CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli
Abstract
CC chemokine ligand (CCL)17 is implicated in the pathogenesis of atopic dermatitis (AD). To study the effect of CCL17 produced by keratinocytes (KC) during inflammation, we created transgenic (Tg) mice in which CCL17 is overexpressed in KC. Th2-type contact hypersensitivity (CHS) was enhanced and Th1-type CHS was suppressed in these mice. Increased numbers of CC chemokine receptor (CCR)4+ cells and mast cells infiltrated in Tg mice. Levels of IL-4 mRNA were higher and those of IFN-γ mRNA were lower in both acute and chronic CHS. Higher levels of serum IgE were observed after CHS. Numbers of CCR4+ cells among PBMC were increased in Tg mice challenged acutely on the trunk. Chronic irritation with croton oil induced dermatitis and an elevation of serum IgE levels. Tg mice showed enhanced ear swelling after tape stripping. CCL17 was thought to modify the inflammation caused by sensitizing reagents as well as irritant reagents by attracting CCR4+ cells into the lesional skin and creating a Th2-dominant condition. AD-like conditions such as increased number of mast cells and elevated levels of serum IgE were observed. Thus, CCL17 may participate in the pathogenesis of skin diseases such as AD by regulating both allergic and irritant inflammation.
Abbreviations:
-
- AD:
-
atopic dermatitis
-
- CCL:
-
CC chemokine ligand
-
- CCR:
-
CC chemokine receptor
-
- CHS:
-
contact hypersensitivity
-
- CLA:
-
cutaneous lymphocyte-associated antigen
-
- CO:
-
croton oil
-
- HE:
-
hematoxylin and eosin
-
- hGH:
-
human growth hormone
-
- hK14:
-
human keratin 14
-
- KC:
-
keratinocyte
-
- LC:
-
Langerhans cell
-
- OX:
-
oxazolone
-
- TARC:
-
thymus and activation-regulated chemokine
-
- UTR:
-
untranslated region
Introduction
Chemokines are a family of polypeptides that govern the chemotaxis and activation of different subsets of leukocytes during immune and inflammatory responses 1–3. Recent studies have revealed that Th1 and Th2 cells differ in the chemokine receptors that they express 1–3.
Thymus and activation-regulated chemokine (TARC)/CC chemokine ligand (CCL)17 is a ligand of CC chemokine receptor (CCR) 4 1–3, which is predominantly expressed on Th2 lymphocytes, basophils, and natural killer cells 1–4. CCR4 is also a receptor for macrophage-derived chemokine (MDC)/CCL22 1–3. Thus, CCL17 and CCL22 are likely to play important roles in Th2-type immune responses by selectively recruiting CCR4+ Th2-polarized memory/effector T cells into inflamed tissues. Cutaneous lymphocyte-associated antigen (CLA) is expressed by the vast majority of skin- infiltrating T cells and plays an important role in leading the skin-associated T cells to inflammatory sites by interacting with the endothelial cell ligand E-selectin, the expression of which is pronounced in inflamed skin 5. Essentially all CLA+ skin-seeking memory effector T cells were found to express CCR4 6, 7.
Atopic dermatitis (AD) is characterized by an expansion of the population of Th2 cells and a decrease in numbers of Th1 cells at least in the initial stages 8, 9. CCL17, CCL22, and CCR4 are highly implicated in the pathogenesis of AD 6, 10–23. The expression of CCL17 and CCL22 in keratinocytes (KC) is up-regulated and the number of CCR4+ cells is increased in the lesional skin 10, 11, 14, 18–21, suggesting that CCL17 and CCL22 play a vital role in inducing Th2-type response by attracting CCR4+ Th2 cells to the lesional skin in AD.
To clarify the contribution of CCL17 produced by KC to skin diseases such as AD, we have created a line of transgenic (Tg) mice that constitutively produce CCL17 in the epidermis under the control of the human keratin 14 (hK14) promoter. Analyses of contact hypersensitivity (CHS) reactions, mRNA expression, histopathological findings, serum levels of IgE, and response to irritation were compared between Tg mice and non-Tg mice.
Results
Establishment of CCL17 Tg mice
Generation and screening of CCL17 Tg mice
Tg mice were generated by the microinjection of a construct including murine CCL17 cDNA 24 into fertilized eggs of C57BL/6 mice as previously described 25. This construct consisted of an hK14 promoter/enhancer, murine CCL17 cDNA, and a portion of the human growth hormone (hGH) gene with a poly A signal 26. Only one founder was successfully bred to yield a line. Mice were screened for the transgene's presence by PCR analysis of tail genomic DNA with the primers F1 and R1 (located in the hK14 gene and in the murine CCL17 gene, respectively). A 498-bp band was detected in Tg mice but not in non-Tg mice because normal mice do not have an hK14-murine CCL17 fusion gene.
Increased expression of CCL17 mRNA in KC of Tg mice
mRNA was extracted from fresh KC and cultured KC 27. The amount of CCL17 mRNA expressed in non-Tg mice and Tg mice was measured using RT-PCR 28. With the primers F3 and R3 (located in the murine CCL17 gene-coding region), the fragment within the CCL17 coding region, which is included both in the transgene and in the genomic DNA was amplified. On the other hand, using F4 and R4 (located in the murine CCL17 gene-coding region and in the murine CCL17 gene 3′-untranslated region (UTR), respectively), the fragment spanning from the CCL17 cDNA region to 3′-UTR that was included only in genomic DNA was amplified. Thus, the PCR product amplified by F3 and R3 reflects mRNA transcribed from the transgene and endogenous genomic DNA while that amplified by F4 and R4 reflects endogenous CCL17 mRNA only. CCL17 mRNA expression detected with F3 and R3 was markedly increased in Tg mice compared with non-Tg mice, while mRNA expression detected with F4 and R4 in Tg mice was very weak and similar to that of non-Tg mice. The same results were obtained in fresh KC and cultured KC. This means that the endogenous CCL17 mRNA level was very low and the transgene was transcribed into mRNA at a high level in Tg mice.
Increased production and secretion of CCL17 protein in KC of Tg mice
To establish that CCL17 mRNA was being translated into the protein, immunohistochemistry, ELISA, and Western blotting were performed. Fig. 1A shows that the epidermis of Tg mice was markedly stained with anti-CCL17 antibody while immunoreactivity in the epidermis of non-Tg mice was very weak. The concentration of CCL17 in the supernatants of cultured KC 27 from Tg mice (70 382 ± 15 407 pg/mL) was markedly higher than that of KC from non-Tg mice (6.78 ± 1.45 pg/mL) (p = 0.02) (Fig. 1B). The CCL17 concentration was significantly higher in the serum of Tg mice (5373 ± 1723 pg/mL) than in that of non-Tg mice (108 ± 32.7 pg/mL) (p = 0.00016) (Fig. 1C). To confirm the molecular weight of the produced and secreted CCL17, Western blotting was performed using cell lysate of Tg KC, supernatant from cultured Tg KC, and recombinant murine CCL17 (as a control). The bands migrated as the band of recombinant murine CCL17 did. This molecular weight is iconsistent with the predicted molecular weight of murine CCL17 protein 24. This means that murine CCL17 mRNA from the transgene was translated into protein and the production and secretion of CCL17 protein was markedly increased in Tg KC.
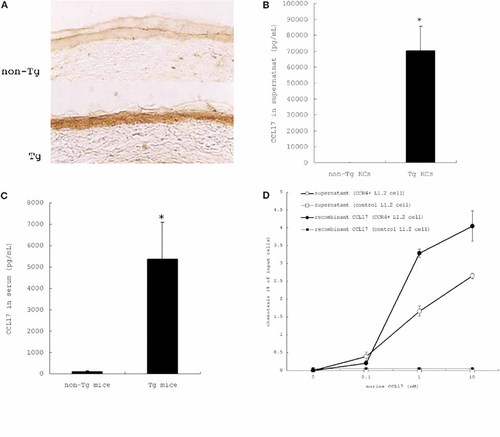
Increased production and secretion of CCL17 protein with biological and functional activity by KC of Tg mice. (A) Epidermal KC in ear skin were strongly stained by anti-CCL17 antibody in Tg mice compared to non-Tg mice. (B) KC from Tg mice produced a significantly higher level of CCL17 than those from non-Tg mice. (C) A significantly higher level of CCL17 was detected in the serum from Tg mice than that from non-Tg mice. The results are presented as the mean ± SD.*p <0.05. (D) The chemoattractant activity of supernatants of cultured KC from Tg mice and recombinant murine CCL17 was determined by chemotaxis assay. CCL17 secreted by KC from Tg mice showed chemotactic activity for CCR4+ cells. The number of migrating cells was expressed as a percentage of the number of cells loaded in the upper chamber (mean ± SD). Supernatant and CCR4+ cells: open circles, supernatant and control cells: open squares, recombinant murine CCL17 and CCR4+ cells: closed circles, recombinant murine CCL17 and control cells: closed squares.
Murine CCL17 produced by Tg KC had biological and functional activity
In a chemotaxis assay 29, the supernatant of cultured Tg KC was tested for its capacity to chemoattract L1.2 cells constitutively expressing CCR4 30 (CCR4+ L1.2 cells). Supernatant of Tg KC and recombinant murine CCL17 displayed strong chemoattractant activity for CCR4+ L1.2 cells, but showed no chemoattractant activity for control L1.2 cells (Fig. 1D). The chemoattraction was blocked by anti- murine CCL17 antibody (data not shown). These results indicate that bioactive murine CCL17 was produced and secreted by Tg KC.
CHS reaction in Tg mice
General remarks
CHS to oxazolone (OX) and FITC was assayed 31, 32. The CHS reaction after a single challenge and that following repeated challenges were designated as “acute CHS” and “chronic CHS”, respectively. The CHS reactions to treatment on the ear and the abdomen were designated as “CHS (ear)” and “CHS (abdomen)”, respectively.
Reduced acute CHS and enhanced chronic CHS to OX in Tg mice
As the ear swelling peaked at 24 h in OX acute CHS in the preliminary experiment, we compared the ear swelling at 24 h between non-Tg and Tg mice. Compared to non-Tg mice, Tg mice showed significantly reduced ear swelling (non-Tg: 39.4 ± 8.9 × 10–2 mm, Tg: 22.8 ± 5.7 × 10–2 mm, p = 0.016) (Fig. 2A). The ear swelling response peaked around day 11, then weakened slightly and reached a plateau level around day 30 in the preliminary experiment in chronic CHS. Thus, we examined the ear swelling from day 0 to day 35. Fig. 2B shows the time course of the ear swelling; a significant difference was detected after day 14 in chronic CHS.
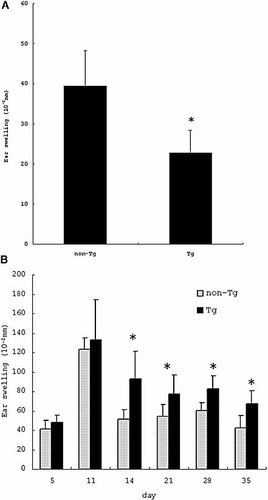
Reduced OX acute CHS and enhanced OX chronic CHS in Tg mice. (A) Compared to non-Tg mice, Tg mice showed significantly reduced ear swelling in OX acute CHS. Ear swelling is presented as the mean ± SD (10–2 mm). (B) There was no significant difference in ear swelling between non-Tg mice and Tg mice until day 11, however, the difference became significant after day 14 in OX chronic CHS. Ear swelling responses before each challenge are shown as the mean ± SD (10–2 mm). *p <0.05 (vs. non-Tg mice).
Pathological findings on the lesional skin after acute and chronic CHS to OX
Without stimulation, there were few inflammatory cells in both non-Tg mice and Tg mice and there was no significant difference (non-Tg: 5.25 ± 1.67/field, Tg: 2.52 ± 1.2/field). In OX acute CHS, a large number of neutrophils and lymphocytes infiltrated the dermis. A significantly smaller number of cells were found in Tg mice than in non-Tg mice (non-Tg: 77.9 ± 14.5/field, Tg: 12.0 ± 7.7/field, p = 1.4 × 10–6) (Fig. 3A). In OX chronic CHS, large numbers of cells infiltrated the dermis and epidermis compared to numbers in acute CHS. Tg mice had significantly more infiltrating cells in the dermis and in the epidermis than non-Tg mice (non-Tg: 245.3 ± 44.0/field, Tg: 508.72 ± 116.5/field, p = 3.2 × 10–5) (Fig. 3B). There was an increased number of mast cells in the dermis in chronic CHS while only a few mast cells in acute CHS. Mast cell counts in chronic CHS were significantly increased in Tg mice compared to non-Tg mice (Fig. 3C) (non-Tg: 44.9 ± 8.5/field, Tg: 62.0 ± 8.5/field, p = 1.3 × 10–5). The number of CCR4+ cells also significantly increased in Tg mice compared to non-Tg mice both in acute CHS (Fig. 3D) (non-Tg: 15.4 ± 5.4/field, Tg: 33.25 ± 7.1/field, p = 4.3 × 10–6) and in chronic CHS (non-Tg: 8.36 ± 4.0/field, Tg: 14.1 ± 4.8/field, p = 0.0067). These CCR4+ cells were stained with anti-CD4 antibody but not anti-CD25 antibody (data not shown).
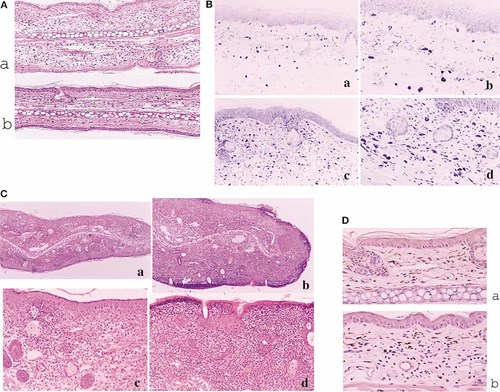
Pathological findings on the lesional skin after OX acute and OX chronic CHS. (A) Many neutrophils and lymphocytes infiltrated the dermis in OX acute CHS. Significantly fewer cells were found in Tg mice than in non-Tg mice (a: non-Tg mice, b: Tg mice, HE stain, x50). (B) Tg mice showed significantly more infiltrating cells in the dermis and in the epidermis than non-Tg mice in OX chronic CHS (HE stain, a: non-Tg mice (x50), b: Tg mice (x50), c: non-Tg mice (x100), d: Tg mice (x100)). (C) Mast cell counts in OX chronic CHS were significantly increased in Tg mice compared to non-Tg mice (toluidine blue stain, a: non-Tg mice (x100), b: non-Tg mice (x400), c: Tg mice (x100), d: Tg mice (x400)). (D) CCR4+ cell counts were significantly increased in Tg mice compared to non-Tg mice in OX acute CHS (a: non-Tg mice, b: Tg mice, x400).
IL-4 mRNA expression was increased and IFN-γ mRNA expression was decreased in Tg mice in acute and chronic CHS to OX
RT-PCR was performed using the primer sets for murine IL-4 and murine IFN-γ 28 with mRNA extracted from the ear skin at 24 h in OX acute CHS and 24 h after the last challenge in chronic CHS at day 35. In the absence of stimulation, neither the mRNA of IL-4 nor that of IFN-γ was detected in non-Tg mice and Tg mice (data not shown). Representative results of mRNA expression in acute CHS are shown in Fig. 4. mRNA expression of IL-4 was increased and that of IFN-γ was reduced in Tg mice compared to non-Tg mice. The same result was obtained in chronic CHS. Tissue cytokine levels were determined by ELISA after extraction of protein from homogenized ear tissue. IL-4 but not IFN-γ was detected by ELISA in acute CHS. In chronic CHS, neither cytokine could be detected. The levels of IL-4 in acute CHS were significantly higher in Tg mice (22.3 ± 10.7 pg/mg protein) than in non-Tg mice (7.80 ± 1.73 pg/mg protein) (p = 0.0045).
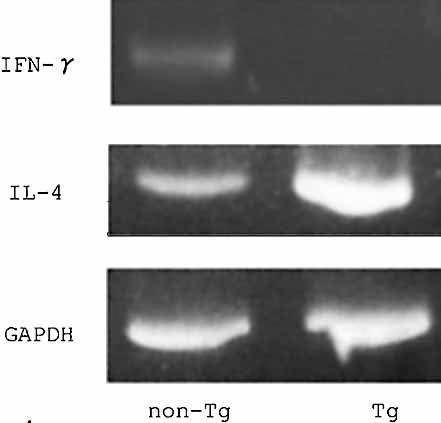
Increased IL-4 mRNA expression and decreased IFN-γ mRNA expression in Tg mice in OX acute CHS. RT-PCR revealed that mRNA expression of IL-4 was increased and that of IFN-γ was reduced in ear skin of Tg mice compared to non-Tg mice in OX acute CHS.
Increased number of CCR4+ lymphocytes among PBMC of Tg mice in OX acute CHS
OX was painted on the trunk of the sensitized non-Tg and Tg mice. Blood was drawn from each mouse 24 h after the first challenge for acute CHS or 24 h after the last challenge for chronic CHS, and the percentage of CCR4+ lymphocytes was determined by flow cytometry. Almost all the CCR4+ lymphocytes expressed CD4. Non-Tg mice and Tg mice showed a similarly low percentage of CCR4+ lymphocytes (non-Tg: 0.29 ± 0.14%, Tg: 0.26 ± 0.13%, p = 0.65) when not stimulated. In both non-Tg and Tg mice, the percentage of CCR4+ lymphocytes was significantly higher in acute CHS (non-Tg: 1.29 ± 0.71%, p = 0.003, Tg: 3.05 ± 1.30%, p = 0.000085) but not in chronic CHS (non-Tg: 0.14 ± 0.06%, Tg: 0.20 ± 0.04%) than without any treatment (Fig. 5). In addition, CCR4+ lymphocytes significantly increased in Tg mice compared to non-Tg mice in acute CHS (p = 0.0023). CD25+CCR4+ lymphocytes were rarely found even in OX acute CHS in both non-Tg mice (0.14 ± 0.056%) and Tg mice (0.15 ± 0.073%).

Increased number of CCR4+ lymphocytes among PBMC of Tg mice in OX acute CHS. The percentage of CCR4+ lymphocytes was determined by flow cytometry. Non-Tg mice and Tg mice showed a similar percentage of CCR4+ lymphocytes when not stimulated. In both non-Tg mice and Tg mice, the percentage of CCR4+ lymphocytes was significantly higher in OX acute CHS but not in OX chronic CHS. The numbers of CCR4+ lymphocytes were significantly increased in Tg mice compared to non-Tg mice in acute CHS *p <0.05 (vs. non-Tg mice).
Increased FITC acute CHS in Tg mice
As in OX CHS, we examined the acute and chronic CHS to FITC. As the ear swelling peaked at 24 h in FITC acute CHS in the preliminary experiment, we compared the ear swelling at 24 h between non-Tg and Tg mice. Tg mice showed significantly increased ear swelling compared to non-Tg mice (non-Tg: 5.67 ± 5.29 × 10–2 mm, Tg: 11.8 ± 5.85 × 10–2 mm, p = 0.049) (Fig. 6). In FITC chronic CHS, the ear swelling tended to be enhanced in Tg mice compared to non-Tg mice, but the difference was not statistically significant.
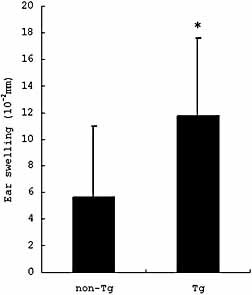
Increased FITC acute CHS in Tg mice. Tg mice showed significantly increased ear swelling compared to non-Tg mice in FITC acute CHS. Ear swelling is presented as the mean ± SD (10–2 mm). *p <0.05.
Enhanced irritant dermatitis with croton oil in Tg mice
Croton oil (CO) was repeatedly painted on the trunk. Tg mice showed severe hair loss and mild erythema while non-Tg mice showed only slight erythema after day 15 (Fig. 7A). Histopathologically, hyperkeratosis, acanthosis in the epidermis, and lymphocytic infiltration of the epidermis and dermis were observed only in Tg mice (Fig. 7B). The number of infiltrating cells was increased in the epidermis (non-Tg: 1.83 ± 1.72/field, Tg: 12.8 ± 5.88/field, p = 0.0039) and in the dermis (non-Tg: 44 ± 9.2/field, Tg: 109 ± 16.0/field, p = 0.0017) of Tg mice.
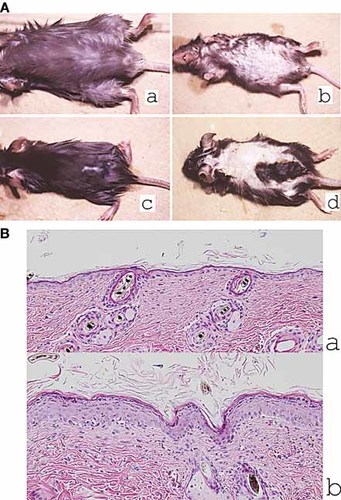
Enhanced irritant dermatitis with CO in Tg mice. (A) Macroscopically, Tg mice showed mild erythema of the skin with severe hair loss on chronic irritation with CO (a: abdomen of non-Tg mice, b: abdomen of Tg mice, c: back of non-Tg mice, d: back of Tg mice). (B) Histopathologically, hyperkeratosis, acanthosis in the epidermis, and lymphocytic infiltration of the epidermis and dermis were observed in Tg mice (a: non-Tg mice, b: Tg mice; HE stain, x100).
Ear swelling was significantly increased in Tg mice by repeated tape stripping
The ear swelling after the sixth stripping is shown in Fig. 8, and was significantly increased in Tg mice (73.8 ± 18.7 × 10–2 mm) compared to non-Tg mice (48.6 ± 19.0 × 10–2 mm) (p = 0.013).
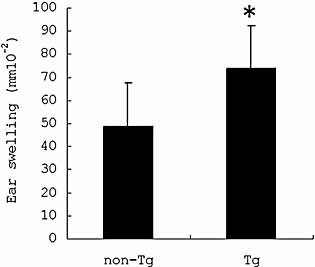
Increased ear swelling in Tg mice in tape stripping. Ear swelling was significantly increased in Tg mice compared to non-Tg mice following tape stripping (p = 0.013).
Increased serum IgE levels after CHS in Tg mice
Without treatment, the serum IgE concentration of Tg mice (319.6 ± 19.2 ng/mL) was significantly higher than that of non-Tg mice (233.4 ± 40.3 ng/mL) (p = 0.0021). After chronic CHS, the serum IgE levels were increased compared with those before the challenge in both non-Tg mice and Tg mice, and Tg mice showed significantly higher levels of serum IgE than non-Tg mice. In chronic irritation with CO on the trunk, the serum IgE levels were significantly higher in Tg mice (797.5 ± 473.3 ng/mL) than non-Tg mice (242.1 ± 45.5 ng/mL ) (p = 0.006) (Fig. 9).
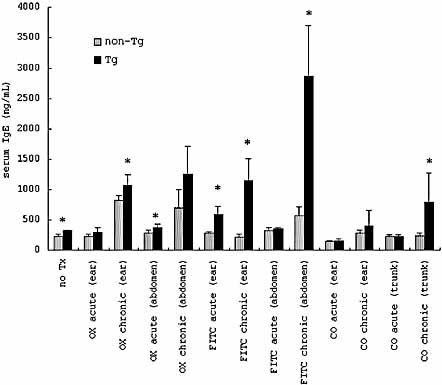
Serum IgE levels before and after the challenge. In acute or chronic CHS, non-Tg and Tg mice were challenged with OX or FITC on the ear or the abdomen. In acute or chronic irritation, CO was painted on the ear or the trunk (abdomen and back). Serum IgE levels are expressed as the mean ± SD (ng/mL). *p <0.05 (vs. non-Tg mice).
Discussion
We created a line of Tg mice in which CCL17 expression is increased in the KC of the epidermis under the control of the hK14 promoter. It was confirmed that the transgene was integrated, mRNA for CCL17 was transcribed from the transgene, a large amount of CCL17 protein was produced and secreted by KC, the molecular weight of the protein is identical to that reported, and the CCL17 protein produced is bioactive for CCR4+ cells.
In this study, CHS reactions 33 were examined. OX, a Th1-type sensitizer 34, and FITC, a Th2-type sensitizer 35, 36, were employed as sensitizers, and two types of challenge, namely acute CHS and chronic CHS, were used. In terms of cytokine levels, an initial challenge with an antigen such as OX, leads to the predominant production of Th1 cytokines like IFN-γ and IL-2, and minimal production of Th2 cytokines such as IL-4 and IL-10 in the lesional skin 28, 37, 38. Continued exposure to antigens induces a down-regulation of the production of Th1 cytokines and an up-regulation of that of Th2 cytokines 28, 37, 38. As Th2 cells produce IL-4 and IL-13, which is closely related to the promotion of IgE production, IgE is a reliable marker of Th2 activity 35. Consistent with the predominant Th2 cytokine phenotype, repeated challenges induce an elevation in levels of serum IgE 28, 37. As mentioned above, OX induces Th1-dominated inflammation in acute CHS and Th2-dominated inflammation in chronic CHS. In this study, the levels of ear swelling and the number of infiltrating inflammatory cells were reduced in OX acute CHS and increased in chronic CHS in Tg mice compared to non-Tg mice. This suggests that the Th1-type response was suppressed and the Th2-type response was enhanced in Tg mice. This was confirmed at the mRNA level. The number of CCR4+ cells in the lesional skin increased in Tg mice compared to non-Tg mice in both acute and chronic CHS to OX. This suggests that CCL17 produced by the epidermal KC in Tg mice attracts CCR4+ cells and these cells secrete Th2 cytokines, inducing a Th2-dominated condition in Tg mice. This Th2-dominated condition is believed to have reduced OX acute CHS, a Th1-type inflammation and enhanced OX chronic CHS, a Th2-type inflammation. Surprisingly, chronic irritation on the trunk by CO induced erythema and hair loss macroscopically and eczematous change histopathologically in Tg mice but not in non-Tg mice. The ear swelling on tape stripping was also increased in Tg mice. These results suggest that CCL17 can modify not only an allergic reaction but also an irritant reaction. Both allergic inflammation induced by antigens and non-allergic inflammation caused by an impaired barrier function of the epidermis are important mechanisms in the pathogenesis of AD 39. CCL17 may function in these two mechanisms. A greater number of mast cells infiltrated in chronic than acute CHS to OX, and Tg mice showed an increased number of mast cells compared to non-Tg mice. A large number of mast cells are found in chronic lesions in AD and thought to be involved in the pathogenesis of AD 40. CCL17 might participate in the formation of chronic lesions by increasing mast cell numbers.
Katou et al. 41. reported that CCR4 was expressed by epidermal Langerhans cells (LC) and dermal CD4+ lymphocytes but not by dermal DC in the inflamed skin, and speculated that CCR4 is involved in the trafficking of epidermal LC at the inflamed sites. Thus, there remains a possibility that the migration of LC in the sensitization phase is altered in Tg mice. The numbers of LC in Tg mice before and after the sensitization were compared with those in non-Tg mice; no difference was found, however (data not shown), suggesting that CCL17 produced by KC does not alter the migration of LC.
Although CCL17 produced by Tg mice had chemoattractant activity for CCR4+ L1.2 cells in this study, no spontaneous infiltration of inflammatory cells into the skin could be identified. However, when inflammation was provoked by CHS, CCL17 modified it. This means that CCL17 does not itself induce local inflammatory responses, but affects the inflammation caused by other stimuli. The mechanism by which lymphocytes invade tissues is a multistep process in which adhesion molecules participate 42. Stimulation by contactants induces the production of proinflammatory cytokines from KC, which in turn induces endothelial activation and expression of adhesion molecules 33. The lack of spontaneous inflammation in Tg mice would be due to an absence of the endothelial activation and expression of adhesion molecules. It is possible that CCR4+ lymphocytes cannot infiltrate when E-selectin is not expressed even if the concentration of CCL17 is sufficient for migration, because CCR4 has been identified as being expressed preferentially on CLA+ skin homing T cells 6, 7, 19, 20.
Simple wounds such as those caused by tape stripping are reported to induce primary cytokines 43. Irritation by CO and tape stripping induced enhanced inflammation in Tg mice. These irritations were believed to have induced the expression of primary cytokines and adhesion molecules and, under these conditions, CCL17 prompted Th2-type inflammation.
Serum IgE levels were increased after CHS to OX and to FITC, especially after chronic CHS. Tg mice and non-Tg mice showed remarkably higher serum IgE levels, particularly in FITC chronic CHS. This is consistent with FITC being a Th2-dominant sensitizer and chronic CHS inducing Th2-type inflammation. Tg mice produced a greater amount of serum IgE than non-Tg mice in chronic CHS, suggesting that CCL17 produced by Tg KC enhanced IgE production. CCL17 might have activated CCR4+ Th2 cells that, in turn, secreted IL-4 and IL-13, which are important in IgE production 44. AD is characterized by chronic dermatitis associated with high levels of serum IgE 8, 40. These results suggest that chronic exposure to various antigens and CCL17 produced by KC plays a role in the elevation of serum IgE levels in human AD. Chronic exposure to various irritant agents due to an impaired skin barrier function is an important factor in the pathogenesis of AD 39. Irritation by CO on the trunk induced a rise in serum IgE levels only in Tg mice, suggesting that chronic irritation can also induce serum IgE levels to increase in the presence of a high concentration of CCL17.
Alferink et al. 45 reported that CCL17-deficient mice mount diminished acute CHS. They employed dinitrofluorobenzene (DNFB) and FITC as sensitizing agents. FITC acute CHS was enhanced in Tg mice in our study. These findings indicate that CCL17 promotes FITC acute CHS. This can be explained by the fact that FITC is a Th2-type sensitizer. We did not examine DNFB CHS, which is believed to be a Th1-type sensitizer. In our study, the acute CHS evoked by OX, a Th1-type agent, was reduced in Tg mice, which conflicted with the result in CCL17-deficient mice. A possible explanation is that some amount of CCL17 is necessary for Th1-type CHS 46 as well as Th2-type CHS but that excess CCL17 suppresses Th1-type responses.
The percentage of CCR4+ cells among PBMC was remarkably increased in OX acute CHS on the trunk but very low without CHS and in OX chronic CHS on the trunk. This suggests that a high concentration of serum CCL17 alone is not sufficient and that other factors are necessary for mobilization of CCR4+ cells. Some cytokines and chemokines produced in the skin during CHS might cooperate with CCL17 to increase number of CCR4+ cells in the PBMC population. Serum CCL17 levels 11, 14–16 and percentages of CCR4+ cells in PBMC 19–22 are high in AD patients. Various cytokines and chemokines are reported to be up-regulated in their expression in AD patients 8 and these factors may function with CCL17 in mobilizing CCR4+ cells. The percentage of CCR4+ cells among PBMC in OX chronic CHS was similar to that without CHS and the number of CCR4+ cells was reduced in OX chronic CHS compared to OX acute CHS in the inflamed skin, suggesting that CCR4+ cells are more important in acute CHS than in chronic CHS. Other cells infiltrating in OX chronic CHS, such as mast cells, may function in the maintenance of the Th2-dominant condition in chronic CHS because mast cells produce Th2 cytokines.
Regulatory T (Treg) cells have been proposed to control peripheral immunological self-tolerance 47, 48. The best-characterized population of naturally occurring Treg cells is CD4+CD25+ T cells 47, 48. These cells are reported to express CCR4 and respond to CCL17 and CCL22 47–49. We studied whether CCR4+ lymphocytes circulating in the blood and infiltrating the skin express CD25, the best available marker for Treg cells 48. However, these CCR4+ lymphocytes did not express CD25, indicating that they are not Treg cells. Thus, we concluded that these CCR4+ lymphocytes are Th2 cells.
In summary, we studied the effect of CCL17 produced by epidermal KC in CCL17-Tg mice. Although CCL17 itself is not sufficient for the induction of inflammation, it has an effect on the inflammation caused by other factors by attracting CCR4+ Th2 cells into the lesional skin and creating a Th2-dominant condition. In addition, AD-like conditions such as increased numbers of mast cells and high levels of serum IgE were observed in chronically challenged Tg mice. Tg mice also displayed an elevation of serum IgE levels and dermatitis on chronic irritation. CCL17 may participate in the pathogenesis of skin diseases such as AD by regulating both allergic and non-allergic irritant inflammation.
Materials and methods
Mice
C57BL/6 (C57BL/6J Jcl) mice were purchased from Japan Clea (Tokyo, Japan). All mice used in the experiments were 6–8 weeks old and only animals of the same sex were used in each experiment. Eight mice in both the non-Tg group and the Tg group were employed in each experiment. They were maintained under a specific pathogen-free environment unless otherwise mentioned and kept under standard conditions with a 12-h day/night rhythm and free access to food and water. All the mice received humane care and the experiments were approved by an internal ethics committee.
Generation of a transgenic construct
A DNA construct including cDNA for murine CCL17 (24, GenBank accession no. AJ242587) was generated and microinjected as previously described 25. The murine CCL17 cDNA coding region was inserted into the Bam HI site (between hK14 and hGH) of a hK14/hGH expression vector containing the hK14 promoter/enhancer and a portion of the hGH gene with a polyA signal 26. The hK14 promoter/enhancer-CCL17-hGH fusion products were used for microinjection.
Genotyping of CCL17 Tg mice
Mice were screened for the transgene by PCR amplification of DNA from tail skin using a forward primer (F1: 5′-ACACCTCCCCCTGTGAATCA-3′) located in the hK14 gene and a reverse primer (R1: 5′-TTTCACCAATCTGATGGCCT-3′) located in the murine CCL17 gene. The murine GAPDH gene was employed as an internal control (F2 forward primer: TGAAGGTCGGTGTGAACGGATTTGGC and R2 reverse primer: CATGTAGGCCATGAGGTCCACCAC). The genomic DNA was extracted with a QIAGEN DNeasy Tissue Kit (Qiagen, Hilden, Germany).
Keratinocyte cell culture
An epidermal KC suspension was made from the ear skin as described previously 27. These KC used just after their isolation were designated as “fresh KC.” The KC were cultured and maintained on collagen type I-coated tissue culture six-well plates (Iwaki, Chiba, Japan) using Keratinocyte-SFM supplemented with 5 ng/mL of epidermal growth factor and 50 μg/mL of bovine pituitary extract (Invitrogen, CA) in humidified 5% CO2, 95% air at 37°C. These KC were designated as “cultured KC.” Supernatant was collected from KC cultures grown for 48 h at 80–90% confluence without an exchange of medium.
RT-PCR
Total RNA was extracted using standard methods 28 from fresh KC isolated from one ear, 106 cultured KC and whole ear excised before or after the CHS reaction. The RNA was treated with Amplification Grade DNase I (Invitrogen) to eliminate any residual genomic DNA. RT-PCR was performed as described previously 28. The primers used in the experiments with fresh KC and cultured KC were as follows: F3 (forward primer in murine CCL17 gene-coding region): 5′-GTCACTTCAGATGCTGCTCCT-3′, R3 (reverse primer in murine CCL17 gene-coding region): 5′-GCCTTGGGTTTTTCACCAAT-3′, F4 (forward primer in murine CCL17 gene-coding region): 5′-CAGGGATGCCATCGTGTTTCT-3′, R4 (reverse primer in murine CCL17 gene 3′-UTR): 5′-GGTCACAGGCCGCTTTATGTT-3′, F5 (forward primer in murine GAPDH gene): 5′-GAGGAGCGAGACCCCACTAA-3′, R5 (reverse primer in murine GAPDH gene): 5′- GGCATCGAAGGTGGAAGAGT-3′. In the experiments on CHS, primer sets for murine IFN-γ and IL-4 described previously 28 were used. The samples without reverse-transcription (negative controls) yielded no bands (data not shown).
Western blot
Western blot was performed in the standard manner using the proteins extracted from cultured KC, KC culture supernatants, and a control sample of recombinant murine CCL17 (529-TR, R & D systems, MN). The antibodies used were polyclonal goat anti- murine CCL17 antibody (AF529, R & D systems) at 0.2 μg/mL as a primary antibody and horseradish peroxidase conjugated donkey anti-goat IgG (sc-2056, Santa Cruz Biotechnology, CA) at 0.2 μg/mL as a secondary antibody.
ELISA for murine CCL17, murine IgE, murine IFN-γ and IL-4
The concentrations of murine CCL17 in the serum and the cultured KC supernatants and of serum IgE were measured with a quantitative sandwich ELISA kit: Quantikine mouse TARC/CCL17 (R & D systems) and mouse IgE kit “Yamasa” (Yamasa corporation, Tokyo, Japan), respectively. The serum was collected 24 h after the challenge in acute CHS and 48 h after the last challenge of chronic CHS. For the determination of IFN-γ and IL-4 levels in ear tissue, the ears were cut and homogenized under liquid nitrogen 24 h after the challenge in acute CHS and 48 h after the last challenge of chronic CHS. The determination of cytokine levels was performed as described previously 50 using a commercial ELISA kit Quantikine mouse IFN-γ (R & D systems) and Quantikine mouse IL-4 (R & D systems).
Chemotaxis assay
The chemotaxis assay was performed as described previously 29. Cell migration was assayed using L1.2 cells 29 (a gift from Dr. Eugene C. Butcher, Stanford University School of Medicine) stably transfected with murine CCR4 cDNA 30 (CCR4+ L1.2 cell). CCR4+ L1.2 cells and control untransfected L1.2 cells were loaded in the upper wells of a Costar Transwell chamber (3 μm pore size, Corning, NY). The murine CCL17 concentration in the supernatants of cultured KC was measured by ELISA. Supernatants of cultured KC or recombinant murine CCL17 (529-TR, R & D systems) were serially diluted with RPMI 1640 medium and added to the lower wells in a volume of 0.6 mL. After 4 h at 37°C, cells in the lower chamber were counted. Values are expressed as the percentage of input cells that migrated through the filter. Polyclonal goat anti-murine CCL17 antibody (AF529, R & D systems) was used for the antibody-blocking experiment. Various dilutions of murine CCL17 (supernatants of cultured KC or recombinant murine CCL17) were incubated with 2.5 μg/mL of the antibody for 30 min at room temperature and used for the chemotaxis assay described above.
CHS
CHS to OX and FITC was assayed as described previously 31, 32. The CHS reaction to a single challenge was designated as “acute CHS”. In the experiment on the CHS reaction to repeated challenges (designated as “chronic CHS”), mice were sensitized in the same way as in acute CHS, and each reagent was repeatedly applied to the same site three times a week. These CHS reactions at the ear were designated as “acute CHS (ear)” or “chronic CHS (ear)”. To examine the serum levels of IgE, elicitation at the abdomen (by painting 100 μL of each reagent) was also performed “acute CHS (abdomen)” and “chronic CHS (abdomen)”. CO (0.5%, Sigma) in 4:1 acetone/olive oil was applied to the ear (20 µL) and trunk (200 µL) to assess the reaction to irritation. Acute irritation and chronic irritation caused by CO was studied as described in OX and FITC CHS.
Tape stripping
Stripping was performed by pressing adhesive tape (Transpore surgical tape, 3 M Health Care, St. Paul, MN) onto the dorsal side of the ear and pulling it off abruptly 20 times. Stripping was performed six times at 2-day intervals. The ear thickness was measured two days after the last stripping.
Flow cytometry
Heparinized blood samples were collected and placed on ice. Two-color analysis was performed by the standard method with a combination of hamster anti-mouse CCR4 mAb (2G12, to be described in detail elsewhere by Nagakubo et al.) and PE-conjugated rat anti-mouse CD4 mAb (RM4–5, Santa Cruz Biotechnology) or PE-conjugated rat anti-mouse CD25 mAb (PC61, BioLegend, CA). For the anti-CCR4 antibody, FITC-conjugated mouse anti-hamster IgG mAb (BD 554011, PharMingen, CA) was used as a secondary antibody. As negative controls, hamster IgG (PN IM3032, IMMUNOTECH, Marseille, France) and PE-conjugated rat IgG (IM1272, IMMUNOTECH) were used. Negative regions were aligned using the negative controls.
Histological and immunohistological analysis
Hematoxylin and eosin (HE) stain, and truisine blue stain were applied to formalin-fixed and paraffin-embedded sections. For detecting murine CCL17, CD4 and CD25, 5 μm cryostat sections were stained with polyclonal goat anti-CCL17 antibody [TARC (N-10), Sc-12271, Santa Cruz Biotechnology] diluted 1:50, rat anti-CD4 mAb (RM4–5, BioLegend) diluted 1:1000, or rat anti-CD25 mAb (PC61, BioLegend) diluted 1:1000 as the primary antibody and biotin-conjugated rabbit anti-goat IgG or biotin-conjugated rabbit anti-rat IgG as the secondary antibody using the VECTASTAIN ABC KIT (Vector Laboratories, CA). Staining was developed by adding a diaminobenzidine solution. For detecting murine CCR4, formalin-fixed and paraffin-embedded sections were stained with the primary antibody, polyclonal goat anti-mouse CCR4 (CI0122, Capralogics, MA), diluted 1:8000, as described above. Ten visual fields were picked up at random under the microscope, and averages of the counted number of cells were recorded. The infiltrating inflammatory cells and CCR4+ cells were counted in fields of x400 magnification. Mast cells were counted in fields of x200 magnification.
Statistical analysis
Data were expressed as the mean ± SD. The statistical significance between two groups was evaluated using Student's t-test and was considered significant if p <0.05.
Acknowledgements
This work was supported by Health Science Research Grants from the Ministry of Health, Welfare and Labor of Japan, and grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. None of the authors has any commercial association that might pose a conflict of interest.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




