A critical lineage-nonspecific role for pTα in mediating allelic and isotypic exclusion in TCRβ-transgenic mice
Abstract
Although it is well established that early expression of TCRβ transgenes in the thymus leads to efficient inhibition of both endogenous TCRβ and TCRγ rearrangement (also known as allelic and “isotypic” exclusion, respectively) the role of pTα in these processes remains controversial. Here, we have systematically re-evaluated this issue using three independent strains of TCRβ-transgenic mice that differ widely in transgene expression levels, and a sensitive intracellular staining assay that detects endogenous TCRVβ expression in individual immature thymocytes. In the absence of pTα, both allelic and isotypic exclusion were reversed in all three TCRβ-transgenic strains, clearly demonstrating a general requirement for pre-TCR signaling in the inhibition of endogenous TCRβ and TCRγ rearrangement. Both allelic and isotypic exclusion were pTα dose dependent when transgenic TCRβ levels were subphysiological. Moreover, pTα-dependent allelic and isotypic exclusion occurred in both αβ and γδ T cell lineages, indicating that pre-TCR signaling can potentially be functional in γδ precursors. Finally, levels of endogenous RAG1 and RAG2 were not down-regulated in TCRβ-transgenic immature thymocytes undergoing allelic or isotypic exclusion. Collectively, our data reveal a critical but lineage-nonspecific role for pTα in mediating both allelic and isotypic exclusion in TCRβ-transgenic mice.
Abbreviations:
-
- DN:
-
CD4– CD8– double negative
-
- DP:
-
CD4+ CD8+ double positive
-
- ic:
-
intracellular
Introduction
T cells can be divided into αβ and γδ lineages based on their expression of TCRαβ or TCRγδ. During development in the thymus, αβ and γδ lineages separate at an early CD4– CD8– double-negative (DN) stage, but the precise mechanism of lineage divergence remains controversial.
One molecule that has been postulated to play a key role in αβ/γδ lineage commitment is the pTα chain. pTα is a critical component of the pre-TCR which consists, in addition, of a functionally rearranged TCRβ chain and CD3 signaling components. Signaling through the pre-TCR has been reported to be responsible for many important functions during the development of αβ lineage cells, including TCRβ allelic exclusion 1–3, TCRγ silencing 4, and extensive proliferation during the transition from DN to CD4+ CD8+ double-positive (DP) stages 5. Since pTα–/– mice have an increased number of γδ cells that harbor an increased frequency of in-frame TCRβ rearrangements 6, it has also been proposed that the pre-TCR favors αβ lineage commitment at the expense of γδ cells 6.
Since functions of pTα are often difficult to study in normal mice, several groups have crossed pTα-deficient mice with TCRβ-transgenic (Tg) mice to assess various putative roles for the pre-TCR during thymus development 7, 8. However, the results of these studies are in many cases controversial and it is still not known whether transgenic “artifacts” caused by overexpression or premature expression of the transgenic TCRβ chain may obscure the contribution of pTα to the observed phenotypes.
In order to re-evaluate this issue in a systematic manner, we have utilized three independent TCRβ Tg mouse strains that express TCRβ over a very wide (>50-fold) range of concentrations, to assess the role of pTα in mediating allelic exclusion at the endogenous TCRβ locus as well as “isotypic” exclusion of TCRγ rearrangement and consequent inhibition of γδ cell development. In addition, we have employed a highly sensitive double intracellular (ic) staining protocol 9 to quantitate allelic exclusion in individual thymocytes of both the αβ and γδ lineages. Our data clearly demonstrate that pTα plays an essential role in mediating both allelic and isotypic exclusion and that both these pTα-dependent functions can potentially occur in either the αβ or the γδ lineage. Implications of these findings for immature thymocyte development and lineage commitment will be discussed.
Results
TCRβ transgenes mediate pTα-dependent allelic exclusion in immature DN3 thymocytes
Although it is well established that early expression of TCRβ transgenes in the thymus leads to efficient inhibition of endogenous TCRβ rearrangement (otherwise known as allelic exclusion), the role of pTα in this process remains controversial 7, 8. One technical problem with previous studies has been that allelic exclusion in the thymus has been assessed either at the population level (via endogenous TCRβ rearrangements) or at the single-cell level in mature T cells (via surface staining for endogenous TCRVβ chains). Since allelic exclusion is first detectable at the immature DN3 stage of thymus development (where endogenous TCRβ rearrangement occurs), we have adapted a previously described double ic staining protocol for TCRβ and Vβ 9, in order to optimize analysis of allelic exclusion in individual DN3 thymocytes. Moreover, since there is some indication that expression levels or developmental timing of TCRβ transgenes may influence the efficiency of allelic exclusion 10, we have utilized three independent strains of TCRβ Tg mice that express icTCRβ very early in development but differ widely in icTCRβ expression. Thus, TCRVβ8.2 and TCRVβ3 Tg mice express approximately six- and twofold more icTCRβ than WT mice, whereas TCRVβ8.1 Tg mice express about tenfold less (according to mean fluorescence intensity of icTCRβ expression; Fig. 1A).
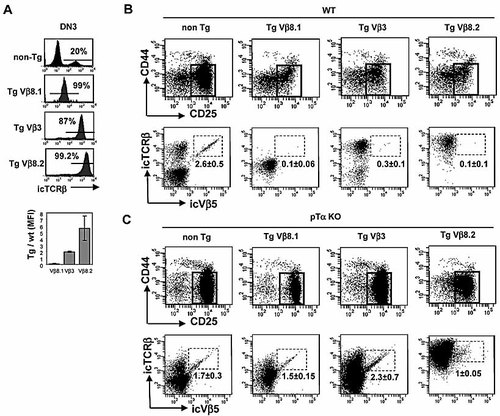
TCRβ transgene-mediated allelic exclusion depends on pTα. (A) Intensity of expression of TCRβ transgenes. Histograms show the icTCRβ staining in DN3 thymocytes from non-Tg or the indicated TCRβ Tg mice on WT background. The mAb used for ic TCRβ expression recognizes the constant region of TCRβ. The percentage of icTCRβ+ DN3 cells is indicated. The bar diagram shows the expression level of each TCRβ trangene relative to endogenous TCRβ expression. Mean fluorescence intensity (MFI) values were used for this calculation. Results are shown as mean ± SD of at least four independent experiments for each transgene. (B, C) CD44 versus CD25 profile in cocktail– thymic populations (see Materials and methods) and icTCRβ versus icVβ5 profile in DN3 thymocytes, from non-Tg or the indicated TCRβ Tg mice, on either WT background (B) or pTα KO background (C). The gate used to analyze DN3 thymocytes is shown in the CD44 versus CD25 dot plot. The number indicated in each icTCRβ versus icVβ5 dot plot corresponds to the percentage of icVβ5+ cells in the total DN3 population (shown as mean ± SD, n ⩾3).
As expected from previous studies 11, 12, all three TCRβ Tg mice exhibited virtually complete inhibition of endogenous TCRβ rearrangements, as evidenced by the absence of icTCRVβ5- (Fig. 1B) or icTCRVβ6-expressing cells (data not shown) in DN3 thymocytes. In order to address the role of pre-TCR signaling in allelic exclusion, the three TCRβ Tg mice were backcrossed onto a pTα–/– 13 or CD3ϵ–/– 14 genetic background. Consistent with an earlier report using CD3ϵ–/– TCRVβ8.1 Tg mice 15, endogenous TCRVβ5 expression was restored to control levels in DN3 thymocytes from all three TCRβ Tg strains on a CD3ϵ–/– background (data not shown), confirming the critical role of the CD3 complex in signaling allelic exclusion. Interestingly, a similar result was obtained when the three TCRβ Tg strains were crossed to pTα–/– mice (Fig. 1C), although restoration of endogenous TCRVβ5 expression was incomplete in pTα–/– TCRVβ8.2 Tg mice expressing the highest levels of transgenic icTCRβ. These data clearly demonstrate that pTα is essential to mediate allelic exclusion by TCRβ transgenes in DN3 thymocytes over a wide range of icTCRβ expression.
TCRβ transgenes mediate pTα-dependent “isotypic” exclusion at the TCRγ locus
In addition to their ability to mediate allelic exclusion, TCRβ transgenes have been shown to efficiently block endogenous TCRγ rearrangements and reduce γδ cell numbers in the thymus 10, 16, 17, a process referred to as “isotypic” exclusion 16. The role of pTα in isotypic exclusion is not clear, since a single study using pTα–/– TCRVβ8.2 Tg mice demonstrated only a modest restoration of endogenous Vγ rearrangements and γδ T cell numbers 8. As shown in Fig. 2A, all three TCRβ transgenes strongly reduced thymic γδ T cell numbers, although the greatest reduction was observed with the highly expressed TCRVβ8.2. As noted previously 13, absolute γδ T cell number increased significantly (threefold) in pTα–/– mice (Fig. 2A, B). Interestingly, γδ cell numbers were increased to an even greater extent (20–60-fold) in the three TCRβ Tg mice in the absence of pTα (Fig. 2A), reaching levels close to those in nontransgenic pTα–/– controls (Fig. 2B). It should be noted that icTCRβ expression was significantly reduced in DN3 thymocytes on the pTα–/– background in both WT and TCRβ Tg mice (see Fig. 1B, C). This effect has been noted previously 18 and may reflect a reduced stability of the TCRβ protein in the absence of pTα. In any event, these data demonstrate that pTα also plays a critical role in mediating isotypic exclusion by TCRβ transgenes.
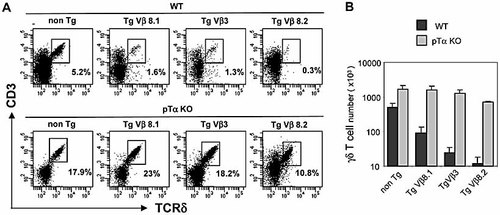
TCRβ transgene-mediated “isotypic” exclusion at the TCRγ locus depends on pTα. (A) Dot plots show the cell surface staining of CD3ϵ versus TCRδ in DN lineage– thymocytes (see Materials and methods) from non-Tg or the indicated TCRβ Tg mice, on WT or pTα KO backgrounds. The percentage of CD3+ TCRδ+ cells is indicated for each case. Data are representative of at least three independent experiments. (B) The bar diagram indicates the absolute number of thymic γδ T cells in non-Tg or the indicated TCRβ Tg mice on WT or pTα KO backgrounds. Values are shown as means ± SD (at least three mice were analyzed for each type).
TCRβ transgenes mediate pTα-dependent allelic exclusion in γδ cell precursors
The ability of pTα to simultaneously mediate allelic and isotypic exclusion by TCRβ transgenes in immature thymocytes suggests that pTα may be active in precursors of both αβ and γδ lineages. Previous studies using pTα ic staining 6, single-cell PCR 19 and reporter mice 20 are consistent with the possibility that most DN3 thymocytes, which have recently been shown to contain a very small subset of γδ precursors 21, express pTα. However, no functional evidence for the presence of pTα in γδ precursors has been reported. To address this issue directly, we took advantage of the fact that a small but distinct subset (10–15%) of thymic γδ cells undergo functional TCRβ rearrangement, resulting in the expression of icTCRβ 6, 22. As shown in Fig. 3A, the TCRβ locus is in fact allelically excluded in γδ cells from all three TCRβ Tg mouse strains, since endogenous TCRVβ5-expressing cells are completely absent. Importantly, allelic exclusion is lost in TCRβ Tg γδ cells in the absence of pTα (Fig. 3B) although restoration of endogenous TCRVβ5 cells is incomplete in TCRVβ8.2 Tg mice, as observed previously for DN3 thymocytes (Fig. 1C). These data clearly demonstrate that pTα is expressed and potentially functional in the γδ cell lineage.
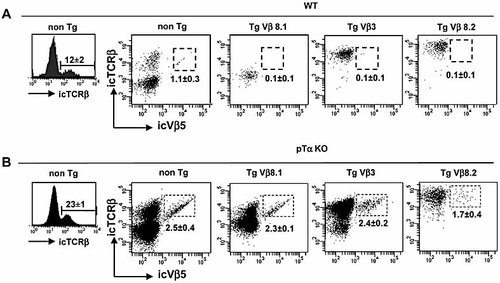
pTα-dependent allelic exclusion also occurs in γδ T cells. (A, B) Histograms show the percentage (mean ± SD, n ⩾5) of icTCRβ+ γδ T cells from WT or pTα KO mice. Dot plots show icTCRβ versus icVβ5 staining in thymic γδ T cells from non-Tg or the indicated TCRβ Tg mice. The percentage of icVβ5+ cells in the total γδ T cell population is indicated (mean ± SD, n ⩾3).
Similar levels of pTα mediate allelic and isotypic exclusion in αβ and γδ lineages
The preceding data indicate that pTα can potentially mediate allelic exclusion in both αβ and γδ cell lineages, as well as isotypic exclusion. However, it is not clear whether the efficiency of pre-TCR signaling differs in each of these situations. To address this important issue, we took advantage of the fact that the TCRVβ8.1 transgene, although expressed at very low levels (Fig. 1A), was nevertheless capable of mediating efficient pTα-dependent allelic and isotypic exclusion in both αβ and γδ lineages (Fig. 1–3). In this situation, the ability of the weakly expressed TCRVβ8.1 transgene to function in pre-TCR signaling might be expected to depend upon pTα gene dosage. Indeed, as shown in Fig. 4A, haploinsufficient pTα+/– TCRVβ8.1 Tg mice showed an intermediate phenotype with respect to their ability to mediate allelic exclusion in both αβ and γδ lineages as well as in their capacity to suppress γδ cell numbers via isotypic exclusion (Fig. 4B). Thus, it appears that inhibition of TCRβ and TCRγ rearrangements in TCRβ Tg mice can occur as a result of similar levels of pre-TCR signaling, independently of lineage.
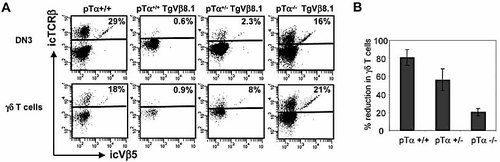
pTα gene dosage affects allelic and isotypic exclusion. (A) icTCRβ versus icVβ5 staining in DN3 thymocytes (upper panels) or thymic γδ T cells (lower panels) from TCRVβ8.1 Tg mice on a pTα+/+, pTα+/– and pTα–/– background. The horizontal line in each dot plot separates endogenous TCRβ+ and TCRβ– cells in non-Tg mice, or endogenous TCRβ+ and Tg TCRβ+ cells in TCRVβ8.1 Tg mice. The percentage of endogenous icTCRβ-expressing cells is indicated. Data are representative of at least three independent experiments. (B) Percentage of reduction in the absolute number of thymic γδ T cells induced by TCRVβ8.1 transgene on pTα+/+, pTα+/– or pTα–/– backgrounds (mean ± SD, n = 3).
pTα-dependent allelic and isotypic exclusion occur despite normal levels of RAG expression
The ability of the pre-TCR to mediate allelic and isotypic exclusion at similar pTα doses in TCRβ Tg mice raises the possibility that a similar mechanism may be involved in shutting off TCRβ and TCRγ rearrangement. One obvious candidate for such a mechanism would be down-regulation of expression of the RAG genes, as has been shown for DP thymocytes undergoing positive selection 23–25. However, as shown in Fig. 5A, expression of RAG1 mRNA in DN3 thymocytes (where the vast majority of TCRβ and TCRγ rearrangements occur) is not affected by the presence of a TCRVβ8.1 transgene that efficiently inhibits endogenous TCRβ and TCRγ rearrangement (10 and Fig. 5B). Furthermore, RAG2 protein, which can be regulated either at the mRNA level 25 or via phosphorylation and subsequent degradation in cycling cells 26, 27, was not down-regulated in DN (Fig. 5C) and DN3 (data not shown) thymocytes from all three strains of TCRβ Tg mice. Control experiments using DP thymocytes from TCRαβ Tg P14 mice confirmed that both RAG1 mRNA (Fig. 5A) and RAG2 protein (Fig. 5C) were dramatically down-regulated upon TCR engagement, as expected from previous reports 23–25. Collectively, these data indicate that allelic and isotypic exclusion in DN3 thymocytes can occur independently of regulation of RAG expression.
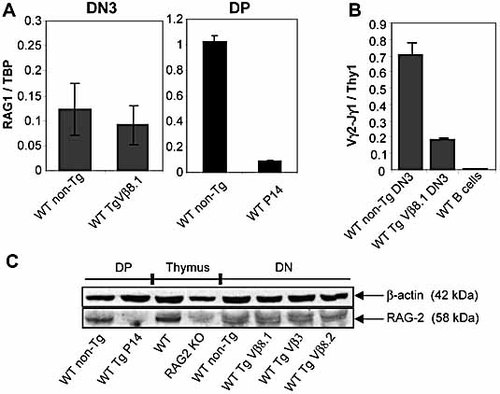
TCRβ transgenes fail to inhibit RAG expression in DN3 thymocytes. (A) Quantitative real-time RT-PCR was performed to compare RAG1 mRNA expression in sorted DN3 thymocytes from non-Tg or TCRVβ8.1 Tg mice. The same analysis was performed in DP thymocytes from WT or P14 Tg mice. Results are shown in arbitrary units (mean ± SD) after normalization to TBP. Note the different scales used for DN3 and DP thymic populations. (B) Genomic DNA was extracted from sorted DN3 thymocytes from non-Tg or TCRVβ8.1 Tg mice and from lymph node B cells. Vγ2-Jγ1 rearrangements were analyzed by real-time PCR. Values correspond to arbitrary units (mean ± SD) after normalization to Thy1. The negative result obtained in B cells shows the specificity of the real-time PCR conditions. (C) Western blot analysis for RAG2 expression. Total thymocytes from WT and RAG2 KO mice were used as positive and negative controls, respectively. Of total protein, 14 µg was loaded for total thymus or DN samples, and 10 µg for sorted DP samples. In DP samples, only one band is specifically recognized by the anti-RAG2 Ab (compared to RAG2 KO sample) whereas DN samples show two specific bands differing only slightly in size, possibly reflecting different phosphorylation states of RAG2. The same result was obtained when sorted DN3 thymocytes were analyzed.
Discussion
Although it has been known for many years that early expression of a transgenic TCRβ chain can efficiently inhibit endogenous rearrangements at the TCRβ 7, 11, 12 and TCRγ 10, 16, 17 loci, the role of pre-TCR signaling in this process has remained controversial 7, 8. The data presented here clearly demonstrate that pTα has a critical role in mediating both allelic and isotypic exclusion in three independent strains of TCRβ Tg mice.
In considering apparent discrepancies with earlier studies 7, 8, it is noteworthy that, whereas we observed complete restoration of endogenous TCRβ expression in the absence of pTα for the TCRVβ3 and TCRVβ8.1 Tg mice, only partial restoration was seen for TCRVβ8.2 Tg mice. Importantly, the TCRVβ8.2 Tg is expressed at nearly six times the level of endogenous TCRβ in immature DN3 thymocytes whereas TCRVβ3 is only expressed at two times the normal levels, and TCRVβ8.1 at tenfold less than normal levels. Indeed, the same highly expressed TCRVβ8.2 transgene was used in a previous study 8 where allelic exclusion was observed to occur (at least partially) in the absence of pTα. The pTα-independent contribution to allelic exclusion in TCRVβ8.2 Tg mice nevertheless requires CD3 components, since endogenous TCRβ expression was completely restored in CD3ϵ–/– TCRVβ8.2 Tg mice (unpublished data). The simplest explanation for these data would be to postulate that allelic exclusion is critically dependent upon pTα as long as TCRβ expression levels remain within the physiological (for TCRVβ3 Tg mice) or subphysiological (for TCRVβ8.1 Tg mice) range. In cases where TCRβ is highly overexpressed (such as in TCRVβ8.2 Tg mice), one could speculate that pTα-independent TCRβ:CD3 complexes reach a threshold level that is capable of partially inhibiting TCRβ rearrangement, perhaps by favoring dimerization of CD3ϵ, as recently reported for pTα 28.
Despite extensive investigation over many years, the mechanism of allelic exclusion at the TCRβ locus remains obscure (reviewed in 29). In this context, our data with TCRβ Tg mice as well as other results with DN3 thymocytes from normal mice 1 clearly implicate pTα as being required for allelic exclusion. Possible consequences of pre-TCR signaling that might regulate allelic exclusion include modifications in chromatin accessibility 30 or contraction 31 at the TCRβ locus itself as well as changes in the expression 32 or stability 26 of the RAG proteins. With regard to the latter point, our failure to observe down-regulation of RAG1 mRNA or RAG2 protein expression in DN3 thymocytes in the presence of a TCRβ transgene does not support a direct role for pre-TCR signaling in the regulation of RAG expression. Further experiments will be required to establish the precise role of pre-TCR signaling in allelic exclusion.
Although a requirement for pre-TCR signaling in TCRβ transgene-mediated allelic exclusion is not unexpected in view of the fact that pTα clearly contributes to allelic exclusion in immature DN3 thymocytes from normal mice 1, our finding that isotypic exclusion of TCRγ genes is also controlled by pTα in three independent strains of TCRβ Tg mice is somewhat more surprising. Indeed, a previous study 8 using the same TCRVβ8.2 Tg mice as described here noted only a slight increase in TCRγ rearrangements and γδ cell numbers in the absence of pTα. In contrast, we observed a dramatic (60-fold) increase in absolute γδ cell numbers in pTα–/– TCRVβ8.2 Tg mice, although the levels reached were still twofold lower than in control pTα–/– mice. Moreover, essentially complete restoration of γδ cell numbers was achieved in both pTα–/– TCRVβ3 Tg and pTα–/– TCRVβ8.1 Tg mice, demonstrating clearly that pTα is essential to mediate isotypic exclusion under conditions where TCRβ transgenes are expressed at near physiological or even subphysiological levels.
The strong pTα dependence of inhibition of γδ T cell development observed in all three TCRβ Tg strains suggests that pTα may be expressed in precursors of the γδ lineage. This speculation is further supported by other studies showing that suppression of γδ cell development in TCRβ Tg mice can be reversed by simultaneous expression of a TCRγδ transgene 33. To obtain more direct evidence that pTα is expressed and potentially functional in the γδ lineage, we analyzed the small fraction of γδ cells that express a functional icTCRβ protein 6, 22. Interestingly, the endogenous TCRβ locus was allelically excluded in such γδ cells in all three TCRβ Tg lines, and this allelic exclusion could be reversed in the absence of pTα. These data provide direct functional evidence that pTα is expressed in precursors of γδ cells, confirming and extending earlier studies of ic pTα 6 and pTα reporter gene expression 20 in immature DN3 thymocytes. Thus, models of αβ/γδ lineage commitment that propose a restricted expression or function of pTα in the αβ lineage 34, 35 are clearly oversimplified.
The ability of pTα to mediate both allelic and isotypic exclusion in TCRβ Tg mice suggests that pre-TCR signaling may have similar consequences at the TCRβ and TCRγ loci. This conclusion is strengthened by our observation that both allelic and isotypic exclusion are pTα dose dependent, as evidenced by the intermediate phenotype in pTα+/– TCRVβ8.1 Tg mice which express very low levels of icTCRβ. Thus, similar levels of pre-TCR signaling are apparently capable of terminating rearrangement at either the TCRβ or TCRγ locus. Furthermore, since allelic exclusion in γδ cells is pTα dependent and TCRγ rearrangements in DN3 thymocytes (which are mostly αβ lineage cells) are inhibited by pre-TCR signaling, the ability of pTα to mediate allelic and isotypic exclusion is not restricted to the αβ or γδ lineage.
The ability of pTα to efficiently signal isotypic exclusion via inhibition of TCRγ rearrangements in TCRβ Tg mice places clear constraints on the sequence of TCR gene rearrangements in normal mice. Thus, whereas it is often presumed that TCRγ, TCRδ and TCRβ rearrangements occur simultaneously 36, 37 during thymus development, it can be inferred from our data that the vast majority of TCRγ (and perhaps TCRδ?) rearrangements must take place prior to VDJ rearrangements at the TCRβ locus, or else normal γδ cell development would be severely compromised by pre-TCR-induced isotypic exclusion. Viewed from this perspective, the fact that some γδ cells escape isotypic exclusion and develop in TCRβ Tg mice may reflect very early rearrangement of γ and δ genes (prior to expression of TCRβ transgenes and/or endogenous pTα). Indeed, it has been shown that pre-TCR expression does limit TCRγ rearrangements in individual γδ cells to some extent 38, thereby potentially explaining (at least in part) the increased numbers of γδ cells in pTα-deficient mice. Nevertheless, other factors such as homeostatic competition and putative lineage commitment functions of pTα 6 may also contribute to the expansion of γδ cells in the absence of pre-TCR signaling.
In contrast to isotypic exclusion, the possible physiological consequence of pTα-mediated allelic exclusion in the γδ lineage is less clear, since pre-TCR signaling cannot occur until after TCRβ protein is expressed. Thus, whereas allelic exclusion can potentially prevent simultaneous expression of two functional TCRβ chains in individual γδ cells, it should not have any effect on the vast majority of γδ T cells that do not express icTCRβ protein. According to this reasoning, lack of allelic exclusion could explain the observed increase in frequency of productive TCRβ rearrangements in pTα-deficient γδ cells 6, but not the increased number of γδ cells in pTα-deficient mice.
In conclusion, our data confirm and extend the already long list of functions mediated (or potentially mediated) by pTα. In addition to the well-established role of pTα in expansion of αβ lineage-committed thymocytes 5, 39 and its related (although indirect) role in TCRγ silencing 4, we confirm with three independent TCRβ Tg mouse lines that pTα is strictly required to mediate allelic exclusion of endogenous TCRβ genes and isotypic exclusion of γδ cell development. The extent to which pTα influences αβ/γδ lineage commitment in normal mice by these latter mechanisms remains to be directly established.
Materials and methods
Mice
C57.BL/6 WT mice were purchased from Harlan Netherlands (Horst, The Netherlands). C57.BL/6 pTα–/– mice 13 were originally provided by Dr. H.-J. Fehling (University Clinics, Ulm, Germany). C57.BL/6 Tg Vβ8.1 11 and C57.BL/6 Tg P14 40 mice were originally obtained from Dr. H.-P. Pircher (University of Freiburg, Germany). C57.BL/6 Tg Vβ3 41 and C57.BL/6 Tg Vβ8.2 12 mice were originally obtained from Dr. M. Dohlsten (Pharmacia, Sweden) and Dr. H. Bluethman (Hoffmann-La Roche AG, Switzerland), respectively. C57.BL/6 Tg Vβ8.1, C57.BL/6 Tg Vβ3 and and C57.BL/6 Tg Vβ8.2 mice were backcrossed to pTα–/– mice. C57.BL/6 RAG2–/– mice were purchased from Taconic. All mice were used at 4–8 wk of age. This study has been reviewed and approved by the Service Vétérinaire Cantonal of Etat de Vaud.
Cell preparation, flow cytometry and sorting
For the analysis of icTCRβ expression on CD25+ CD44– DN (DN3) thymocytes, or DN3 sorting, WT DN-enriched 22 or total pTα–/– thymocyte cell suspensions were incubated with a cocktail of FITC-conjugated Ab to CD4, CD8, CD3ϵ, TCRβ, TCRδ, B220, CD11c, Ter119, CD161, Gr-1 and F4/80, together with anti-CD44-Alexa647, anti-CD25-APC-Cy7 and anti-CD117-PE-Cy7 Ab. DN3 thymocytes were defined as cocktail– CD117– CD25+ CD44– cells. Staining for γδ T cells was performed by incubating WT DN-enriched or total pTα–/– thymocyte cell suspensions with a cocktail of PE-Cy7-conjugated Ab to CD4, CD8, B220, Ter119, TCRβ, GR-1, NK1.1 and CD11b, together with anti-CD3ϵ-Alexa647 and anti-TCRδ-FITC Ab. γδ T cells were defined as cocktail– CD3ϵ+ TCRδ+ cells. icTCRβ staining was performed as described 9, 22. For the isolation of DP thymocytes, WT or P14 Tg thymocyte cell suspensions were incubated with a cocktail of FITC-conjugated Ab to B220, CD11c, Ter119, CD161, Gr-1 and F4/80, together with anti-CD4-Alexa647 and anti-CD8-PE-Cy5 Ab. DP cells were defined as cocktail– CD4+ CD8+. For the isolation of B cells, lymph node cell suspensions were incubated with anti-F4/80-FITC, anti-CD19-PE and anti-CD3-PE-Cy5 Ab. The B cell population was sorted as F4/80– CD3– CD19+ cells.
Cells were analyzed on a FACSCanto flow cytometer using FACSDiva software (Becton Dickinson). Dead cells were gated out by their forward and side scatter profile. Sortings were performed on a FACSAria flow cytometer (Becton Dickinson).
Real-time PCR
Real-time PCR using SYBR Green was performed on a LightCycler (Roche, Rotkreuz, Switzerland) according to the manufacturer's instructions. Total RNA from sorted DN3 cells was purified using a High Pure RNA tissue kit (Roche) that includes DNase treatment. Total RNA was reverse-transcribed using random nonamers and AMV reverse transcriptase (Roche). Genomic DNA was extracted from sorted DN3 cells and quantification of Vγ2-Jγ1 rearrangements was performed as described 4. For the PCR reaction, the LightCycler-FastStart DNA Master SYBR Green I kit (Roche) was used following the instruction manual. RAG1 transcripts were normalized to TATA-binding protein (TBP) and Vγ2-Jγ1 rearrangement was normalized to Thy-1. All primer sequences are available upon request. Amplification plots were analyzed using the second-derivative method with LC data analysis 3.5 software (Roche), and the relative quantification was determined using the LightCycler relative quantification software 1.0 (Roche). Sextuplet analysis showed that measurement errors were always <9%.
Immunoblot analysis
Total thymocyte and isolated DP or DN populations were lysed in lysis buffer (Sigma) and the protein concentration was determined by BCA assay (Pierce). Protein extracts (14 µg for total thymus and DN cells, 10 µg for DP cells) were applied to SDS-PAGE and analyzed by Western blot for RAG2 expression. To ensure that equal amounts of protein were loaded, the membrane was reprobed with anti-β-actin Ab. The anti-RAG2 (C-19) Ab was from Santa Cruz Biotechnology and the anti-β-actin Ab from Sigma.
Acknowledgements
We thank Steven Merlin for FACS sorting, Catherine Fumey for technical assistance, Rose Lees for her effort in maintaining the breeding of the transgenic models used in this study and Sabine Morand and Donata Rimoldi for their useful advice on Western blotting. E.F. was supported by a Marie Heim-Vögtlin Fellowship (PMP DB-115611/1).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




