GGAGTA |
|
AT |
0 |
JH6 |
|
DH2-21 |
0 |
GGAATA |
AG |
|
0 |
JH1 |
|
DH2-15 |
–3 |
|
TCCGGAGTAGTGGGAAA |
|
0 |
JH1 |
|
DH2-2 |
–3 |
|
GCCGGCAGCTCTCAGCA |
|
–8 |
JH6 |
|
DH3-10 |
0 |
G |
GT |
AT |
0 |
JH6 |
|
DH3-10 |
0 |
GTTAT |
|
|
0 |
JH6 |
|
DH3-22 |
0 |
GTAGT |
|
|
0 |
JH4 |
|
DH3-10 |
–28 |
|
TCCCTTACTACTTCCCCT |
AAAGTAGT |
0 |
JH4 |
|
DH3-10 |
0 |
GTTATAATAACTCCCCGAACCATAGTAATACC |
C |
CATCA |
0 |
JH3 |
|
DH3-16 |
–1 |
|
TCTT |
GTAGT |
0 |
JH4 |
|
DH4-23 |
0 |
GGA |
A |
|
0 |
JH6 |
|
DH4-17 |
0 |
GTAG |
|
|
0 |
JH2 |
|
Artemis-5 |
||||||
|
DH2-15 |
0 |
GG |
|
|
0 |
JH4 |
|
DH2-21 |
0 |
|
|
ATCA |
0 |
JH3 |
|
DH5-12 |
0 |
GT |
|
|
0 |
JH4 |
|
DH5-24 |
0 |
GTAATTG |
AGGG |
|
0 |
JH4 |
|
Artemis-8 |
||||||
|
DH1-26 |
0 |
GTAGTAG |
|
T |
0 |
JH4 |
|
DH1-26 |
0 |
|
TA |
|
–7 |
JH4 |
|
DH2-15 |
0 |
GGAGTAG |
|
|
–7 |
JH6 |
|
DH2-21 |
–8 |
|
CCT |
TAGT |
0 |
JH4 |
|
DH2-21 |
0 |
GGAATA |
AG |
|
–1 |
JH3 |
|
DH3-9 |
–1 |
|
AC |
|
–6 |
JH4 |
|
DH3-16 |
–4 |
|
C |
TAGT |
0 |
JH4 |
|
DH3-9 |
0 |
GTTATAA |
|
GTAGT |
0 |
JH4 |
|
DH3-9 |
0 |
GTTATAATAACCAG |
CCTC |
AAGTAGT |
0 |
JH4 |
|
DH3-9 |
–16 |
|
TGAGCTGGTTATT |
AAGTAGT |
0 |
JH4 |
|
DH3-22 |
–3 |
|
CCTCCGGA |
|
–8 |
JH6 |
|
DH4-4 |
0 |
GTAGT |
|
AGT |
0 |
JH4 |
|
DH4-17 |
0 |
GTAGTC |
T |
AAGTAGT |
0 |
JH4 |
|
DH5-5 |
0 |
GTAACC |
G |
|
–12 |
JH4 |
|
DH5-5 |
–4 |
|
CCCTT |
|
–4 |
JH4 |
|
DH6-19 |
0 |
TG |
|
|
–4 |
JH3 |
|
DH6-25 |
–2 |
|
G |
AGTAGTAAT |
0 |
JH6 |
|
DH6-19 |
0 |
GTACCAG |
|
TAGT |
0 |
JH4 |
- a) Underlined nucleotides can be derived from both D and J side.
|
Patients (number of junctions) |
3′del DHa) |
Total P-nucleotides |
N-nucleotides |
5′-del JH |
% of junctions with microhomology in P-region |
|---|---|---|---|---|---|
|
Artemis-deficient (53) |
–1.9 |
6.5 |
5.9 |
–1.1 |
25 |
|
Healthy control (15) |
–3.1 |
0.4 |
5.6 |
–6.0 |
0 |
- a) Average per junction.
A second characteristic of DH-JH junctions of Artemis-deficient patients was a high frequency of microhomology in the P-nucleotide regions. In 25% of the analyzed junctions, two to six P-nucleotides could be derived either from the DH segment or from the JH segment. These junctions most probably occur via annealing of the long 3′overhangs that are generated through aberrant hairpin opening (Fig. 1). Apparently, microhomology of P-nucleotide stretches plays an important role in DH-JH junction formation in Artemis-deficient patients, presumably by facilitating ligation of two ends with long (P-nucleotide) overhangs.
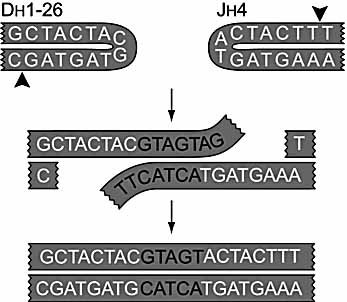
Rearrangement of a DH1–26 to a JH4 gene segment with microhomology in the P-nucleotide region. Hairpin opening occurred seven nucleotides downstream of the tip resulting in large 3′ overhang, which annealed prior to ligation leading to microhomology in the P-nucleotide region (indicated with black letters).
V(D)J recombination assay
We developed a V(D)J recombination assay to further investigate the role of hairpin opening and P-nucleotide microhomology use in coding joint formation. The V(D)J recombination substrate contains two RSS in inversion orientation that were flanked by a four-base pairs direct repeat (Fig. 2A). This recombination substrate was cotransfected with RAG1 and RAG2 expression constructs into wild-type or Artemis-deficient fibroblasts. The excised DNA fragment is inverted, resulting in a signal joint and a coding joint in the same plasmid. The coding joints can be amplified by a nested PCR approach (Fig. 2A). Depending on the way of hairpin opening and coding joint formation, three different types of coding joints can be generated and discriminated from each other by restriction enzyme digestion (Fig. 2B). Coding joints with long stretches of homologous P-nucleotides give an NgoMI restriction site, while a four-base pair microhomology of the coding ends creates a NotI restriction site. Junctions that have been processed differently will not contain either of the two restriction sites.
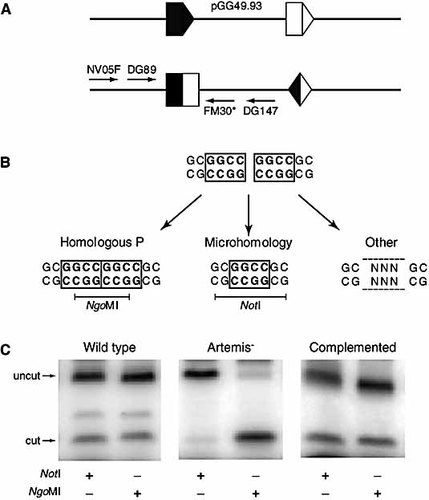
V(D)J assay for testing hairpin opening activity. (A) pGG49.93 containing two RSS in inversion orientation. The PCR primers, which were used for amplification of the coding joints, are indicated. (B) Depending on the way of hairpin opening three different types of coding joints can be formed. (C) PCR and restriction enzyme digestion of coding joints recovered from C5RO wild-type fibroblasts after cotransfection of pGG49.93 and RAG1 and RAG2 expression constructs (left panel), Artemis-1 fibroblasts (middle panel) or Artemis-1 fibroblasts complemented with wild type Artemis (right panel). Without restriction enzyme digestion, only PCR products were found with the size of uncut products.
Coding joints in C5RO wild type were of diverse nature: of these junctions, approximately 30% were NgoMI sensitive, approximately 30% were NotI sensitive and the rest had a different composition (Fig. 2C). Artemis-deficient fibroblasts (including fibroblasts of Artemis-8) yielded only NgoMI-sensitive coding joints with microhomologous P-nucleotide stretches, consistent with the high frequency of microhomology of P nucleotides in BMMC-derived DH-JH junctions. Cotransfection of a wild-type Artemis expression construct restored the normal distribution of junctions.
This assay can be used for easy discrimination between fibroblasts derived from Artemis-deficient SCID patients and LIG4-deficient SCID patients, because the latter did not show the characteristic shifted pattern (data not shown).
Validation of the V(D)J recombination assay for Artemis activity studies
To evaluate whether our assay can be used to test Artemis mutants, which can be either generated via site-directed mutagenesis or identified in a patient and subsequently cloned into an expression vector, we performed complementation experiments with Artemis mutants that have been described in the V(D)J recombination literature 23, 24. These two independent studies have shown that eight conserved amino acids are indispensable for the Artemis endonucleolytic activity. In addition, they showed that the C terminus could be deleted without losing V(D)J recombination activity, whereas small N-terminal deletions are already deleterious for Artemis function. We therefore generated four N-terminal deletion mutants (NΔ7, NΔ16, NΔ45, and NΔ66), four C-terminal mutants (CΔ80, CΔ135, CΔ232, and CΔ331), and four point mutants (D17A, H33A/H35A, H115A, and H319A) and tested whether these mutants could restore the shifted junction pattern of 100% NgoMI-sensitive to a normal distribution (Fig. 3A, B).
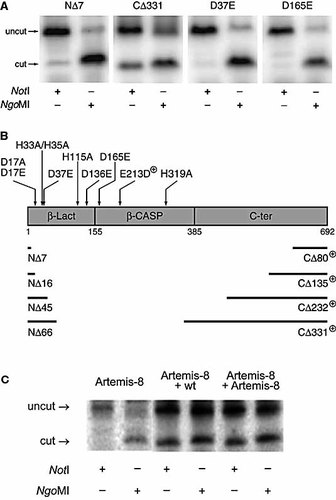
Mutational analysis of the Artemis gene. (A) Analysis of coding joints from Artemis-1 fibroblasts after cotransfection of pGG49.93 and RAG1 and RAG2 expression constructs and four Artemis mutants (NΔ7, CΔ331, D37E and D165E). (B) Schematic representation of Artemis with the metallo-β-lactamase (β-Lact) homology region, the β-CASP motif and the C-terminal region. The mutants that were able to complement the defect in Artemis-deficient cells are indicated with a + sign. CΔ331 could almost fully complement the Artemis defect. (C) V(D)J recombination assay with Artemis-8 fibroblasts. PCR and restriction enzyme digestion of coding joints recovered after cotransfection of pGG49.93 and RAG1 and RAG2 expression constructs (left panel), complemented with wild-type Artemis expression construct (middle panel), and complemented with Artemis-8 expression construct.
In line with published data, the N-terminal deletion or point mutants did not show complementation, indicating that the N-terminal region is indispensable for V(D)J recombination. All C-terminal deletion mutants complemented the Artemis defect. The C-terminal mutant with the largest deletion (CΔ331), however, did not show full complementation (Fig. 3A), probably because a small part of the β-CASP domain was lost. Based on these results, we conclude that our V(D)J recombination assay can reliably be used for Artemis activity studies.
Further mapping of the Artemis-active site
Artemis is assigned to the metallo-β-lactamase superfamily, based on sequence homology. Therefore, the mechanism of this structure-specific endonuclease has been proposed to function similarly. In this model, H33, H35, D37, and H38 form the conserved metallo-β-lactamase HxHxDH signature. H115 and H319 have been proposed to coordinate two active site metals, while D17, D136 and/or D165 may form salt bridges to the HxHxDH motif and stabilize its conformation. However, alternative models may also be considered to explain some observations that cannot be explained easily by this model. First, D136 and D165 have not been found in the sequences of available metallo-β-lactamase crystal structures. Furthermore, metallo-β-lactamases coordinate Zn2+ ions in the active site, whereas biochemical experiments show that Mg2+ ions are required for activity 23.
Therefore, we considered another possible nuclease mechanism. Retroviral integrases and several transposases coordinate Mg2+ in the active site for DNA cleavage using an array of three acidic amino acids, i.e. two aspartates (D) and one glutamate (E) residue, referred to as a DDE motif 27. The acidic amino acids in this motif cannot be exchanged for any other amino acid residue without loss of activity. Even the most conservative mutations (D to E or E to D) result in inactive proteins. We reasoned that changing D to E or E to D would inactivate the Artemis protein if an array of acidic amino acids is required for divalent metal ion coordination, while a metallo-β-lactamase active site would probably not be disrupted (completely) by such a conservative substitution.
Therefore, we generated five Artemis mutants in the D and E amino acids that have been conserved between Artemis and Snm1 (D17E, D37E, D136E, D165E, and E213D). Except for E213D, cotransfection of these Artemis mutants did not result in complementation of the Artemis defect (Fig. 3A, B), suggesting that they may indeed be involved in metal ion coordination. The importance of D37 is supported by the finding of a mutation in this amino acid in SCID Artemis-9, i.e. p.D37G, also results in radiosensitive SCID. We therefore propose in an alternative model that the Artemis-active site might contain an array of conserved acidic amino acids that coordinate Mg2+ ions. This motif might be stabilized by the metallo-β-lactamase fold, in which the histidines could aid in coordination of the acidic residues and/or accept a proton from the water molecule that acts as the nucleophile that cleaves the phosphodiester bond in the DNA.
SCID patient with a mutation in the Artemis C terminus
One of the SCID patients, Artemis-8, had a mutation affecting the C-terminal region of the Artemis protein resulting in a frame-shift and a premature stop codon (p.G464AfsX18). This seemingly contradicts the observation that C-terminal deletion mutants complement the Artemis defect. We hypothesized that the expression level of the C-terminally mutated Artemis protein in patient cells is insufficient to carry out non-homologous end joining (NHEJ) and V(D)J recombination resulting in radiosensitive SCID. Therefore, we cloned this mutation in the pDVG90 expression vector and tested this Artemis-8 mutant in our V(D)J recombination assay. The Artemis-8 mutant was indeed able to complement the Artemis defect in Artemis-1 fibroblasts and even in Artemis-8 fibroblasts (Fig. 3C), showing that complementation was caused by overexpression. Therefore, the results of activity assays with transient overexpression of mutant Artemis protein should be interpreted with caution, since disease-causing Artemis mutations with residual activity might be masked by the transient overexpression.
Concluding remarks
This study demonstrates the value of different assays in the identification of genetic defects in T–B–NK+ SCID. If a T–B–NK+ SCID patient does not have a mutation in the RAG1 or RAG2 genes, patient fibroblasts can be tested for ionizing radiation sensitivity. In case of radiosensitivity, analysis of the coding joints of residual DH-JH rearrangements provides additional insight in the type of V(D)J recombination defect. Defects in the Artemis gene result in aberrant hairpin opening and give rise to strongly increased numbers of P-nucleotides in the coding joints with a high frequency of microhomology in the P-region (25%). Sequence analysis of the Artemis gene will always be required to identify the genetic defect. Finally, our V(D)J recombination assay can be used to determine whether the identified Artemis mutation is indeed disease causing. In this assay the fibroblasts of the patient will show the characteristic Artemis defect, which can be complemented by cotransfection of the wild-type Artemis expression construct. This approach is preferred over complementation of Artemis-deficient cells with an Artemis expression construct containing the mutation identified in the patient, because overexpression might mask disease-causing mutations that allow low levels of residual Artemis activity.
Materials and methods
Patient samples
Five radiosensitive (RS) SCID patients were studied. Informed consent for patient material was obtained according to the guidelines of the medical ethics committees of Hacettepe University Children's Hospital and Erasmus MC. All patients were diagnosed with SCID within the first 6 months of life based on failure to thrive, recurrent infections and absence of B and T cells. The previously described Artemis-2 patient carries a homozygous G118V mutation 16, 28, Artemis-4 and 5 had a homozygous deletion of exons 1 and 2. Artemis-8 has a homozygous deletion of five nucleotides (c.1391_1395delGAATC) resulting in a frame shift and a premature stop codon (p.G464AfsX18) and Artemis-9 had a homozygous missense mutation (c.A110G; p.D37G).
Analysis of DH-JH junctions in Artemis-deficient SCID patients
DNA was isolated from BMMC of four Artemis-deficient SCID patients and healthy donors. From the fifth Artemis-deficient patient (Artemis-9) no BMMC were available. Analysis of DH-JH gene rearrangements was performed as previously described 20. Due to the low frequency of DH-JH junctions, a second PCR was performed to generate sufficient amounts of PCR product for cloning and sequencing.
V(D)J recombination assay
C5RO wild type or Artemis-1 fibroblasts containing a homozygous deletion of exons 10–12 16 were transfected with a recombination substrate (pGG49.93), RAG1 and RAG2 expression constructs and one of the Artemis mutant constructs 16, 29. The pGG49.93 was made by cloning a 1.4-kb AflIII (blunted)-BamHI fragment of pDvG93 into the SpeI (blunted)-BamHI digested vector pGG49 16, 29, 30. After 48 h, the fibroblasts were harvested and extrachromosomal DNA was isolated 29. To amplify the coding joints, a nested PCR of 2 × 25 cycles was performed. The first round of PCR was performed with oligonucleotides NV05F and DG147. Subsequently, 1 µL of this PCR reaction was used as a template for the second round of PCR using oligonucleotides DG89 and radioactively labeled FM30 31. PCR products were digested with NotI or NgoMI. After electrophoresis, the gel was dried and PCR products were visualized by phosphorimaging.
Artemis mutant expression constructs
For in vitro PCR-based mutagenesis, the Artemis wild-type construct pDVG190 (based on pCDM8, Invitrogen) was used 16. Several deletion and point mutants were generated. Details are available upon request. The protein expression levels of the Artemis-mutants were checked by Western blot analysis of transiently transfected COS cells.
Acknowledgements
The authors thank Mr. T. van Os for making the figures. This work was supported by grants from the Dutch Organization for Scientific Research (NWO/ZonMw veni grant 916.56.107; MvdB), the Dutch Cancer Society (KWF, grant EMCR 2002–2734; NSV, MvdB), and the European Union (RISC-RAD and DNA repair; DCvG).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




