Characterization of mouse CD4 T cell subsets defined by expression of KLRG1
Abstract
The mouse killer cell lectin-like receptor G1 (KLRG1) is an inhibitory receptor known to be expressed on a subset of NK cells and antigen-experienced CD8 T cells. Here, we have characterized expression of KLRG1 on CD4+ T cells from normal mice. While a polyclonal TCR repertoire suggests thymic origin of KLRG1+ CD4+ cells, KLRG1 expression was found to be restricted to peripheral CD4+ T cells. Based on phenotypic analyses, a minority of KLRG1+ CD4+ cells are effector/memory cells with a proliferative history. The majority of KLRG1+ CD4+ cells are, however, bona fide Treg cells that depend on IL-2 and/or CD28 and express both FoxP3 and high levels of intracellular CD152. KLRG1-expressing Treg are contained within the CD38+ subset but are only partially overlapping with the CD25+ CD4+ Treg subset. In functional assays, KLRG1+ CD4+ cells were anergic to TCR stimulation with respect to proliferation, and sorted KLRG1+ CD25+ CD4+ cells were equal or superior to KLRG1+ CD25– CD4+ cells, which were more potent than KLRG1– CD25+ CD4+ cells in suppressing responder cell proliferation. Together, our results demonstrate that KLRG1 expression defines novel and distinctive subsets of senescent effector/memory and potent regulatory CD4+ T cells.
Abbreviation:
-
- KLRG1:
-
killer cell lectin-like receptor G1
Introduction
It is well accepted that peripheral CD4+ Treg maintain peripheral T cell tolerance and prevent the development of autoimmune/inflammatory diseases 1–4. FoxP3 is the signature marker of Treg, as its expression causally relates to suppressive/regulatory T cell activity and since many other molecules constitutively expressed by Treg - such as CD25, CD152 or GITR - can also be induced by activation of conventional T cells 5, 6. “Natural” FoxP3+ CD4+ CD25+ Treg arise in the thymus 7, and their homeostasis depends on IL-2 8, 9 and CD28 10–12. In peripheral lymphoid tissues, Treg are subjected to further differentiation/activation processes and can acquire expression of immunomodulatory cytokines such as IL-10 13, 14 or tissue homing receptors such as CD103 15.
In addition to changes in the expression patterns of activation markers, cytokine receptors and homing molecules during the generation and differentiation of “conventional” effector/memory cells, it has been recognized that this process is also marked by the de novo expression of so-called NK cell receptors 16. These activating or inhibitory cell surface molecules are critical for controlling NK cell effector functions and, when expressed by T cells, have been shown to either act as co-stimulators of TCR stimulation or inhibitors of T cell activation 16.
Killer cell lectin-like receptor G1 (KLRG1) belongs to a family of lectin-like type II transmembrane proteins. Initially, mouse KLRG1 expression was described for an NK cell subset 17 and virus-activated CD8+ T cells 18. On mouse NK cells, expression of KLRG1 is more frequent in MHC class I-proficient mice than in class I-deficient mice 19, 20, and it is further up-regulated upon mouse cytomegalovirus (MCMV) infection 21. KLRG1+ CD8+ T cells have an effector/memory phenotype 22, and it has been shown that frequent cell division after repetitive antigenic stimulation of CD8+ cells in a transgenic mouse model induces KLRG1 expression on senescent T cells 23, revealing a link between the proliferative history of a CD8+ T cell and the expression of KLRG1. A similar mechanism probably underlies the in vivo induction of KLRG1 expression among CD8+ cells during viral or parasitic infections 18, 24.
Recently, members of the cadherin family have been identified as ligands for KLRG1 25, 26, and it has been shown that recombinant cadherins or anti-KLRG1 antibodies can mediate inhibition of effector functions of KLRG1-expressing CD8+ T cells 25, 26 and NK cells 26.
While it was speculated that inhibitory lectin-like NK receptors may ‘optimally shape CTL responses to infection’ 27, expression of NK cell receptors on CD4+ T cells - with the notable exception of CD4+ NK T cells 28 - has not been studied in great detail. It has been reported that KLRG1 is present on a small subset of splenic CD4+ T cells and that its expression is up-regulated after Toxoplasma gondii infection 24. Here, we investigated phenotypic and functional properties of KLRG1+ CD4+ T cells in normal mice and show that most KLRG1 expression marks CD4+ cells that either belong to the effector/memory compartment or constitute a subset of Treg that bears distinctive functional features.
Results
Distribution of KLRG1 on CD4+ cells
As shown in Fig. 1A, KLRG1 is present on the surface of 1–2% of CD4+ splenocytes from adult B6 mice. Four-color flow cytometric analysis revealed that the vast majority of KLRG1+ CD4+ cells express high levels of CD3 and lack expression of NK1.1 (Fig. 1A). Comparable TCR Vβ distribution patterns among splenic KLRG1+ and KLRG1– CD4+ cells (Fig. 1B) are indicative of a polyclonal TCR repertoire and thymic origin of most if not all KLRG1+ CD4+ cells.
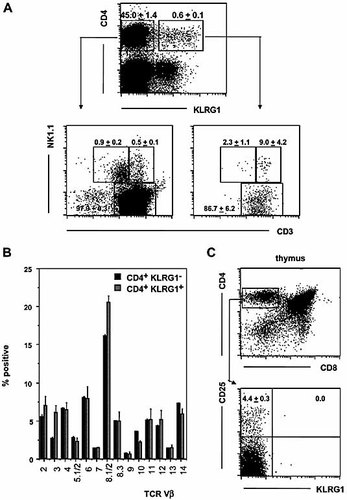
KLRG1 expression by splenic CD4+ T cells. (A) Nylon wool non-adherent splenocytes were stained for CD4, KLRG1, NK1.1 and CD3 expression by four-color flow cytometry. Representative dot plots are shown for ungated cells (upper panel), CD4+ KLRG1– cells (lower left panel) or CD4+ KLRG1+ cells (lower right panel). (B) Nylon wool non-adherent splenocytes were stained with a panel of TCR Vβ-specific mAb. Percentages of CD4+ KLRG1– (black bars) and CD4+ KLRG1+ (gray bars) cells expressing a given TCRVβ chain are shown. (C) Thymocytes from young adult mice were stained for CD4, CD8, KLRG1 and CD25 expression. Dot plots are shown for ungated cells (upper panel) or gated CD4+ CD8– cells (lower panel). (A–C) Mean frequencies ± SD were determined from three individual values.
We also studied whether KLRG1 expression on CD4+ cells is already induced in the thymus. While the subset of CD25+ Treg progenitors was readily detectable among CD4+ CD8– thymocytes, KLRG1 expression was completely absent on CD4+ CD8– cells from young adult mice (Fig. 1C) or on fetal thymocytes (data not shown). These findings suggest that KLRG1 is induced on CD4+ T cells exclusively after emigration from the thymus.
In newborn mice, a high frequency of peripheral T cells and Treg undergo marked proliferation 29. Analysis of KLRG1 expression on CD4+ cells from mice between 1 day and 6 months of age revealed that more than 10% of CD4+ cells express KLRG1 immediately after birth and that the number decreases to 1–2% during adult life (Fig. 2A). While the role of IL-2 in the expansion of KLRG1+ CD4+ cells in neonatal mice remains to be determined, we noticed that in adult mice, the KLRG1+ and CD25+ CD4 subsets are partially overlapping, as approximately 60% of CD4+ KLRG1+ cells express CD25, and among CD25+ cells, approximately 6% co-express KLRG1 (Fig. 2B, C).
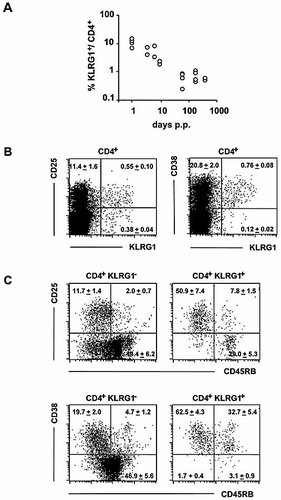
Expression of KLRG1 during ontogeny and correlation with cell surface markers for Treg. (A) KLRG1-specific cell surface expression among CD4 cells was determined by flow cytometric analysis of splenocytes from mice of the indicated ages (p.p.: postpartum). Results from three independent experimental series are summarized. (B, C) Nylon wool non-adherent splenocytes were analyzed for co-expression of the indicated cell surface receptors by four-color staining. Dot plots are shown for gated CD4+ cells (B) or gated CD4+ KLRG1+ or – cells (C). Numbers indicate the mean percentage of cells ± SD in the respective quadrant of three mice analyzed in parallel.
It has been shown that IL-2 deficiency 8, 9, 30 or CD28 deficiency 10–12 impairs the generation of CD25+ CD4+ cells. While reduced numbers of CD25+ KLRG1– cells were observed in both IL-2- and CD28-deficient mice, KLRG1+ CD25+ cell numbers were reduced in CD28–/– but not in IL-2–/– mice, and KLRG1+ CD25– cell numbers were normal in CD28–/– mice and even enhanced in IL-2–/– mice (Table 1).
|
|
|
|
Subsets of CD4+ cells |
||
|---|---|---|---|---|---|
|
Mouse strain |
Age (weeks) |
# |
CD25+KLRG1– |
CD25+KLRG1+ |
CD25–KLRG1+ |
|
C57BL/6 |
6–12 |
n=8 |
7.9±0.7a) |
0.5±0.2 |
0.6±0.2 |
|
IL-2–/– |
3–5 |
n=5 |
2.6±1.7 |
0.4±0.2 |
1.5±0.3 |
|
CD28–/– |
8–11 |
n=5 |
3.1±0.9 |
0.2±0.0 |
0.7±0.5 |
- a) Numbers indicate mean percentages ± SD of indicated subsets among CD4+ cells.
We next studied whether antigen recognition is essential for KLRG1 expression on CD4+ cells in OT2 TCR-tg mice, which express a clonotypic receptor (Vα2) specific for OVA 31. Since KLRG1 was expressed by 3.5% of Vα2+ TCR-tg CD4+ cells but only by 1.1% of CD4+ cells from non-tg mice (Fig. 1 and data not shown), cognate antigen recognition is not necessary to induce KLRG1 expression on CD4+ cells.
KLRG1+ CD4+ cells have the phenotype of regulatory or effector/memory cells
To more closely examine the phenotype of KLRG1+ CD4+ cells, splenocytes were co-stained for KLRG1 expression and other markers of CD4 cell activation and/or differentiation. Expression of the early activation antigen CD69 was comparable on KLRG1+ and KLRG1– cells, and all KLRG1+ cells expressed high levels of CD44 (data not shown). Interestingly, most if not all splenic KLRG1+ CD4+ cells were contained within a subpopulation of CD38+ CD4+ cells (Fig. 2B, C). CD38 has been reported to mark a subset of suppressive/regulatory CD4+ cells that has largely a CD45RBlow phenotype and is unresponsive to TCR triggering in vitro 32. Our analyses showed that approximately two-thirds of KLRG1+ CD4+ cells were CD38+ CD45RBlow, with a minority of KLRG1+ cells being CD38+ CD45RBhigh (Fig. 2C, lower panel).
In summary, the splenic KLRG1+ CD4+ subpopulation partially overlaps with the regulatory CD25+ and CD45RBlow subsets and is almost exclusively contained within the CD38+ subset. Similar results were obtained for CD4+ cells from the lymph node, where, however, the overall expression frequency of KLRG1 among CD4+ T cells was lower (data not shown).
In addition to CD25, high levels of intracellular CD152 and the transcription factor FoxP3 have been found to most reliably identify Treg 7. When studied by four-color flow cytometry, approximately 60% of KLRG1+ cells co-expressed FoxP3 (Fig. 3), marking them as bona fide Treg. Among all FoxP3+ cells, KLRG1 was expressed by approximately 6% of cells, all of which co-expressed high levels of CD152. Our data also show that most if not all KLRG1+ CD25+ and KLRG1– CD25+ cells were FoxP3+ and that FoxP3– KLRG1+ cells lacked co-expression of CD25 and lacked CD152 expression. Therefore, KLRG1 marks peripheral CD4+ cells that display the phenotype of either Treg (i.e. FoxP3+, most of which also express CD25) or antigen-experienced conventional T cells.
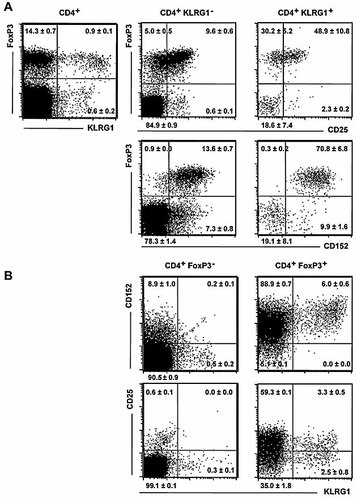
Co-expression of FoxP3, CD25 and CD152 with KLRG1. Freshly isolated splenocytes were stained for cell surface expression of CD4, CD25 and/or KLRG1 followed by staining for intracellular CD152 and FoxP3 expression. Dot plots are shown for gated CD4+ cells or for cells gated according to CD4 and KLRG1 (A) or FoxP3 staining (B), as indicated. Mean percentages of cells per quadrant ± SD of three mice analyzed in parallel are shown.
Unresponsiveness of KLRG1+ CD4+ cells to TCR stimulation
We next sought to analyze functional properties of KLRG1+ CD4+ cells. Initially, nylon wool-passed splenocytes were stimulated ex vivo by immobilized anti-TCRαβ mAb, and proliferative responses were determined by CFSE analysis. Similar to results obtained for CD8+ T cells 22–24, anti-TCR stimulation did not up-regulate KLRG1 levels (data not shown) or increase the relative number of KLRG1+ CD4+ cells but rather reduced the relative frequency of KLRG1+ CD4+ cells among splenocytes over a culture period of up to 3 days (Fig. 4A). This result suggests either down-regulation of KLRG1 expression, an impaired proliferative response of KLRG1+ cells or enhanced cellular death among KLRG1+ cells. While TCR triggering induced profound cellular division among KLRG1– CD4+ cells (Fig. 4B), KLRG1+ cells proliferated poorly: 58% of KLRG1– cells but only 17% of KLRG1+ cells had divided two or more times after 3 days of stimulation. In addition, we found a comparable degree of apoptosis among TCR-stimulated KLRG1+ and KLRG1– cells (data not shown). Therefore, KLRG1+ CD4+ cells are refractory to induction of proliferation by TCR triggering in a T cell culture.
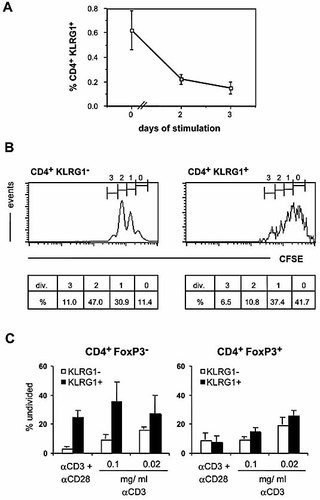
In vitro response of KLRG1+ CD4+ cells to TCR stimulation. (A, B) Nylon wool non-adherent splenocytes were stimulated for 48 h with immobilized anti-TCR mAb. (A) Percentages of CD4+ KLRG1+ cells before (day 0) and after (day 2, day 3) culture ± SD (n=3) are shown. (B) Proliferative dilution of CFSE label. Histograms show log10 green fluorescence intensities for gated CD4+ KLRG1– cells (left panel) or CD4+ KLRG1+ cells (right panel) after 2 days of culture. Numbers indicate apparent number of cell divisions (div.) and percentage of cells (%). In cultures without TCR stimulation, CFSE intensity was uniformly high at “0” divisions. Data are representative of three independent experiments. (C) CFSE-labeled splenocytes were stimulated with the indicated concentrations of anti-CD3 mAb +/– anti-CD28 mAb. After 3 days, the percentage of undivided cells among subsets gated for CD4, FoxP3 and KLRG1 expression was determined. Bars represent means ± SD of three or four individual determinations.
To compare the proliferative capacity of KLRG1+ “conventional” T cells with KLRG1+ Treg, we stimulated splenocytes with soluble anti-CD3 mAb in the absence or presence of anti-CD28 mAb and analyzed CFSE dilution and FoxP3 expression of CD4+ KLRG1+ and CD4+ KLRG1– cells. Proliferation of KLRG1+ FoxP3+ cells was similar to that of KLRG1– FoxP3+ and KLRG1– FoxP3– CD4+ cells, while KLRG1+ FoxP3– cells proliferated less well than KLRG1– FoxP3– cells under all conditions tested (Fig. 4C). Therefore, KLRG1+ FoxP3– cells were distinct from Treg cells expressing or lacking KLRG1, as they were refractory to mitogenic stimuli.
Regulatory capacity of CD25+ and KLRG1+ cell subsets
To study the suppressive activity of KLRG1+ cells in an in vitro inhibition assay and compare it to the established suppressive effects of the partially overlapping CD25+ subset, splenic CD4+ cells were sorted into KLRG1+ CD25+, KLRG1+ CD25–, KLRG1– CD25+ and KLRG1– CD25– populations. As shown in Fig. 5A, the purity of sorted cells was routinely >90%. In a post-sort analysis, FoxP3 staining of the different subsets equaled the results obtained for unseparated cells as shown in Fig. 3.
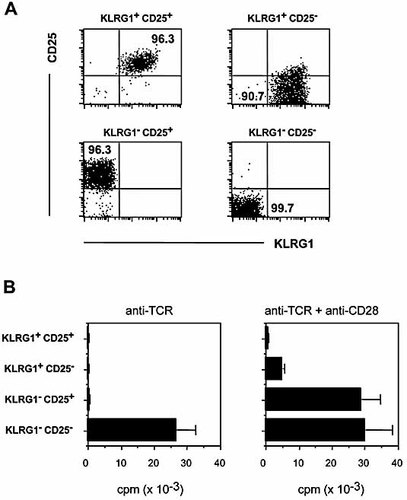
Proliferative responsiveness of sorted KLRG1+ CD25+, KLRG1+ CD25– and KLRG1– CD25+ CD4 cells. CD4+ splenocytes were sorted into the four subsets defined by KLRG1 and CD25 expression, as indicated; purities of >90% were routinely achieved. Representative data from a post-sort analysis are shown in (A). (B) Sorted cells were cultured for 3 days in the presence of immobilized anti-TCR mAb (left) or immobilized anti-TCR mAb plus soluble anti-CD28 mAb (right), and 16 h prior to harvesting, cells were pulsed with [3H]-thymidine. Data show means ± SD and are representative of four independent experiments.
In a first set of experiments, cells were stimulated for 3 days with immobilized anti-TCR mAb in the absence or presence of soluble anti-CD28 mAb. CD4+ KLRG1– CD25– cells proliferated substantially upon TCR stimulation alone, while KLRG1+ and/or CD25+ cells did not proliferate (Fig. 5B). For the KLRG1– CD25+ subset, unresponsiveness to TCR triggering could readily be overcome by addition of soluble anti-CD28 mAb. Interestingly, we observed only marginal proliferation of KLRG1+ CD25– cells and complete absence of proliferation of KLRG1+ CD25+ cells in response to costimulation (Fig. 5B), which could not be reverted by increased amounts of anti-TCR or anti-CD28 mAb (data not shown). Therefore, KLRG1+ CD25+ or – CD4+ cells are distinct from KLRG1– CD25+ CD4+ cells, as they are completely (CD25+ fraction) or almost completely (CD25– fraction) anergic to stimulation via TCR plus CD28.
To test their suppressive capacity, CD4+ cells sorted for KLRG1 and CD25 expression were co-cultured with KLRG1– CD25– CD4+ responder cells on anti-TCR-coated plates, and [3H]-thymidine incorporation was determined after 3 days. As shown in Fig. 6A, all three subsets defined by KLRG1 and CD25 expression efficiently reduced proliferation of responder cells, i.e. displayed suppressive activity. When data from four or five independent experiments were compared using an “inhibition index” that allows comparison of individual experiments (see the Materials and methods), it became evident that the KLRG1+ CD25+ population tended to be most effective in suppressing responder cell proliferation, followed by the KLRG1+ CD25– and KLRG1– CD25+ subsets (Fig. 6B).
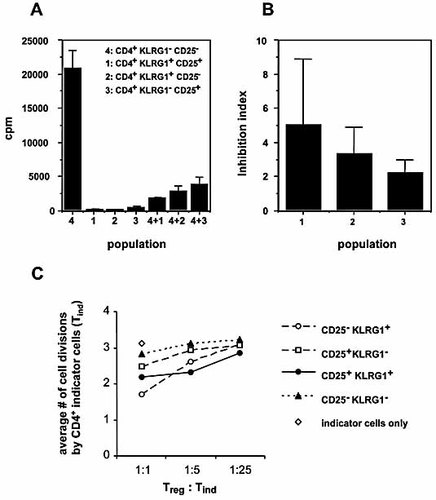
Suppressive capacity of KLRG1+ CD25+, KLRG1+ CD25– and KLRG1– CD25+ CD4 cells. (A) Nylon wool non-adherent spleen cells sorted for the indicated subsets defined by expression of CD4, KLRG1 and CD25 (see Fig. 5) were cultured either alone (left) or at a 1:1 ratio together with CD4+ CD25– KLRG1– responder cells (right) at a final concentration of 2 × 105 cells/mL. After 3 days, [3H]-thymidine incorporation was determined. Results (mean cpm ± SD) are representative of four or five independent experiments. (B) Inhibition indices were determined as described in the Materials and methods and represent means ± SD of four or five independent determinations. (C) CFSE dilution profiles were determined for primary CD4+ CD25– KLRG1– indicator cells stimulated with anti-CD3 in the presence of antigen-presenting cells and the CD4+ cell subsets defined by KLRG1 and CD25 expression (as described in the Materials and methods). The graph shows the average number of cell divisions among indicator CD4+ cells from one out of two experiments with comparable outcome.
Comparable results were obtained in CFSE dilution experiments in which different Treg:indicator ratios were employed (Fig. 6C). Depending on the Treg:indicator ratio, either KLRG1+ CD25+ cells or KLRG1+ CD25– cells were more prominent in suppressing indicator cell proliferation. As both KLRG1– CD25+ and KLRG1+ CD25+ cells contain >90% FoxP3+ cells (Fig. 3), our results further suggest that the suppressive activity per FoxP3+ cell was greater among KLRG1+ than among KLRG1– cells.
Discussion
“Natural” Treg with a CD4+ CD25+ phenotype are probably the most extensively studied subset of regulatory/suppressor T cells. Based on the differential cell surface expression of KLRG1 on CD4+ T cells, we have now been able to functionally subdivide CD4+ CD25+ T cells. We could also identify a new subset of KLRG1+ Treg outside of the CD25+ compartment and show that KLRG1 is also expressed by non-Treg.
The complete absence of KLRG1 expression on CD4+ CD8– thymocytes (Fig. 1C) together with the finding that KLRG1 could not be induced on CD4 cells in short-term in vitro culture (Fig. 4 and 22–24) indicates that KLRG1 expression in vivo is not an early activation marker but a consequence of CD4+ T cell stimulation in the appropriate microenvironment of a secondary lymphoid organ or peripheral tissue. Since it takes at least 7 days to detect a pronounced increase of splenic KLRG1+ CD4+ T cell numbers in mice infected with T. gondii 24, the expression of KLRG1 in vivo apparently does not mark cellular activation of a CD4+ cell but rather indicates its proliferative history, a result of either repetitive encounter with self-antigen and homeostatic maintenance (as assumed for Treg) or clonal expansion after exogenous antigen recognition in an inflammatory environment (as in the case of T. gondii infection). It remains, however, to be determined whether the induced or expanded KLRG1+ cells in T. gondii-infected mice belong to the Treg or the effector/memory lineage.
A peripheral T cell-specific induction mechanism similar to the one discussed for KLRG1 has been proposed for the population of CD38+ CD4+ cells 32, although CD38 can also be expressed by some CD4+ CD8– cells in the thymus 33. While cell surface expression of KLRG1 is confined to peripheral T cells, it remains possible that its expression is programmed in the thymus and that it is only expressed in the periphery after the cell has received appropriate proliferative/inflammatory signals.
The high frequency of CD45RBlow cells among KLRG1+ CD4+ cells (Fig. 2C) provides additional evidence that KLRG1 expression occurs after stimulation of peripheral T cells. A minor proportion of KLRG1+ CD4+ cells expresses high levels of CD45RB yet also expresses other markers for antigen-experienced cells such as CD44 and therefore probably represents memory cells that have re-enforced CD45RB expression 34, 35 rather than naive CD4 T cells.
A CD45RBlow phenotype not only marks conventional memory cells but also characterizes CD4+ CD25+ Treg 36. Indeed, co-stainings of extracellular KLRG1 and CD25 versus intracellular FoxP3 and CD152 clearly showed that approximately 60% of KLRG1+ cells are FoxP3+ CD152bright Treg, with 60% of FoxP3+ KLRG1+ cells also expressing CD25 (Fig. 3). Conventional FoxP3– KLRG1+ cells are all negative for CD152 and CD25 expression, indicating that while the majority of KLRG1+ cells are bona fide Treg, KLRG1 expression is not confined to this compartment.
In keeping with this interpretation, we also found that the number of KLRG1+ CD25– cells was unaltered in mice lacking functional expression of CD28 (Table 1), which is critical for the generation of natural Treg 10–12, and even increased in IL-2-deficient mice (Table 1), which are also devoid of natural Treg 9, 30 and in which conventional CD4+ T cells proliferate unchecked and contribute to the development of a lethal lymphoproliferative disorder 37. While formally not addressed, it is tempting to speculate that the higher numbers of KLRG1+ CD25– cells in young adult IL-2–/– mice are indeed those cells that have escaped homeostatic control due to the lack of thymus-derived and IL-2-dependent natural Treg. On the other hand, frequencies of KLRG1– CD25+ cells were diminished in IL-2-deficient mice, while normal frequencies of KLRG1+ CD25+ cells are maintained in the absence of IL-2, suggesting that KLRG1+ CD25+ Treg are less dependent on (presumably exogenous) IL-2 than are KLRG1– CD25+ Treg.
When assessing a possible requirement of antigenic recognition for KLRG1 induction on CD4+ cells in the OT2-tg system 31, we observed rather increased frequencies of KLRG1+ cells in the absence of the cognate antigen. These data are in accordance with the lack of in vivo induction of KLRG1 on Vβ11+ cells by the superantigen SEA (data not shown) and underline that KLRG1 should not be considered a marker for activation or antigen-experienced cells.
KLRG1 expression among CD4+ cells appears to be confined to those subsets with a constitutively high turnover under steady-state conditions, i.e. effector/memory T cells 38 and Treg 11. Interestingly, KLRG1 expression frequencies among CD4+ cells of mice and humans show a first peak shortly after birth (Fig. 2A and 39), when the available niches within secondary lymphoid organs are being filled up by proliferating thymic emigrants 29. Therefore, KLRG1 expression is not confined to “aged cells” but rather appears to reflect the number of cell cycles a cell has undergone, be it in a short or prolonged period of time.
At the molecular level, the hypo-responsiveness of KLRG1+ CD4+ cells towards TCR-mediated stimulation does not appear to be mediated by KLRG1 itself functioning as an inhibitory receptor, as extensive cross-linking of KLRG1 on primary CD4+ T cells from KLRG1-transgenic or non-transgenic mice by anti-KLRG1 mAb did not inhibit ex vivo T cell proliferation (data not shown). However, it cannot be ruled out that constitutive inhibitory signaling through KLRG1 can be triggered in vivo by the physiological cadherin ligand(s).
Pircher and colleagues have reported that KLRG1+ CD8+ cells of mice and KLRG1+ CD4+ and CD8+ cells of humans are proliferatively senescent, i.e. refractory to antigenic re-stimulation in vivo or in vitro, respectively 23, 40. In our experiments, all KLRG1+ CD4+ cells were anergic to isolated TCR stimulation, while proliferative anergy of KLRG1+ Treg could be readily overcome, and only KLRG1+ FoxP3– “conventional” T cells exhibited a truly senescent phenotype under conditions of “optimal” ex vivo stimulation in the presence of APC (Fig. 4C).
In addition to the intrinsic proliferative potential of the different subsets of CD4+ cells defined by KLRG1 expression, we also analyzed their suppressive capabilities. KLRG1+ CD25+ CD4+ cells not only displayed the ‘deepest’ degree of anergy but also appeared to be the strongest suppressors of responder cell proliferation on a per-cell basis (Fig. 6). While the high prevalence of FoxP3+ cells among KLRG1+ CD25+ CD4+ cells could suffice to explain this observation, the somewhat stronger suppressive function of KLRG1+ CD25– versus KLRG1– CD25+ cells was not related to the frequency of FoxP3+ cells within the respective subsets (Fig. 3 and Fig. 6): FoxP3 was expressed by only ∼60% of KLRG1+ CD25– cells, while it was expressed by >90% of KLRG1– CD25+ cells. Therefore, it is tempting to speculate that KLRG1+ CD25– FoxP3+ cells might be more suppressive on a per-cell basis. On the other hand, we cannot formally exclude that KLRG1+ FoxP3– cells were also suppressive in our assay system.
While the mechanism of suppression exerted by the CD4+ cell subsets defined by KLRG1 and/or CD25 expression was not experimentally addressed in our assays, we consider it most likely that it is mediated by cell-cell contact rather than by suppressive cytokines such as IL-10, as this has been convincingly shown for CD25+ cells, which contain the largest fraction of cells defined by KLRG1 and CD25 expression, i.e. CD25+ KLRG1+ cells 41, and since TCR stimulation did not induce substantial secretion of IL-10 by KLRG1+ cells (data not shown). However, the nature of the molecules involved in the cell-cell interaction remains to be determined.
In summary, we have identified KLRG1 as a marker for a subpopulation of highly suppressive but otherwise “normal” FoxP3+ Treg that partially overlaps with the CD4+ CD25+ subset. Outside of the Treg cell compartment, KLRG1 expression marks senescent “conventional” CD4+ cells. We believe that assessment of KLRG1 expression in future functional studies will help to more precisely define activation/differentiation steps occurring within Treg as well as effector/memory CD4+ T cell populations.
Materials and methods
Animals
C57BL/6J, IL-2–/– 42 and OT2-tg 31 mice were bred at the animal facilities of the Institute for Virology and Immunobiology, University of Würzburg, in accordance with institutional guidelines. Young adult mice were used throughout the study.
Antibodies
The following primary mAb were used: unconjugated anti-TCRαβ (H57), anti-CD3 (145–2C11), and anti-CD28 (37.51); anti-CD3-FITC, anti-CD4-PE, anti-CD4-allophycocyanin (APC), anti-CD4-Alexa647, anti-CD25-FITC, anti-Vα2-FITC, anti-CD25-biotin, anti-CD38-FITC, anti-CD38-PE, anti-CD45RB-FITC, anti-CD152-PE, anti-NK1.1-PE, anti-KLRG1-biotin, rat-IgG-PE and a panel of FITC-conjugated anti-TCR Vβ mAb (all from BD/Pharmingen); anti-FoxP3-PE or anti-FoxP3-APC (FJK-16s; eBioscience). Unconjugated anti-KLRG1 (2F1 or 4.21) or FITC-conjugated anti-KLRG1 (2F1) was prepared from hybridoma supernatant according to standard techniques. As second-step, control or blocking reagents, hamster IgG (Sigma) and unconjugated or FITC-conjugated goat anti-hamster IgG (Dianova) were used.
Cell surface staining and flow cytometry
For flow cytometry, spleen cells were routinely passed through nylon wool to enrich for CD4+ cells and reduce background staining. The cells (1 × 106) were stained in PBS/0.1 %BSA/0.02% NaN3 at 4°C using saturating amounts of reagents. Samples were washed once between steps or before analysis and three times after staining with biotinylated mAb. Stainings with biotinylated mAb were developed with SA-Cychrome (Pharmingen) or SA-Tricolor (Caltag). Samples were analyzed on a FACSCan® or FACSCalibur® (Becton Dickinson) flow cytometer using Cell Quest® software with live gate settings based on scatter profile. Dot plots and histograms are shown as log10 fluorescence intensities on a four-decade scale. For cell sorting, nylon wool-passed spleen cells were stained with mAb 2F1 and anti-hamster Ig-FITC. Positive and negative fractions were separated using anti-FITC-beads and MACS technology (Miltenyi) according to the manufacturer's instructions. Subsequently, cells were stained with anti-CD4-APC and anti-CD25-PE and sorted on a FACSVantage® or a FACSDiva® (Becton Dickinson). The purity of sorted cells as determined by post-sort analysis was routinely between 88 and 99%. Viability of sorted cells was confirmed by trypan blue exclusion, electronic forward vs. side scatter profile and, in some cases, by propidium iodide exclusion.
In vitro stimulation of T cells
(A) Stimulation by plate-bound anti-TCR mAb: 96-well flat-bottom plates were routinely coated with goat anti-hamster IgG (Sigma; 50 μg/mL in 50 mM carbonate buffer pH 9) and, after extensive washing, 5 μg/mL anti-TCRαβ mAb H57. Following washing, 2 × 105 to 5 × 105 cells/mL (unseparated splenocytes) or 1 × 105 to 2.5 × 105 cells/mL (sorted cells) were cultured in supplemented RPMI 1640 medium (Gibco BRL). Where indicated, 2 μg/mL soluble anti-CD28 mAb (BD Pharmingen) was added. (B) Stimulation by soluble anti-CD3 mAb: Unseparated spleen cell preparations (2 × 106 cells/mL) were labeled with CFSE (5 μM; MoBiTec) and stimulated with anti-CD3 mAb alone or in conjunction with soluble anti-CD28 mAb. After 3 days, cells were stained for CD4, KLRG1 and FoxP3 expression prior to flow cytometric analysis. (C) Suppression assays using [3H]-thymidine incorporation to determine T cell proliferation: For co-cultures, equal numbers of “regulator” cells sorted for KLRG1 and/or CD25 expression and KLRG1– CD25– “responder” cells were co-cultured at a final concentration of 105 cells/mL. Proliferation was measured by determining [3H]-thymidine incorporation (12.5 μCi [3H]-thymidine; ICN) during the final 16–20 h of a 3-day culturing period. Radioactive incorporation was determined by β-counting. Counts per minute (cpm) are given as means ± SD. (D) Suppression assay with CFSE-labeled cells: 5 × 104 CFSE-labeled CD8-, KLRG1- and CD25-depleted mixed spleen and lymph node cells (50% CD4+ cells) were co-cultured with either 2.5, 0.5 or 0.1 × 104 CD4+ cells sorted according to CD25 and KLRG1 expression. T cells were activated by addition of 0.5 μg/mL anti-CD3 mAb. After 3 days, CFSE dilution was determined among CD4+ indicator cells, and the average number of cell divisions was calculated from histogram analysis.
Intracellular staining
For intracellular staining of FoxP3 and CD152, cells were fixed for 30 min at room temperature with fixation buffer (eBioscience) prior to permeabilization (permeabilization buffer; eBioscience). The cells were blocked with rat serum before staining with the appropriate mAb for 30 min at room temperature.
Calculation of inhibition indices
The inhibition indices shown in Fig. 6 were calculated by dividing the sum of the cpm from separately cultured responder cells and regulator cells by two times the cpm obtained in co-culture (since the total cell number was identical in cultures and co-cultures). Low proliferation in co-cultures indicates a strong regulatory activity and a high inhibition index.
Acknowledgements
We wish to thank Sonja Rotzoll and Christian Linden for expert cell sorting, Karen Balbach, Nadine Freyer and Sandra Werner for technical assistance and Thomas Hünig for critical reading of the manuscript. This work was supported by DFG grant HA 2456 / 3–1 (to T. H.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




