c-Abl is required for the signaling transduction induced by L-selectin ligation
Abstract
Lymphocyte recruitment onto inflamed tissues requires cells tethering to and rolling on vascular surfaces under flow. L-selectin is constitutively expressed on leukocytes to mediate the leukocytes’ initial capture and subsequent rolling along the vessel. Apart from its adhesive function, engagement of L-selectin also results in cell activation, which is related to the completed signaling transduction. Here we show that ligation of L-selectin with its mAb increases c-Abl kinase activity, and that the activated c-Abl kinase can be recruited to and phosphorylate the cytoplasmic domain of L-selectin. In addition, the activated c-Abl kinase can regulate Zap70 kinase by increasing the phosphorylation of the Y319 site of Zap70 kinase and connect with Zap70 kinase through its SH2 domain. These results indicate that c-Abl kinase plays an important role in accepting and transferring the upstream activation events induced by L-selectin ligation.
Introduction
Recruitment of leukocytes from the blood into tissues is controlled by a variety of adhesion molecules expressed on the surface of the endothelium and circulating leukocytes 1, 2. L-selectin, a member of selectin family and constitutively expressed on leukocytes, plays a fundamental role in the immune system by mediating lymphocyte homing, and in the recruitment of leukocytes to inflammatory sites 3–7.
Apart from its role in adhesion, L-selectin can function as a signal transduction molecule. Cross-linking of human L-selectin with its antibody leads to neutrophil activation as measured by Ca2+ flux 8, superoxide generation 9, increased adhesiveness 10, and activation of intracellular protein pathways, such as tyrosine phosphorylation and mitogen-activated protein kinase (MAPK) activation 11, 12. Ligation of neutrophil L-selectin with sulfatides has been shown to induce increased expression of TNF-α and IL-8 8. Cross-linking of L-selectin on lymphocytes or Jurkat cells with anti-L-selectin mAb or stimulated with fucoidan or sialyl-Lewisx initiate a signaling cascade from L-selectin via the tyrosine kinase p56lck to Sos, Ras, MAPK, and Rac2, resulting in O2- generation 13, 14. In the lymphocytes, L-selectin triggering results in a src-tyrosine kinase-dependent activation of the small G-proteins Ras, Rac1/2 and MAPK, ERK1/2 and JNK, and NFAT and NF-κB transcription factors 15–17. Clustering of L-selectin by mAb leads to a rapid induction of actin assembly and CD18 co-localization with L-selectin in the plasma 18, 19.
Previous reports indicated that the signaling function of L-selectin was dependent on different kinases activity. c-Abl, a non-receptor protein tyrosine kinase, has been shown to be very important for cell signal transduction. c-Abl contains a catalytic domain, polyproline-rich regions, and SH2 and SH3 domains that are involved in protein-protein interactions and may also regulate the kinase activity itself 20, 21. Additionally, the C terminus of c-Abl contains nuclear localization and export signals (NLS and NES), as well as F- and G-actin-binding domains 22, 23. The mammalian c-Abl can shuttle between the nuclear and cytoplasmic compartments because of its NLS and NES 22, 23. Nuclear c-Abl plays a role in transcription regulation, particularly in response to DNA damage and apoptosis 24, 25. Cytoplasmic c-Abl kinase activity is under stringent control within the cell and likely to be held in an inactive conformation through intramolecular and intermolecular restraints 26–28. It has been reported that c-Abl kinase could be activated by growth factors and integrin engagement 29–31. In our previous work, we demonstrated that c-Abl was stimulated after L-selectin and PSGL-1 (P-selectin glycoprotein ligand-1) cross-linking with their mAb, and could co-localize with F-actin in the activated neutrophils 32, 33.
In this study, we investigated the role of c-Abl kinase in the L-selectin ligation-stimulated lymphocytes. Results showed that in Jurkat cells L-selectin ligation with mAb could increase c-Abl kinase activity. The activated c-Abl kinase could regulate Zap70 kinase in the stimulated cells. After ligation with DREG56, the cytoplasmic tail of L-selectin, c-Abl and Zap70 formed a complex that transfers upstream signal events triggered by L-selectin ligation.
Results
L-selectin ligation induces CSF-1 gene transcriptional activation
The activation of T cells via the interaction of L-selectin and its mAb leads to the stimulation of multiple intracellular events. In our study, we investigated whether the ligation of L-selectin was able to induce CSF-1 gene transcriptional activation. Jurkat cells were transfected with the CSF-1-luc vector, and after 24 h, the transfected cells were incubated at 37°C for 6 h without antibody or with mouse IgG, W6/32 or DREG56. The cells were then lysed to measure both the firefly and the Renilla luciferase. The data shown indicated that the CSF-1 gene transcription was clearly induced in anti-L-selectin mAb-treated cells. In contrast, no significant signal was detected in the cells treated with the control antibodies (Fig. 1A). We also tested the endogenous CSF-1 gene transcription. Jurkat cells were incubated with DREG56 at 37°C for the indicated time. Cells were then lysed and analyzed by RT-PCR for CSF-1 gene transcription. As shown in Fig. 1B, treatment of cells with an anti-L-selectin antibody for 30 min resulted in the maximal activation of CSF-1 gene transcription. The activation decreased when the cells were treated with DREG56 for 60 min. Treatment of the isolated primary T lymphocytes with anti-L-selectin antibody for 30 min also resulted in the maximal activation of CSF-1 gene transcription (Fig. 1C). To better understand the physiological role of L-selectin, we also tested the CSF-1 gene transcriptional activation in the sulfatide-stimulated primary T lymphocytes. The isolated cells were incubated with 100 μg/mL or 250 μg/mL sulfatides for different times. As shown in Fig. 1D, CSF-1 mRNA increased about tenfold when the lymphocytes were stimulated with sulfatides, while CSF-1 mRNA increased about threefold when cells were stimulated with DREG56 ligation (Fig. 1C). The results suggested that sulfatides ligation induced CSF-1 gene transcription more strongly than L-selectin engagement with DREG 56.
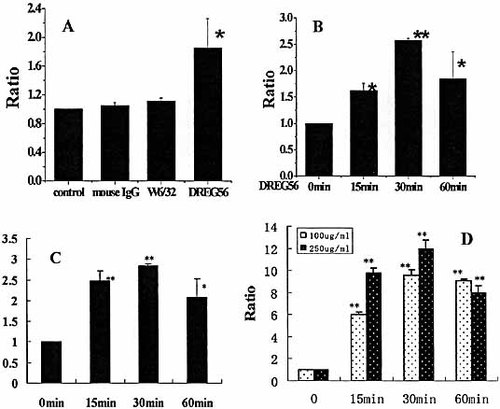
L-selectin ligation induces CSF-1 transcriptional activation. (A) Jurkat cells were transfected with CSF-1-luc vector and were left unstimulated or stimulated with the indicated antibodies for 6 h after transfection. Cells were then lysed and analyzed for luciferase activity. Results shown are the mean ± SD of three independent experiments performed in triplicate. All Firefly luciferase values are corrected for the Renilla luciferase values obtained in each case. (B) Jurkat cells or (C) the isolated primary T lymphocytes were incubated at 37°C for 0, 15, 30 or 60 min with 10 µg/mL anti-L-selectin antibody (DREG56). RNA isolated with the TRIzol isolation system from the treated cells was used for RT-PCR for both CSF-1 and β-actin expression. The fold change in CSF-1 gene transcription was calculated using the ΔΔ Ct method, with β-actin mRNA as an internal control. (D) The isolated primary T lymphocytes were incubated at 37°C for 0, 15, 30 or 60 min with 100 µg/mL or 250 µg/mL sulfatides. RNA isolated with the TRIzol isolation system from the treated cells was used for RT-PCR for both CSF-1 and β-actin expression. The fold change in CSF-1 gene transcription was calculated as above. The significant difference from the control was determined by One-way ANOVA (* p<0.05, ** p<0.01).
c-Abl kinase regulates CSF-1 gene transcription induced by L-selectin ligation
c-Abl kinase has been reported to play an important role in the cytoskeleton reorganization induced by adhesion molecules cross-linking. To assess whether c-Abl kinase could function as a necessary kinase in CSF-1 gene transcriptional activation triggered by L-selectin ligation, WT c-Abl expression vector or control vector and the CSF-1-luc vector were co-transfected in Jurkat cells. As shown in Fig. 2A, the overexpression of WT c-Abl kinase could increase CSF-1 gene transcription, while overexpression of a KD mutant c-Abl kinase was not able to regulate CSF-1 transcription (Fig. 2B). We also tested the endogenous CSF-1 transcriptional level in the Jurkat cells pretreated with STI571 for 30 min before they were activated by antibody ligation. STI571 is a potent and specific blocker of c-Abl kinase with an IC50 of ∼650 nM for inhibition of its kinase activity. The CSF-1 gene transcription of the STI571-treated cells was decreased dramatically compared to the cells without STI571 pretreated (Fig. 2C).
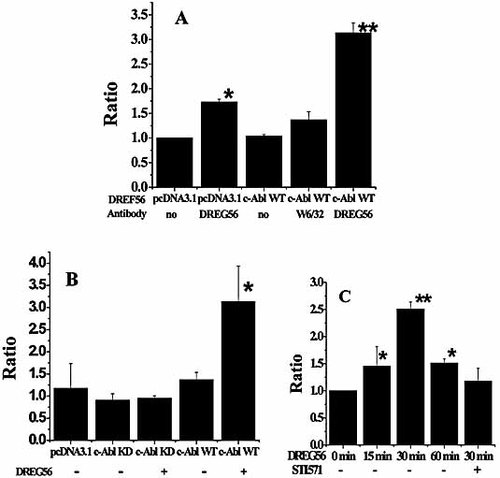
c-Abl kinase regulates CSF-1 gene transcription induced by L-selectin ligation. (A) Jurkat cells were transfected with empty pcDNA3.1 or the same vector expressing c-Abl WT in the presence of CSF-1-luc vector. After 24 h, transfected cells were incubated for 6 h without an antibody or with the indicated antibodies, and cells were lysed and analyzed for luciferase activity. (B) Jurkat cells were transfected with empty pcDNA3.1 or the same vector expressing WT c-Abl or KD c-Abl in the presence of CSF-1-luc vector. After 24 h, transfected cells were incubated for 6 h without antibody or with the indicated antibody, and then the cells were lysed and analyzed for luciferase activity. For (A) and (B), results shown are the mean ± SD of three independent experiments performed in triplicate. All the Firefly luciferase values were corrected for the Renilla luciferase values obtained in each case. (C) Jurkat cells, pretreated with STI571 for 1 h before L-selectin ligation, were incubated with DREG56 for 30 min. The RNA of the treated Jurkat cells was isolated and analyzed by RT-PCR for both CSF-1 and β-actin expression. The fold change in CSF-1 gene transcription was calculated using the ΔΔ Ct method with β-actin mRNA as an internal control. The significant difference from the control was determined by One-way ANOVA (* p<0.05, ** p<0.01).
L-selectin ligation increases c-Abl kinase activity
We next examined the c-Abl kinase activity in response to L-selectin ligation stimulation. The endogenous c-Abl kinase activity was tested by analyzing the phosphorylation status of the endogenous c-Abl substrate Crk. The results showed that the level of Crk phosphorylation was increased within 5 min of L-selectin stimulation (Fig. 3A). To determine if the phosphorylation of Crk was dependent on c-Abl kinase activity, we treated Jurkat cells with STI571 prior to activation and found that STI571 inhibited the Crk phosphorylation induced by L-selectin ligation (Fig. 3B). We then tested the c-Abl kinase activity using GST-CrKII-CTD as a substrate in an in vitro kinase assay. The results demonstrated that c-Abl kinase activity was elevated after L-selectin ligation (Fig. 3C), and STI571 incubation could reduce the phosphorylation of GST-CrKII-CTD (Fig. 3D). The data described above indicates that c-Abl kinase activity was increased after L-selectin ligation.
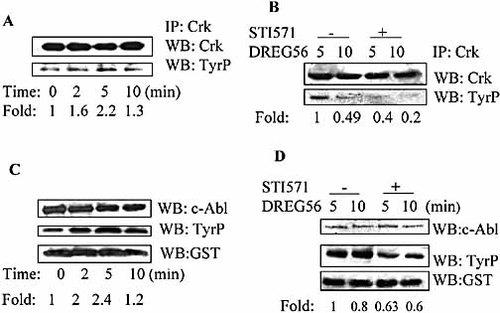
L-selectin ligation increases c-Abl kinase activity. (A) Jurkat cells were stimulated with DREG56 for the indicated time and then the cells were lysed. (B) Jurkat cells were stimulated with DREG56 for the indicated time in the presence or absence of STI571, and cells were then lysed. For (A) and (B), phosphorylation of Crk was assessed by immunoprecipitation of Crk and Western blotting with PY99. Blots were stripped and re-probed with an anti-Crk antibody to demonstrate equal loading. (C) Jurkat cells were stimulated with DREG56 for the indicated time and cells were then lysed. The c-Abl kinase activity was determined using an in vitro kinase assay with GST-CrKII-CTD as a substrate. (D) Jurkat cells were stimulated with DREG56 for the indicated time in the presence or absence of STI571, and cells were then lysed. For (C) and (D), phosphorylation of GST-CrKII-CTD was tested by using Western blotting with PY99. The level of GST-CrKII-CTD was detected by Western blotting with the anti-GST antibody to demonstrate equal loading. The Western blot analysis was quantified by densitometry and data were normalized with respect to controls.
Activated c-Abl kinase enhances Zap70 kinase activity downstream of L-selectin ligation
The activation of Zap70 kinase is one of the first events following T cell activation. So we were interested to discover if Zap70 kinase was involved in the L-selectin ligation-induced signal transduction in which c-Abl kinase was necessary. We first investigated Zap70 kinase activity after L-selectin ligation. As shown in Fig. 4A, Zap70 phosphorylation was greatly increased in the L-selectin ligation-stimulated cells, and the kinase activity remained elevated for 20 min after which it decreased to baseline values. It has been reported that phosphorylation of Tyr319 of Zap70 (Y319) serves an important role for the Zap70 kinase activity. We next tested the Y319 phosphorylation of Zap70 kinase after L-selectin ligation. The results showed that the phosphorylation of Y319 site of Zap70 kinase was elevated in the stimulated cells (Fig. 4A). To define if c-Abl kinase could regulate Zap70 kinase activity, Jurkat cells were pre-incubated with piceatannol (the syk/Zap70-specific kinases inhibitor) before ligation with DREG56. The results showed that c-Abl kinase activity was not affected in the piceatannol-treated cells (Fig. 4B). We also assessed the tyrosine phosphorylation of Zap70 kinase after the cells were treated with STI571. As shown in Fig. 4C, the phosphorylation of Y319 site of Zap70 kinase, other than the total phosphorylation of Zap70, was greatly inhibited in the presence of STI571. All these results indicate that the activated c-Abl kinase could regulate Zap70 kinase activity by phosphorylating the Y319 site downstream of L-selectin ligation.
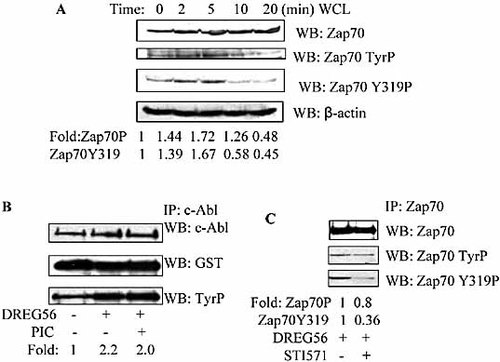
The activated c-Abl kinase enhances Zap70 kinase activity downstream of L-selectin ligation. (A) Jurkat cells were stimulated with DREG56 for the indicated time and then the cells were lysed. Phosphorylation of Zap70 was assessed by Western blotting with PY99. Blots were stripped and re-probed with an anti-Zap70 antibody to demonstrate equal loading. Blots were stripped and re-probed with Zap70 Y319 tyrosine phosphorylation antibody to detect the Zap70 Y319 phosphorylation. β-actin also was assessed by Western blotting with its mAb. (B) Jurkat cells were stimulated as above for 5 min in the presence or absence of piceatannol (PIC). c-Abl kinase activity was determined using an in vitro kinase assay with GST-Crkα-CTD as a substrate. The phosphorylation of GST-Crkα-CTD was tested using Western blotting with PY99. The level of GST-Crkα-CTD was detected by Western blotting with the anti-GST antibody to demonstrate equal loading. (C) Jurkat cells were stimulated in the presence or absence of STI571. The phosphorylation of Zap70 was assessed by immunoprecipitation of Zap70 and Western blotting with PY99. Blots were stripped and re-probed with an anti-Zap70 antibody to demonstrate equal loading. Blots were re-stripped and re-probed with an anti-Zap70 tyrosine 319 antibody. The Western blot analysis was quantified by densitometry and data were normalized with respect to controls.
c-Abl kinase increases CSF-1 gene transcription via regulating Zap70 kinase activity
c-Abl kinase can increase CSF-1 gene transcriptional activity and regulate Zap70 kinase activity in the L-selectin-stimulated Jurkat cells. To determine whether Zap70 kinase was involved in the CSF-1 gene transcription triggered by L-selectin ligation, Jurkat cells were transfected with WT Zap70, KD Zap70 or Y319F Zap70 expression vector in the presence of CSF-1-luc vector. After 24 h, the transfected cells were incubated with DREG56 at 37°C for 6 h. The cells were then lysed and analyzed by a double luciferase reporter assay. The results showed that overexpression of WT Zap70, other than KD Zap70 or Y319F Zap70, could increase the CSF-1 gene transcription (Fig. 5A). The increased CSF-1 gene transcriptional activity was reduced if the WT Zap70-transfected cells were preincubated with STI571 or piceatannol before stimulation with L-selectin ligation (Fig. 5B). With pretreatment of the WT c-Abl kinase-overexpressing cells with piceatannol or STI571 before L-selectin ligation, the CSF-1 gene transcriptional activation was also reduced (Fig. 5C). Consistently, the endogenous increased CSF-1 gene transcriptional activity induced by L-selectin ligation was inhibited by STI571 and piceatannol incubation (Fig. 5D). The results above indicate that Zap70 kinase was involved in the CSF-1 gene transcription, which was dependent on the c-Abl kinase activity.
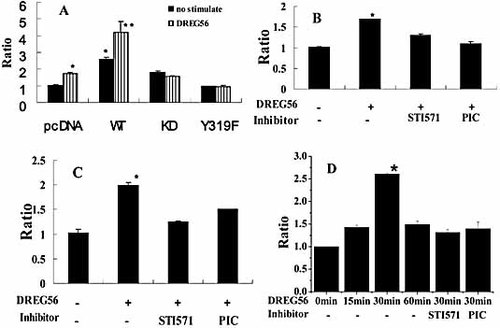
c-Abl kinase increases CSF-1 gene transcription via regulating Zap70 kinase activity. (A) Jurkat cells were transfected with empty pcDNA3.1 or the same vector expressing WT Zap70, KD Zap70 or Y319F Zap70 in the presence of CSF-1-luc vector. After 24 h, transfected cells were incubated for 6 h without antibody or with the indicated antibody, and cells were lysed and analyzed for luciferase activity. Results shown are the mean ± SD of three independent experiments performed in triplicate. (B) Jurkat cells were transfected with the pcDNA3.1 WT Zap70, in the presence of CSF-1-luc vector. After 24 h, the transfected cells were pre-incubated with STI571 or piceatannol for 1 h before stimulating with DREG56, and cells were lysed and analyzed for luciferase activity. (C) Jurkat cells were transfected with the pcDNA3.1 WT c-Abl, in the presence of CSF-1-luc vector. After 24 h, transfected cells were pre-incubated with STI571 or piceatannol for 1 h before stimulating with DREG56, and treated cells were lysed and analyzed for luciferase activity. For (B) and (C), results shown are the mean ± SD of three independent experiments performed in triplicate. All the Firefly luciferase values were corrected for the Renilla luciferase values obtained in each case. (D) Jurkat cells, pretreated with STI571 or piceatannol for 1 h before L-selectin ligation, were incubated with DREG56 for 30 min. RNA of the treated Jurkat cells was isolated and analyzed by RT-PCR for both CSF-1 and β-actin expression. The fold change in CSF-1 gene transcription was calculated using the ΔΔ Ct method with β-actin mRNA as an internal control. The significant difference from the control was determined by One-way ANOVA (* p<0.05, ** p<0.01).
The cytoplasmic tail of L-selectin is associated with c-Abl kinase and Zap70 kinase in activated Jurkat cells
We have shown that c-Abl and Zap70 kinases were activated in the L-selectin-stimulated Jurkat cells. Next we investigated the relationship of L-selectin with c-Abl kinase and Zap70 kinase. Jurkat cells were stimulated and lysed, and the lysates were used for co-immunoprecipitation. As shown in Fig. 6A, c-Abl kinase and Zap70 kinase were present in the L-selectin immunoprecipitated complex. L-selectin was also detected in the c-Abl kinase and Zap70 kinase immunoprecipitated complexes (Fig. 6B and C). STI571 preincubation reduced the association of L-selectin and c-Abl kinase and also decreased the association between L-selectin and Zap70 kinase (Fig. 6A–C). These results indicate that L-selectin was associated with c-Abl kinase and Zap70 kinase in the L-selectin ligation-stimulated cells.
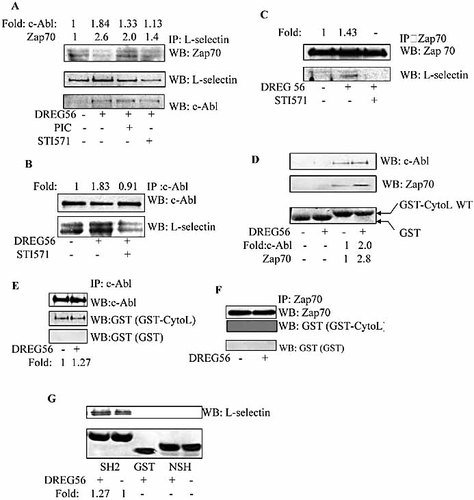
The cytoplasmic domain of L-selectin is associated with c-Abl and Zap70 in the activated Jurkat cells. (A) Jurkat cells were either left untreated or pre-treated with piceatannol or STI571 for 1 h prior to stimulation with DREG56, then the cells were lysed and the lysates were immunoprecipitated with anti-L-selectin antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for L-selectin (top) and c-Abl (middle). Blots were also probed with anti-Zap70 antibody (bottom). (B) Jurkat cells were either left untreated or pretreated with STI571 for 1 h prior to stimulation with DREG56. The cells were lysed and the lysates were immunoprecipitated with anti-c-Abl antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for c-Abl (top). Blots were also probed with anti-L-selectin antibody (bottom). (C) Jurkat cells were stimulated as in (A). The cells were lysed and the lysates were immunoprecipitated with anti-Zap70 antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for Zap70 (top). Blots were stripped and re-probed with anti-L-selectin antibody (bottom). (D) The GST pull down assay was done as described in the Materials and methods. The bound proteins were analyzed by SDS-PAGE and Western blotted for c-Abl (top) and Zap70 (middle). The lower panel is the Coomassie-stained gel of GST and GST fusion proteins. (E) Jurkat cells were either left untreated or treated with DREG56. The cells were lysed and the lysates were immunoprecipitated with anti-c-Abl antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for c-Abl (top). Blots were stripped for far Western blot. Bound proteins were detected by Western blotting with a rabbit anti-GST mAb. (F) Jurkat cells were either left untreated or treated with DREG56. The cells were lysed and the lysates were immunoprecipitated with anti-c-Zap70 antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for Zap70 (top). Blots were stripped for far Western blot. Bound proteins were detected by Western blotting with a rabbit anti-GST mAb. (G) The GST pull down assay was done as described in the Materials and methods and the bound proteins were analyzed by SDS-PAGE and Western blotted for L-selectin antibody (top). The lower panel is the Coomassie-stained gel of GST and GST fusion proteins. The Western blot analysis was quantified by densitometry and data were normalized with respect to controls.
We also examined the association of L-selectin with c-Abl kinase and Zap70 kinase in vitro using a GST pull-down assay. GST-CytoL WT fusion protein was incubated with Jurkat cell lysates and the extensively washed protein complexes were separated by SDS-PAGE. c-Abl kinase and Zap70 kinase were found in the GST pull-down complexes (Fig. 6D). To detect whether GST-CytoL directly binds to c-Abl kinase and Zap70 kinase, a far Western blot analysis was performed. The results showed that GST-CytoL fusion protein directly interacted with c-Abl kinase (Fig. 6E), while it could not interact with Zap70 kinase directly (Fig. 6F). To determine which domain of c-Abl kinase was responsible for the interaction of c-Abl kinase and L-selectin, GST-c-Abl SH2, GST-c-Abl NSH were incubated with the lysates from unstimulated or stimulated Jurkat cells. As shown in Fig. 6G, L-selectin was found in the GST-c-Abl SH2, but not GST or NSH pull-down complexes. We therefore concluded that L-selectin connects directly with the c-Abl kinase SH2 domain, but indirectly with Zap70 kinase in Jurkat cells.
L-selectin cytoplasmic tail is a substrate of c-Abl kinase
We have shown that c-Abl kinase could interact with the cytoplasmic domain of L-selectin in the activated Jurkat cells triggered by L-selectin ligation. To determine whether the interaction between c-Abl kinase and L-selectin led to a modification of L-selectin, we examined the ability of endogenous c-Abl kinase to phosphorylate the cytoplasmic tail of L-selectin by carrying out a kinase assay using GST-CytoL fusion protein as a substrate and immunoprecipitated c-Abl kinase derived from unstimulated or L-selectin engagement-stimulated Jurkat cells. As shown in Fig. 7A, the c-Abl kinase obtained from stimulated cells was able to phosphorylate the cytoplasmic tail of L-selectin. There is only one tyrosine residue (Y372) in the cytoplasmic domain of L-selectin (Fig. 7B). To test whether this site is responsible for the association of L-selectin and c-Abl kinase, GST-CytoL WT, GST-CytoL Y372A and GST-CytoL Y372F were used for GST pull-down assays. The results showed that all these GST fusion proteins could associate with c-Abl kinase, with the GST-CytoL Y372F having a lower interaction with c-Abl kinase (Fig. 7C). These results suggest that c-Abl kinase could phosphorylate L-selectin Y372, and this modification plays a role, although not a critical, in the association between c-Abl kinase and L-selectin.
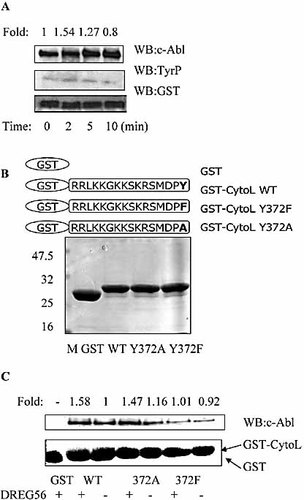
L-selectin is a substrate of c-Abl. (A) Jurkat cells were stimulated with DREG56 for the indicated time and cells were lysed. The c-Abl kinase assay was conducted using an in vitro kinase assay with GST-CytoL as a substrate. The immunoprecipitated c-Abl kinase was Western blotted with the anti-c-Abl kinase antibody (top). The phosphorylation of GST-CytoL was tested using Western blotting with PY99. The level of GST-CytoL was detected by Western blotting with anti-GST antibody to demonstrate equal loading. (B) Mutants of L-selectin fusion proteins. (C) Immobilized GST and GST-CytoL fusion proteins were incubated with lysates from untreated or DREG56-treated Jurkat cells to allow binding of interacting proteins. The bound proteins were analyzed by SDS-PAGE and Western blotted for c-Abl (top). The lower panel is the Coomassie-stained gel of GST and GST fusion proteins. The Western blot analysis was quantified by densitometry and data were normalized with respect to controls.
c-Abl kinase associates with Zap70 in the activated Jurkat cells
We have shown that L-selectin interacts directly with c-Abl SH2 domain, but not directly with Zap70 kinase in the activated Jurkat cells. Next we tested the relationship between c-Abl kinase and Zap70 kinase using a co-immunoprecipitation assay. The results showed that c-Abl kinase could be found in the immunoprecipitate of Zap70 kinase, and Zap70 kinase was present in the c-Abl kinase immunoprecipitated complexes (Fig. 8A and B). STI571 could reduce the association between c-Abl kinase and Zap70 kinase, indicating that c-Abl kinase stimulation was responsible for the association between c-Abl kinase and Zap70 kinase. To better understand the physiological role of L-selectin, we tested the association of c-Abl and ZAP70 in primary T lymphocytes stimulated by L-selectin engagement with DREG56 or sulfatides. We first tested the association between c-Abl and Zap70 in primary T lymphocytes. Before the primary T lymphocytes were stimulated with DREG56 or sulfatides, the cells were pre-incubated with STI571 or piceatannol. The treated cells were lysed, and the lysates were used for co-immunoprecipitation. As shown in Fig. 8C, c-Abl appeared in the Zap70 kinase immunoprecipitated complexes. Zap70 was detected in the c-Abl kinase immunoprecipitated complexes (Fig. 8D). STI571 and piceatannol pre-incubation reduced the association of c-Abl kinase and Zap70 kinase (Fig. 8C and D). These results indicated that c-Abl kinase was associated with Zap70 kinase in normal primary T cells stimulated by L-selectin ligation. Our GST-c-Abl SH2 pull-down assay showed that Zap70 kinase was associated with the SH2 domain of c-Abl kinase (Fig. 8E), and the far Western blot assay results further demonstrated that Zap70 kinase was connected with c-Abl SH2 domain directly (Fig. 8F). As mentioned above, both L-selectin and Zap70 kinase could interact with the SH2 domain of c-Abl kinase. To test whether these two molecules interacted with c-Abl kinase simultaneously, the association between c-Abl SH2 domain and Zap70 kinase or c-Abl SH2 domain and L-selectin was analyzed using a GST pull-down assay. As shown in Fig. 8G, c-Abl kinase was connected with L-selectin constitutively and the connection between c-Abl kinase and Zap70 kinase was enhanced after L-selectin stimulation. These data indicate that the activated c-Abl kinase interacted with Zap70 kinase in the L-selectin-stimulated cells. The connection between c-Abl kinase and Zap70 kinase was strengthened following L-selectin ligation.
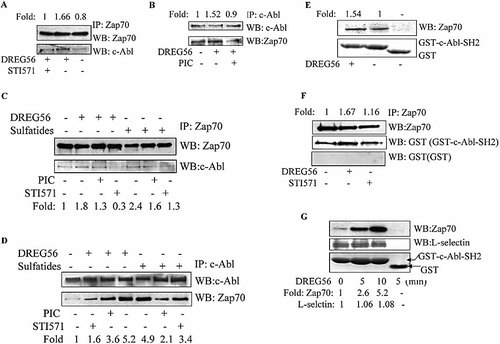
c-Abl kinase associates with Zap70 in the activated Jurkat cells. (A) Jurkat cells were either left untreated or pretreated with STI571 for 1 h prior to stimulation with DREG56. Cells were lysed and the lysates immunoprecipitated with anti-Zap70 antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for Zap70 (top). Blots were also probed with anti-c-Abl (bottom). (B) Jurkat cells were stimulated as in (A). Cells were lysed and the lysates immunoprecipitated with anti-c-Abl antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for c-Abl (top). Blots were also probed with anti-Zap70 (bottom). (C) The isolated primary T lymphocytes were either left untreated or pre-treated with piceatannol or STI571 for 1 h prior to stimulation with DREG56 or sulfatides. Cells were lysed and the lysates immunoprecipitated with anti-Zap70 antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for Zap70 (top) and c-Abl (bottom). (D) The isolated primary T lymphocytes were stimulated as in (C). Cells were lysed and the lysates immunoprecipitated with anti-c-Abl antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for c-Abl (top). Blots were also probed with anti-Zap70 antibody (bottom). (E) GST pull down assay was done as described in the Materials and methods. The bound proteins were analyzed by SDS-PAGE and Western blotted for Zap70 (top). The lower panel is the Coomassie-stained gel of GST and GST fusion proteins. (F) Jurkat cells were stimulated as in (A). Cells were lysed and the lysates immunoprecipitated with anti-Zap70 antibody. The immunoprecipitates were separated by SDS-PAGE and Western blotted for Zap70 (top). Blots were stripped for Far Western blot assay by using the GST or GST-c-Abl SH2 fusion proteins. Bound proteins were detected by Western blotting with the anti-GST antibody. (G) GST pull down assay was done as described in the Materials and methods. The bound proteins were analyzed by SDS-PAGE and Western blotted for Zap70 (top) and L-selectin (middle). The lower panel is the Coomassie-stained gel of GST and GST fusion proteins. The Western blot analysis was quantified by densitometry and data were normalized with respect to controls.
Discussion
Leukocyte rolling, the initial step in the recruitment of leukocytes to the sites of inflammation, is followed by leukocyte activation, firm adhesion, and transmigration into the interstitial tissues 1, 2. L-selectin contributes to physiological leukocyte rolling and plays a central role in the signal transduction initiated by the interactions of leukocytes and endothelial cells 3–7. Cross-linking of L-selectin on lymphocytes or Jurkat cells with anti-L-selectin mAb or stimulated with fucoidan or sialyl-Lewisx initiates several signaling cascades in which many kinases and adaptor proteins are involved 13–17. Here we reported that c-Abl kinase was required in the signal transduction induced by L-selectin engagement. Our conclusion is based on the following evidence: c-Abl kinase regulates CSF-1 gene transcription triggered by L-selectin ligation. After L-selectin engagement, the kinase activity of c-Abl is increased and the activated c-Abl kinase connects with and phosphorylates the cytoplasmic domain of L-selectin. c-Abl kinase also regulates Zap70 kinase and connects with it in the activated Jurkat cells.
L-selectin ligation on neutrophils with sulfatides has been shown to induce increased expression of TNF-α and IL-8, which are the markers of inflammation 8. Our data established that L-selectin ligation with its mAb increases CSF-1 gene transcription (Fig. 1A–C). However, sulfatides engagement with L-selectin could increase CSF-1 gene transcription more strongly in the primary T lymphocytes (Fig. 1D). CSF-1, long known as a regulator of macrophage growth and differentiation, has immunomodulatory roles, which makes it a potential marker of tumor, inflammation, or both 34–36. It modulates inflammatory response by stimulating the production of several cytokines and growth factors 37. Our results demonstrate that overexpression of WT c-Abl kinase increases CSF-1 gene transcription (Fig. 2A). Alternatively, CSF-1 gene transcription is blockaded by STI571 incubation as well as by overexpression of the KD mutant of c-Abl, suggesting that c-Abl kinase regulates CSF-1 gene transcription in a kinase activity-dependent manner. It has been reported that the kinase activity of the cytoplasmic c-Abl is tightly regulated within the cells 26–28. PDGF receptor binding to fibroblasts, fibroblast adhesion to fibronectin or integrin cross-linking all lead to the increase of c-Abl kinase activity 29–31. Here we found, using in vivo and in vitro kinase assays, that L-selectin ligation with its mAb increases c-Abl kinase activity within 5 min. Pre-incubation of cells with STI571 before L-selectin ligation dramatically reduces the kinase activity (Fig. 3). Our findings suggest that the cytoplasmic c-Abl kinase might play a necessary role in the initial adhesion events triggered by L-selectin engagement in the inflammation response.
c-Abl kinase can be activated by many stimuli and the activated c-Abl kinase has been shown to activate many cellular proteins to regulate cytoskeletal dynamics, cell proliferation, survival adhesion and migration 29–33, 38. Zap70 kinase is a key regulator of signaling in T lymphocytes and its absence leads to a complete loss of the ability of T cells to respond to antigenic stimuli 39–43. Multiple tyrosine phosphorylation sites (Y292, Y315 and Y319) within interdomain B of Zap70 kinase might regulate Zap70 kinase function during TCR signaling 44–47. Y319 is a TCR inducible phosphorylation site and plays a critical role in Zap70 kinase function 47. Our experiments demonstrated that L-selectin ligation with its mAb increases Zap70 kinase activity by phosphorylating Zap70 Y319. Preincubation with STI571 before L-selectin ligation reduces the phosphorylation of this site, while the increase of c-Abl kinase activity triggered by L-selectin ligation is not inhibited by pretreating the cells with piceatannol (Fig. 4). Overexpression of WT Zap70, other than for KD Zap70 or Y319F Zap70, enhances CSF-1 gene transcription, which is greatly reduced in the presence of STI571 (Fig. 5). These data suggest that c-Abl kinase could regulate Zap70 kinase activity in the L-selectin ligation-stimulated cells and that Zap70 kinase acts as a substrate of the c-Abl kinase in the CSF-1 gene transcriptional events triggered by L-selectin ligation.
It has been reported that α-actinin, calmodulin (CaM) and members of ERM (ezrin/radirin/moesin) family of cytoskeletal proteins interact with the cytoplasmic tail of L-selectin 48–51. When the cells are stimulated, the Grb2/sos complex can be recruited to the cytoplasmic tail of L-selectin 14. Recent studies reported that PKCθ and PKCτ can interact with L-selectin and phosphorylate the serine residue of L-selectin cytoplasmic domain 52. As a cytoplasmic tyrosine kinase, c-Abl is mainly linked to the transduction of extracellular signals through interaction with cell surface receptors. In our previous studies, we have shown that c-Abl kinase co-localized and interacted with L-selectin to regulate F-actin assembly induced by L-selectin cross-linking in neutrophils. Here we further showed that c-Abl kinase interacts with the cytoplasmic tail of L-selectin directly after L-selectin ligation with its mAb (Fig. 6E). The association between c-Abl kinase and L-selectin raises the possibility that c-Abl could phosphorylate the cytoplasmic domain of L-selectin. This hypothesis is supported by our in vitro kinase assay using the GST-CytoL as a substrate and immunoprecipitated c-Abl kinase derived from unstimulated or L-selectin ligation-stimulated Jurkat cells (Fig. 7A). Although the tyrosine phosphorylation of L-selectin downstream of some signals has been reported, little is known regarding the mechanisms of L-selectin activation by the upstream signals. The properties previously described as a p56lck function are responsible for the phosphorylation of L-selectin on tyrosine residue in the L-selectin ligation-stimulated Jurkat cells. Our studies now indicate that c-Abl kinase connects with the cytoplasmic tail of L-selectin and phosphorylates the Y372 site of L-selectin. The complex of c-Abl kinase and L-selectin might initiate the upstream signaling events to regulate CSF-1 gene transcription triggered by L-selectin ligation.
Recruitment of substrates to the c-Abl kinase may be a common event induced by the activation of several receptors tyrosine kinase 31, 53. The SH3 and SH2 domains of c-Abl kinase are thought to function by mediating the interaction between c-Abl kinase and the substrates 54. The SH3 domain preferentially interacts with the proteins containing a proline-rich region to inhibit c-Abl kinase activity 55. The SH2 domain, ∼100 amino acids long, has been shown to bind specifically and with high affinity to tyrosine-phosphorylated proteins 56. In our studies, we found that c-Abl kinase could regulate Zap70 Y319 phosphorylation in the L-selectin ligation-stimulated Jurkat cells. Since the sequences downstream of Zap70 Y319 match the binding sequences of SH2 domain for the c-Abl kinase (YXXP) 54, 57, 58, we postulate that c-Abl kinase and Zap70 kinase might be connected in the L-selectin ligation-activated cells. The results we obtained confirmed our hypothesis that c-Abl kinase associates with Zap70 kinase directly though its SH2 domain (Fig. 8D).We also tested the association of c-Abl and Zap70 in the activated primary T lymphocytes induced by DREG56 or sulfatides engagement (Fig. 1D). The association between c-Abl and Zap70 can be detected in the L-selectin ligation-activated cells without using the secondary antibody cross-linking. As a result of this, we think that L-selectin cross-linking is not necessary for the c-Abl-Zap70 association. We have shown that the cytoplasmic tail of L-selectin is also connected with c-Abl SH2 domain directly in the stimulated Jurkat cells (Fig. 6E). To determine whether the c-Abl SH2 domain can interact with the two molecules simultaneously, we stimulated Jurkat cells at different points in time and tested the connection of c-Abl kinase and Zap70 kinase or L-selectin. The results indicate that c-Abl kinase connected with L-selectin and Zap70 kinase in the resting Jurkat cells. Following the stimulation triggered by L-selectin ligation, the interaction between c-Abl kinase and Zap70 kinase is strengthened. Although much is known about L-selectin-stimulated signaling pathways, there are still gaps in our knowledge regarding the kinetics and sequence of events during early activation involved in the proximal signaling pathways. Our results provide evidence for understanding the signaling dynamics in the T cell initial activation triggered by L-selectin ligation.
In summary, the findings of this study suggest a model for signaling events downstream of the L-selectin engagement. In the resting Jurkat cells, c-Abl kinase is connected with the cytoplasmic domain of L-selectin constitutively. After L-selectin ligation, c-Abl kinase is activated and forms a complex with L-selectin and Zap70 kinase. The complex transfers the upstream signaling to regulate the cell's physiological events, such as CSF-1 gene transcription.
Material and methods
Cell culture, reagents and antibodies
The human leukemic Jurkat T cell line was grown in RPMI 1640 (Gibco) containing 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine. The primary T lymphocytes were isolated as described previously 59.
W6/32 (anti-human HLA-A,B,C mAb, mouse IgG2a, 14–9983) was purchased from eBioscience; DREG56 (anti-L-selectin mAb, mouse IgG1, sc-18851), K12 (anti-c-Abl polyclonal antibody, rabbit IgG, sc-131), PY99 (anti-phosphotyrosine mAb, mouse IgG2b, sc-7020), 1E7.2 (anti-Zap70 mAb, mouse IgG, sc-32760) from Santa Cruz Biotechnology, Inc (Santa Cruz, CA); G7781 (anti-glutathione S-transferase antibody, rabbit IgG) and piceatannol (syk/Zap70-specific kinases inhibitor) from Sigma; 8E9 (anti-c-Abl mAb, mouse IgG) from BD biosciences PharMingen; 65E4 (anti-phospho-Zap70 Tyr319 mAb, Rabbit IgG) from cell signaling technology; and anti-Crk antibody (mouse IgG2a) from BD transduction laboratories. STI571 (a special inhibitor to non-receptor tyrosine kinase c-Abl) was a gift of Novartis Pharma Switzerland AG (Basel, Switzerland). ECL Plus Western blotting detection reagents (RPN2132) and Glutathione Sepharose 4BTM (17–0756–01) were purchased from Amersham Biosciences.
Recombinant DNA constructs
The luciferase reporter vector with a CSF-1 promoter, pREP4-CSF-1-luc, and the pREP7-Renilla-luc vector, were kindly provided by Dr. Keji Zhao (NIH, Maryland). The expression vectors encoding WT c-Abl and KD c-Abl were the gifts of Dr. Hidesaburo Hanafusa (The Rockefeller University, Japan). The GST-Crk-CTD plasmid was the gift of Dr. Giorgio Scita (European Institute of Oncology, Italy). The GST-c-Abl-NSH (489–543) and GST-c-Abl-SH2 plasmids were kindly given by Dr. Nicolas Foray (European Synchrotron Research Facility, France). The expression vectors encoding WT Zap70, KD Zap70 and Y319F Zap70 were kindly provided by Dr. Oreste Acuto (the Molecular Immunology Unit, Department of Immunology, Institute Pasteur, France). We sub-cloned Zap70 fragments in the pcDNA3.1 expression vector. The sequence encoding the cytoplasmic domain of L-selectin was amplified by PCR using the full-length L-selectin cDNA in pcDNA3 as a template. BamHI and EcoRI restriction sites were introduced by the PCR primers, and the fragment was sub-cloned in pGEX vector. Inactivating mutations of putative phosphorylation sites in the cytoplasmic domain of L-selectin were introduced by use of altered PCR primers. The fragments obtained were sub-cloned into pGEX as described for the WT sequence.
GST and GST-fusion protein purification
E. coli strain BL-21 (DE3) transformed with GST or GST-fusion protein expression vectors were incubated in 200 mL LB culture medium with 50–100 µg/mL ampicillin and induced with 0.3 mM IPTG for 3 h at 37°C. Cells were harvested in lysis buffer (20 mM HEPES, pH 7.5, 120 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM PMSF, 10 µg/mL each of aprotinin and leupeptin) and disrupted by sonication. Lysates were cleared by centrifugation at 15 000 × g at 4°C for 30 min. The supernatants were applied to a GSH-Sepharose column. After extensive washing with the lysis buffer, the isolated proteins were stored at 4°C for experiments (for not more than 1 week).
RT-PCR
Jurkat cells were incubated at 37°C for the indicated time with 10 µg/mL DREG56 or control antibody. The total RNA of the cells was extracted with the TRIzol isolation system. Total RNA (1 μL) was reverse transcribed in a total volume of 20 μL and real-time PCR using SYBR green fluorescence was performed. Each real-time PCR reaction consisted of 1 μL RT product, 12.5 μL SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA), and 500 nM forward and reverse primers. Reactions were carried out on an ABI PRISM 7000 Sequence Detection System (AB Applied Biosystems) for 40 cycles (95°C for 15 s, 60°C for 1 min) after initial 10 min incubation at 95°C. The following primers were used: β-actin forward: 5′-ATGCCAGGGTACATGGTGGT-3′, β-actin reverse: 5′-TCGTGCGTGACATTAAGGAG-3′; CSF-1 forward: 5′-GTCATATGTTGAGCCTGTGG-3′, CSF-1 reverse: 5′-GGCTACGGAGATGACAGAAT-3′.
Luciferase reporter assay
For luciferase assays, transfected cells were stimulated with the indicated antibodies for 6 h and then lysed in 25 μL of an appropriate reporter lysis buffer to measure both the Firefly and the Renilla luciferase (Promega). Luciferase activity was assayed in triplicate by luminometry using the Promega double-luciferase assay system. Luciferase activity was expressed as fold increase relative to the basal activity seen in un-stimulated cells.
Kinase assay
For activation, 1 × 107 Jurkat cells per sample were washed twice in sterile HEPES saline (H/S; 132 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, 0.8 mM MgSO4) and stimulated at 37°C with DREG56. Cell stimulation was terminated by lysis in 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM each NaF, Na3VO4, and β-glycerophosphate, and 20 μg/mL aprotinin/leupeptin. After 15-min incubation on ice, cells were centrifuged at 15 000 X g for 25 min, and lysates were incubated with anti-c-Abl antibody (K12). After 2 h, 20 μL protein A-Sepharose beads (50% slurry) was added to the antibody/lysated mixture for 1 h. Immunoprecipitates were washed at least four times (1 mL) with lysis buffer, and then washed three times with the kinase buffer (25 mM Tris, pH 7.5, 2 mM DTT, 5 mM β-glycerophosphate, 1 mM Na3VO4, 10 mM MgCl2). After 5-min pre-incubation at 30°C, 30-μL reactions were initiated by the addition of 3 μg GST-CrKII-CTD or GST-CytoL and 5 μM ATP. After 30 min, reactions were terminated by the addition of 20 μL 3X SDS sample buffer, and resolved by SDS-PAGE.
Immunoprecipitation and Immunoblotting
Jurkat cells were stimulated and lysed as above. Lysates were centrifuged at 15 000 × g for 25 min, and the supernatants were incubated with the indicated antibodies. After 2 h, 20 μL protein A/G-Sepharose beads (50% slurry) was added to the antibody/lysated mixture for 1 h. Immunoprecipitates were washed three to six times with lysis buffer and resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes using the chilled transfer buffer (25 mM Tris base, 192 mM glycerin, and 20% methanol) at 100 V for 1 h at 4°C. After protein transfer, the nitrocellulose membranes were washed with TBST (20 mM Tris base, 500 mM NaCl, 0.05% Tween-20, pH 7.5) at least three times and immediately incubated with 5% non-fat milk, the indicated primary antibody and the HRP-conjugated secondary antibody at 37°C for 1 h. Chemiluminescent detection was performed by using ECL plus Western blotting reagents.
Isolation of cellular proteins interacting with GST fusion protein (pull-down)
Jurkat cells were stimulated and lysed as mentioned above. Cell lysates were incubated with 10 µL GSH-Sepharose coated with GST or GST fusion proteins. After 2 h, beads were collected by centrifugation, washed four times with lysis buffer, and the bound proteins were eluted by boiling in SDS-sample buffer and analyzed by SDS-PAGE.
Far Western analysis
To determine direct interaction of either c-Abl or selected c-Abl domains with the electrophoresed, nitrocellulose-bound proteins, SDS-PAGE and Western blotting were performed as described above, followed by blocking of the membranes with phosphate-buffered saline containing 3% BSA and 0.1% Tween-20. Membranes were then incubated overnight at 4°C with a blocking buffer containing 10 μg/mL of the indicated GST fusion protein or GST protein. Bound GST proteins were detected by incubation of the membrane with a rabbit anti-GST mAb for 1 h, followed by an HRP-conjugated sheep anti-rabbit IgG and ECL development.
Acknowledgements
We thank Novartis Pharma Switzerland AG for providing inhibitor STI571 and Dr. Keji Zhao, Dr. Hidesaburo Hanafusa, Dr. Giorgio Scita, Dr. Nicolas Foray, Dr. Oreste Acuto provided the vectors or plasmids that made this work possible. This work was supported by grants from the National Basic Research Program of China (2002CB513006), and the National Natural Science Foundation of China (30570928).Conflict of interest: The authors declare no financial or commercial conflicts of interest.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




