CD4+CD25+ regulatory T cells control the magnitude ofT-dependent humoral immune responses to exogenous antigens
Abstract
CD4+CD25+ T reg cells are critical for peripheral tolerance and prevention of autoimmunity. Here we show that CD4+CD25+ T reg also regulate the magnitude of humoral responses against a panel of T-dependent antigens of foreign origin during both primary and secondary immune responses. Depletion of CD4+CD25+ T cells leads to increased antigen-specific antibody production and affinity maturation but does not affect T-independent B cell responses, suggesting that CD4+CD25+ T reg exert a feedback mechanism on non-self antigen-specific antibody secretion by dampening the T cell help for B cell activation. Moreover, we show that CD4+CD25+ T reg also suppress in vitro B cell immunoglobulin production by inhibiting CD4+CD25– T cell help delivery, and that blockade of TGF-β activity abolishes this suppression.
Abbreviations:
-
- ALK:
-
activin receptor-like kinase
-
- KLH:
-
keyhole limpet hemocyanin
Introduction
The B cell response to protein Ag requires cognate interactions between Ag-specific B cells and activated Ag-specific Th cells within the microenvironment of secondary lymphoid tissues 1–3. These effector Th cells control the immunoglobulin (Ig) isotype class switch, regulate the emergence of short-lived plasma cells and help to establish the germinal center (GC) reaction. Short-lived plasma cells secrete germline-encoded specific antibodies that enhance the rapid clearance of Ag. In contrast, the GC reaction underpins memory B cell development and is a more delayed response to Ag. This dynamic microenvironment couples clonal expansion and BCR hypermutation with affinity-based selection to produce Ag-specific B cell memory 4, 5. This complex developmental pathway predicts extensive cellular heterogeneity during an active immune response in vivo, but the precise mechanisms by which Ag-specific Th cells control B cell fates are still poorly understood.
In recent years it has become evident that subsets of T cells [called suppressor or T reg cells] are able to suppress various immune responses. One such type of T reg is present in the 5–10% of unstimulated CD4+ T cells of adult mice that express CD25, the α-chain of IL-2R 6. CD4+CD25+ T cells play critical roles in the prevention of organ-specific autoimmunity and allograft rejection, and in the maintenance of self-tolerance. Their depletion results in the development of autoimmune diseases such as thyroiditis, type 1 diabetes and inflammatory bowel disease 7. Furthermore, the depletion of CD25+ T reg enhances some forms of tumour and microbial immunity 8, 9, indicating that these cells function to suppress a wide range of immune responses. Active suppression mediated by the naturally occurring CD4+CD25+ T reg is therefore, in some circumstances, considered as an obstacle to successful vaccination.
A potential role of suppressor T cells in the regulation of humoral responses has recently been proposed based on the facts that a subset of the CD4+CD25+ T cell population expressed GC-homing properties 10, and that depletion of CD4+CD25+ T reg led to the production of autoimmune antibodies 11. However, little is known about their role in the regulation of T-dependent foreign Ag-specific humoral responses during vaccination.
In this work, we show that specific in vivo depletion of CD25+ cells leads to increased production of both IgG1 and IgG2a antibodies in response to various non-self proteins, indicating that weakening suppressor effects at the time of vaccination can result in a more effective humoral immunity. CD25+ T cell depletion does not affect T-independent humoral responses. Moreover, CD4+CD25+ T cells also reduce T-cell mediated B cell help during in vitro co-cultures, further suggesting that T reg could interfere with CD4+ Th cells to reduce the amplitude of T cell-dependent humoral responses.
Results
In vivo depletion of CD25+ cells enhances humoral responses against a panel of foreign protein antigens
We have previously shown that inoculation of anti-CD25 mAb in mice led to a selective loss of CD4+CD25+ T cells in blood and lymphoid organs for at least 30 days, and that a replenishment of the population was observed 50 days after treatment. FACS analysis of splenic populations indicated that T cell, B cell, NK cell, granulocyte, DC and macrophage populations were unaltered in CD25-treated mice 12.
To test whether the CD4+CD25+ T cell population controls the humoral response against foreign protein Ag, control and CD25-depleted BALB/c mice were inoculated with keyhole limpet hemocyanin (KLH) in CFA. Specific anti-KLH antibody production was measured at different time points during both primary and secondary responses. As shown in Fig. 1, CD25-depleted mice produced higher titers of IgG1, IgG2a and IgE anti-KLH antibodies, compared to control mice. Increased Ag-specific antibody titers in CD25-depleted mice were also observed in mice immunised with Ag in saline (Fig. 2C, D) or aluminium hydroxide (data not shown) vehicles, suggesting that the T reg-mediated control of humoral response did not affect the adjuvant delivery signals to immune cells. The total serum levels of IgG1 and IgG2a antibodies were either unchanged or slightly increased following immunisation of CD25+ cell-depleted mice (Fig. 2E–H).
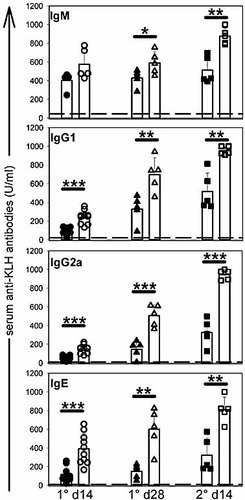
Kinetics of the antibody response in control and CD25-depleted mice. KLH-specific serum antibody titers of CD25-depleted (open symbols) or control (filled symbols) BALB/c mice were determined at various time points after i.p. immunisation with 50 µg of KLH in CFA adjuvant (primary response) and challenge in PBS (secondary response) by standard ELISA method. Dotted lines represent the Ag-specific serum antibody levels detected in untreated mice (normal serum). Results are representative of three independent experiments; *p<0.05; **p<0.01; ***p<0.001.
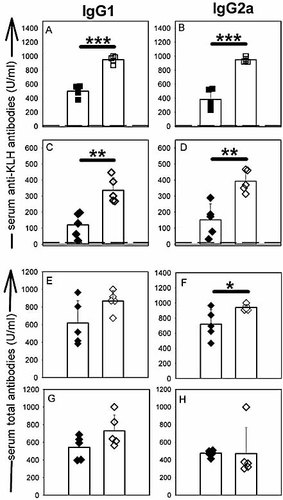
Increased Ag-specific antibody titers do not result from increased total antibody secretion in CD25-depleted mice. CD25-depleted (open symbols) or control (filled symbols) mice were immunised with KLH in CFA (A, B, E, F) or KLH in PBS (C, D, G, H). (A–D) Serum anti-KLH IgG1 (A, C) and IgG2a (B, D) antibody titers (secondary response on day 14). (E–H) Serum total IgG1 (E, G) and IgG2a (F, H) titers. *p<0.05; **p<0.01; ***p<0.001.
KLH immunisation in CD25-depleted mice led to a similar increase in both Ag-specific IgG1and IgG2a isotypes (mean fold increase 2.1±0.4 for IgG1 and 2.67±0.41 for IgG2a, for three independent experiments, Fig. 2A, B; 3A, B). However, when CD25-depleted mice were inoculated with human gammaglobulin or OVA Ag in CFA, we reproducibly observed a stronger increase of Ag-specific IgG2a versus IgG1 secretion (Fig. 3C–F). OVA is a poor inducer of IgG2a antibody secretion in BALB/c mice, despite co-administration of the pro-Th1 CFA adjuvant (our own unpublished observations). Of note, in this particular experimental model, CD25 depletion led to a strong increase in OVA-specific IgG2a antibody secretion (Fig. 3F).
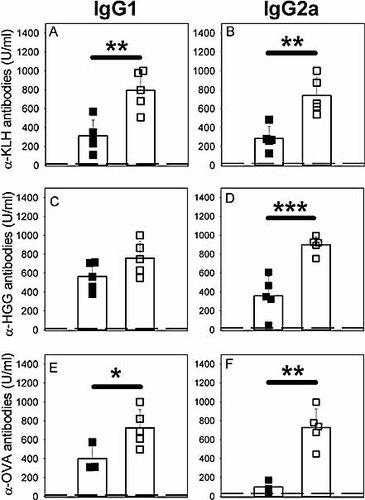
Depending on the Ag inoculated, CD25-depleted mice show an increase in Ag-specific IgG2a/IgG1 ratio or a similar increase in both Ag-specific IgG1 and IgG2a isotypes. CD25-depleted (open symbols) or control (filled symbols) BALB/c mice were immunised with 50 µg KLH (A, B), 15 µg human gammaglobulin (C, D) or 100 µg OVA (E, F) in CFA. Ag-specific antibody contents were determined in sera at day 14 (secondary response). (A, C, E) Serum Ag-specific IgG1 titers. (B, D, F) Serum Ag-specific IgG2a titers. *p<0.05; **p<0.01; ***p<0.001.
In vivo depletion of CD25+ cells selectively enhances T cell-dependent humoral responses and antibody affinity
In the next set of experiments, control and CD25-depleted mice were inoculated with the T-independent Ag NP-Ficoll, and the production of NP-specific serum antibodies was determined 5 and 14 days later. As previously described for T-independent Ag, the maximum anti-NP specific IgM and IgG3 antibody response was observed on day 5 and thereafter declined until day 14. Neither IgG1 nor IgG2a were detected at any time point following NP-Ficoll injection (Fig. 4C, D). Our data showed that neither the production of IgM nor IgG3 NP-specific antibodies was increased following anti-CD25 treatment (Fig. 4A, B), suggesting that T reg depletion did not have significant impact on antibody responses to the T-independent NP-Ficoll Ag. As a control, immunisation with the same hapten coupled to the KLH carrier protein led to a significant increase in the secretion of NP-specific IgG1, IgG2a and IgG3 antibodies in CD25-depleted mice (Fig. 4F–H). Of note, IgG3 has been described as an isotype produced during both T-dependent and T-independent humoral responses. Our results showed that CD25+ cell depletion led to increased in NP-specific IgG3 antibody production following immunisation with the T-dependent NP-KLH Ag only (compare Fig. 4B, F).
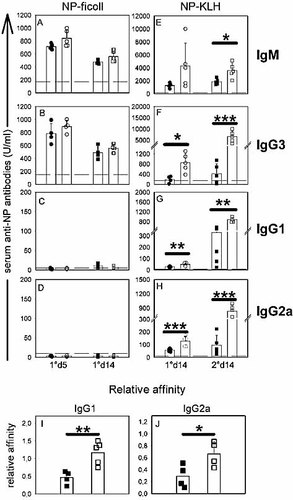
CD25-depleted mice secrete antibodies displaying higher affinities for T-dependent Ag. (A–H) NP-specific serum antibody titers of CD25-depleted (open symbols) or control (filled symbols) mice were determined at various time points after i.p. immunisation with 100 µg NP-Ficoll in PBS (A–D) or 100 µg NP-KLH in CFA (E–H). Dotted lines represent the Ag-specific serum antibody levels detected in untreated mice (normal serum). (I, J) Sera from (G, H) (secondary response on day 14) were tested for NP affinity. Relative affinities are expressed as ratio of 50% binding on NP17-BSA and NP2-BSA. *p<0.05; **p<0.01; ***p<0.001; similar results were obtained in two independent experiments.
Affinity maturation is a hallmark of T-dependent antibodies. We therefore tested whether CD25+ T cell depletion could affect antibody affinity maturation during a T-dependent antibody response. Control and CD25-depleted mice were immunised twice with NP-KLH and the relative affinities of the anti-NP antibodies were determined by comparing their binding on the same carrier protein coupled to different amount of NP (the heavily coupled NP17-BSA and the lightly coupled NP2-BSA), as previously described 13. The data are expressed as the ratio of the amounts of serum required for 50% binding to NP17-BSA and NP2-BSA. Results presented in Fig. 4 clearly showed that the anti-NP antibodies produced by CD25-depleted mice displayed significantly greater relative affinities than those obtained from control mice. Similar results were obtained for both the anti-NP IgG1 and IgG2a antibody fractions (Fig. 4I, J).
CD4+CD25+ T cells inhibit CD4+ T cell-induced antibody production by B lymphocytes in vitro
We examined whether the presence of CD4+CD25+ T reg could suppress Th cell-induced B cell IgG1 and IgG2a production during in vitro T-B cells co-culture experiments. CD4+CD25– and CD4+CD25+ T cells were purified from naive BALB/c mice according to standard MACS procedure (see Fig. 5A for a representative experiment) and activated by plastic-coated anti-CD3 mAb and anti-CD28 mAb for 2 days. Thereafter cells were recovered and cultured in the presence of naive syngenic B cells for 7 days. T cells were irradiated to prevent their outgrowth during the 7-day co-culture with the B lymphocytes. Preliminary experiments showed that pre-activated CD4+CD25– Th cells induced Ig production by co-cultured B cells whereas resting T cells did not (data not shown). CD4+CD25+ T reg, by themselves, did not induce B cell Ig secretion. Moreover, addition of CD4+CD25+ T reg to the pre-activated CD4+CD25– Th cells in the B cell co-culture efficiently suppressed IgG1 and IgG2a secretion (Fig. 5B, C).
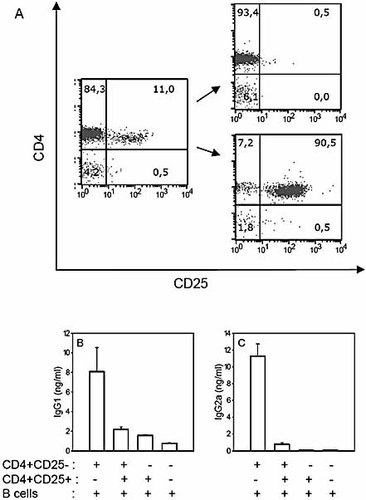
CD4+CD25+ T reg suppress Th cell-dependent antibody secretion in vitro. (A) FACS profil analysis of total CD4+ (left panel), CD4+CD25– (upper right panel) and CD4+CD25+ (lower right panel) T cells. (B, C) CD4+CD25– and CD4+CD25+ T cells were pre-activated with plastic-coated anti-CD3 mAb + anti-CD28 mAb for 2 days. T cells were recovered and cultured (105 cells/well) with 5×105 purified B cells in 250 µL culture for 7 days. Culture supernatant was tested for IgG1 (B) and IgG2a (C) concentration by ELISA. The results shown are the means ± SEM of triplicate wells and are representative of three independent experiments.
In order to test whether T reg inhibited antibody secretion at the level of T cell help delivery, the following experiment was designed. CD4+CD25– Th cells purified from CD45.1+ C57BL/6 mice were pre-activated by plastic-coated anti-CD3 mAb for 48 h, in the presence or absence of equal numbers of CD4+CD25+ T reg isolated from CD45.2+ C57BL/6 mice (Fig. 6A, D). Cells were recovered and CD45.2+ T reg were further removed from the cell mixture by MACS, with anti-CD45.2 specific antibodies (Fig. 6B, E). CD45.1+ Th cells were therefore cultured with syngenic B lymphocytes for 7 days. Results presented in Fig. 6C, F clearly showed that CD45.1+CD4+CD25– Th cells pre-activated in the presence of CD45.2+CD4+CD25+ T reg were deficient in the delivery of B cell help for IgG1 and IgG2a secretion. Of note, T reg were absent during the Th-B cell co-culture, precluding a direct effect of T reg on B cells. Moreover, CD4+CD25+ T reg did not suppress B cell-mediated Ig production induced in the absence of Th help (in response to a combination of anti-IgM antibodies and IL-4, Fig. 6G), further suggesting that CD4+CD25+ T reg interfered at the level of the CD4+CD25– T cell help delivery to B cells, thereby inhibiting IgG1/IgG2a class switch and secretion.
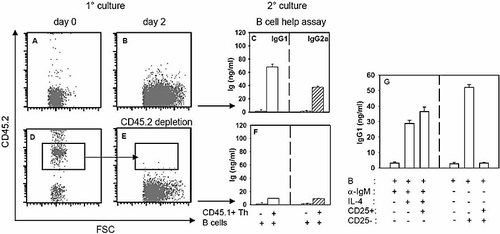
CD4+CD25+ T reg suppress the Th cell activation required for B cell Ig secretion. (A, B, D, E) CD45.1+CD4+CD25– Th cells were pre-activated with plastic-coated anti-CD3 mAb + anti-CD28 mAb in the absence (A) or presence of CD45.2+CD4+CD25+ T reg (D). On day 2, cells were recovered and CD45.2+ T reg were removed from the T cell mixture (E). FACS profile analysis shows the composition of the cell cultures on day 0 (A, D) and before secondary culture (B, E). (C, F) Cells from (B, E) were cultured for 7 days in the presence of purified B cells, as described in Fig. 5. Open histograms: IgG1 secretion; dashed histograms: IgG2a secretion. (G) Purified B cells (5×105 cells/well) were stimulated with anti-IgM (10 µg/mL) and IL-4 (25 ng/mL, left histograms) or with CD4+CD25– Th cells (105 cells/well, right histograms) in the presence or absence of anti-CD3 and anti-CD28 mAb pre-activated CD4+CD25+ T reg (105 cells/well). IgG1 secretion was tested in the culture supernatants on day 7. Results are representative of at least three independent experiments.
When appropriately stimulated, CD4+CD25+ T reg express the active form of the membrane-bound TGF-β and secrete high amount of this regulatory cytokine in the culture medium 14. We therefore tested whether T reg inhibited Th cell-dependent Ig synthesis by B cells via TGF-β. CD4+CD25– Th cells or total CD4+ T cells (comprising 90% CD4+CD25– Th cells and 10% CD4+CD25+ T reg) were cultured for 48 h in the presence of anti-CD3 + anti-CD28 mAb (an experimental condition known to induce a strong expression and secretion of TGF-β by T reg 14). Anti-TGF-β or control mAb were added in some wells. Cells were then recovered, extensively washed and cultured with syngenic B cells. We reproducibly observed that, as shown in Fig. 7, addition of anti-TGF-β mAb during the first 48 h of co-culture completely abolished the suppression mediated by the CD4+CD25+ T reg.
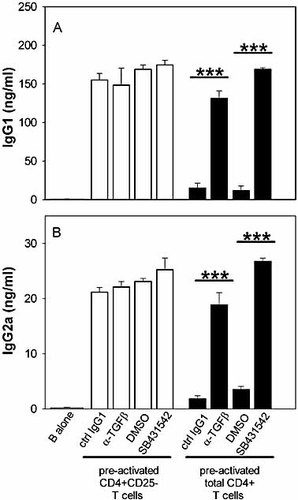
CD4+CD25+ T reg inhibit in vitro T cell help delivery through TGF-β. Purified CD4+CD25– (open histograms) or total CD4+ (90% CD4+CD25– and 10% CD4+CD25+, black histograms) from BALB/c mice were pre-activated with plastic-coated anti-CD3 mAb + anti-CD28 mAb in the presence of control or TGF-β-blocking agents, as indicated. Thereafter, recovered T cells were washed and cultured with B cells. Supernatant IgG1 (A) and IgG2a (B) contents were measured on day 7. Results are representative of at least three independent experiments (***p<0.0001).
TGF-β initiates signalling by binding to and bringing together type II and type I receptor serine/threonine kinases on the cell surface. TGF-β binding enables the type II receptor to phosphorylate activin receptor-like kinase (ALK)-4, ALK-5 and ALK-7 type I receptors which, in turn, propagate signalling through phosphorylation of the effector proteins, including Smad2 and Smad3 15. CD4+CD25– Th cells and total CD4+ T cells (Th + T reg) were therefore activated, as above, in the presence or absence of SB-431542, a selective inhibitor of the receptor kinases ALK-4, ALK-5 and ALK-7 16. Results presented in Fig. 7 showed that blocking TGF-β signalling during the Th-T reg co-culture also abolished the suppression mediated by CD4+CD25+ T reg, further suggesting that T reg suppress CD4+CD25– T cell help through TGF-β.
Discussion
Although the active suppression mediated by naturally occurring CD4+CD25+ T reg cells on CD4+CD25- Th cells, CD8+ cytotoxic cells, APC and NKT cells have been extensively described 17–19, little is known about their regulation of humoral responses. Our study showed that the magnitude of humoral immune responses to exogenous Ag is also subject to a control by CD4+CD25+ T reg. Depletion of CD4+CD25+ T cells before inoculation of a panel of haptens or proteins of foreign origin led to increased Ag-specific antibody secretion during both primary and secondary responses in vivo. Changes in humoral responsiveness are both quantitative and qualitative, as we observed increased amount of antibodies (Fig. 1–4) and increased affinity (Fig. 4I, J), following immunisation of CD4+CD25+ cell-depleted mice.
Accordingly, Lim et al. 10 recently described a subset of T cells (CD4+CD25+CD69– T reg) in human tonsils that displayed potent suppressive activities toward CD57+ GC-Th cells. Upon activation, tonsil CD4+CD25+CD69– T reg changed their chemotactic behaviour so that they potentially could migrate into the GC 10. Taken together, these data and our study suggest that T reg could interfere with the GC-mediated affinity maturation, isotype switching and B cell clonal expansion.
CD4+CD25+ T reg produce TGF-β when stimulated in an appropriated fashion and, in addition, express high levels of TGF-β on their cell surfaces 14. TGF-β plays dual roles in Ig synthesis: It is a switch factor for IgA secretion and a potent immunosuppressive cytokine that inhibits Ig synthesis 20, 21. Nakamura et al. 14 elegantly demonstrated that CD4+CD25+ T cells suppressed T and B cell functions through cell surface presentation of TGF-β to TGF-β receptors on target cells. That study, however, did not allow concluding whether surface-bound TGF-β mediated antibody suppression by acting directly on B cells or on CD4+CD25– Th cells.
Our results confirmed the role of TGF-β in the suppressive capacity of CD4+CD25+ T cells and strongly suggested that CD4+CD25+ T reg did not interfere directly with B cells but instead suppressed CD4+CD25– T cell help delivery as (1) the anti-TGF-β antibody and the TGF-β signalling inhibitor SB-431542 abolished T reg suppression when present during the 48-h pre-incubation of CD4+ T cells only (Fig. 7); (2) B cells cultured with CD45.1+CD4+CD25– Th cells that have been pre-activated in the presence of CD45.2+CD4+CD25+ T reg did not secrete optimal amounts of Ig, even if the CD45.2+CD4+CD25+ T reg were removed before co-culture with B cells (Fig. 6A–F) and (3) T reg did not inhibit T cell-independent Ig secretion, neither in vitro (Fig. 6G) nor in vivo (Fig. 4A–D).
However, Lim et al. recently reported that T reg purified from human tonsils directly suppressed B cell IgG response without having to suppress Th cells 22. In Lim's study 22, the B cell IgG response was inhibited in the presence of a 1:1 ratio of T reg:B cells. It is therefore possible that in our in vitro settings, a lower T reg:B cell ratio (1:5) was not sufficient to show a direct effect on B cells. Of note, this number of T reg was shown to inhibit the Th cell function in T-dependent B cell help (Fig. 5; 6G). Therefore, we would propose that, depending on their number and localization, T reg recruited in the GC could interfere with Th cells, B cells or both to suppress Ig secretion. Interestingly, our data showed that T reg inhibited in vitro Ig secretion when added to Th cells at the beginning of the primary culture (Fig. 6, 7) or when added at the time of the co-culture with B cells (Fig. 5), suggesting that T reg could interfere with T cell help delivery of both naive and effector Th cells.
Several studies have suggested that T reg specifically control the production of autoimmune antibodies in mice 23. Accordingly, Seo et al. 24 reported that CD4+CD25+ T reg down-modulated the response of autoreactive anti-double-stranded DNA-specific B cells 24. Moreover, depletion of a potent chemoattractant for CD4+CD25+ T reg, CCL4, by neutralizing antiserum led to the deregulated production of autoantibodies, further suggesting that recruitment of T reg might constitute a mechanism preventing the generation of autoimmune antibodies 11.
However, CD4+CD25+ T reg express a repertoire of Ag specificities as broad as that of naive CD4+CD25– Th cells, and are capable of recognizing both self and non-self Ag, enabling them to inhibit immune responses against microbes and innocuous Ag in different experimental settings 12, 25. Our results extended these findings and clearly showed that CD4+CD25+ T reg inhibited humoral responses against foreign Ag. Interestingly, we observed an increased in vivo antibody production in CD25+-depleted mice following vaccination with the inflammatory CFA adjuvant (Fig. 1–4) or with endotoxin-free Ag formulations (Fig. 2C, D).
Lee et al. 26 recently reported that depletion of CD4+CD25+ T reg did not affect the acute humoral response to polysaccharides and proteins expressed by intact Streptococcus pneumoniae 26. We do not have definitive explanation for the discrepancies between the results presented herein and the absence of T reg effect on the acute humoral response against intact S. pneumoniae. However, the nature of the Ag or the infectious context might further dictate the susceptibility of humoral responses to the regulatory effect of CD4+CD25+ T cells.
Depending on the Ag inoculated, T reg depletion could lead to an increase in both IgG1/IgE and IgG2a Ag-specific antibodies (Fig. 1) or to a specific overproduction of Ag-specific IgG2a (Fig. 3C–F). This is reminiscent of works showing that T reg influence the character of Th cell differentiation by selectively dampening Th1-type responses 12.
The finding that depletion of CD4+CD25+ T reg enhanced the efficacy of humoral responses (increased Ag-specific IgG concentration and affinity) might have functional consequences for the generation of polyclonal antiserum and the derivation of mAb-secreting B cell hybridomas. Indeed, we would propose that in vivo depletion of CD25+ T cells before immunisation could increase the frequency of Ag-reactive B cells, therefore facilitating the generation of Ag-specific B cell hybridomas. This might be particularly relevant in the case of poorly immunogenic or self-mimetic Ag. In summary, our data suggest that CD4+CD25+ T cells could exert a feedback mechanism on Ag-specific antibody secretion by dampening the T cell help for B cell activation.
Materials and methods
Mice and immunisations
Six- to eight-week-old BALB/c and C57BL/6 mice were purchased from Harlan (Horst, The Netherlands). CD45.1+ C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in our pathogen-free environment.
Groups of mice received one i.p. injection of 0.5 mg anti-CD25 mAb (clone PC61, rat IgG1 mAb produced in house 27). Control mice were either untreated or inoculated with equal amount of control rat IgG1 mAb (clone LoDNP2; Lo-Imex, Bruxelles, Belgium).
Seven days after PC61 or control mAb treatment, mice were injected i.p. with KLH (endotoxin-free formulation, 50 µg/mouse; Calbiochem), human gammaglobulin (15 µg/mouse; Sigma-Aldrich), OVA (100 µg/mouse; Sigma-Aldrich), NP-KLH or NP-Ficoll (100 µg/mouse; Biosearch Technologies, Inc.). Except when otherwise stated, mice were inoculated with Ag emulsified in CFA (primary response) and challenged 14 days later with the same amount of Ag solubilised in saline (secondary response).
Antibody titer and relative affinity determinations
Serum levels of Ag-specific antibodies were determined by ELISA according to standard procedures using mouse isotype-specific rat mAb, as described 28. Total isotype serum antibody levels were determined using rat anti-mouse isotype mAb, as depicted in section In vitro cultures.
In order to measure the relative affinities of anti-NP responses, we used a variation of the procedure described by Herzenberg et al. 29. The relative affinities of NP-immune sera were calculated by comparing their binding to differently haptenised carrier proteins (heavily haptenised NP17-BSA versus lightly haptenised NP2-BSA; Biosearch Technologies, Inc), as described 13. ELISA plates were coated with 5 µg/mL of either NP17-BSA or NP2-BSA. The same serial dilutions of each serum sample were allowed to bind on NP17-BSA and NP2-BSA and the 50% maximum binding on NP17-BSA was determined. The relative affinities of the anti-NP serum antibodies are expressed as a ratio of the serum volumes required to give the 50% of maximum binding on NP17-BSA divided by that required for same binding on NP2-BSA (serum relative affinity = vol50% binding on NP17-BSA / vol50% binding on NP2-BSA; see supplemental Fig. S1 for a detailed example). Larger values (close to 1) indicate a greater affinity.
Cell isolations
CD4+ T cells were purified from naive animals by magnetic depletion of B cells, macrophages, DC, NK cells, granulocytes and CD8+ T cells. Spleen cells (2×107/mL) were incubated (30 min at 4°C) in a depletion cocktail medium containing 1 µg/mL of the following biotinylated mAb: anti-CD19, anti-pan NK, anti-GR1, anti-TER119 (all from BD Bioscience, Mountain View, CA), anti-CD11c (clone N418), anti-MHC class II (anti-I-Ed: clone 14.4.4; anti-I-Ab: clone AF6) and anti-CD8 (clone H35). The cells were then centrifuged and resuspended in 70 µL of anti-biotin-conjugated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) per 108 spleen cells, for 30 min at 4°C before centrifugation and separation over an autoMACS column (Miltenyi Biotec) using the DepleteS program, according to the manufacturer's recommendations.
CD4+CD25– T cell fraction was obtained by adding biotinylated anti-CD25 mAb (clone PC-61) in the depletion cocktail medium. CD4+CD25+ T reg were further purified from the CD4+ T cell fraction by positive selection of CD25+ T cells with PE-coupled anti-CD25 mAb and magnetic beads (Miltenyi Biotec). CD45.2+ cell depletion was obtained by incubating the CD45.1+/CD45.2+ mixed cell population with PE-coupled anti-CD45.2 mAb (BD Bioscience), followed by anti-PE beads incubation and MACS separation.
B cells were isolated by positive selection with anti-CD19-coupled magnetic beads. The percentage of purified cell fractions in all experiments ranged between 90 and 98%, as estimated by flow cytometry using a FACSsort cytometer (Becton Dickinson, Mountain View, CA)
In vitro cultures
All cell cultures were performed in RPMI 1640 supplemented with 5% FCS, penicillin, streptomycin, glutamine, nonessential amino acids, 1 mM sodium pyruvate and 5×10–5 M 2-mercaptoethanol. CD4+CD25– Th cells or CD4+CD25+ T reg (106 cells/ well in 24-well plates) were pre-activated for 2 days with plastic-coated anti-CD3 mAb (10 µg/mL) and anti-CD28 mAb (dilution 2000× from ascetic fluid). T cells were recovered, washed in fresh medium and co-cultured (105 cells/well) for 7 days with purified syngenic B cells (5×105 cells/well). T cells were irradiated (2000 rad) to prevent their outgrowth during the 7-day culture. IgG1 and IgG2a antibodies in the supernatants of B cells cultured with CD4+CD25– Th cells, CD4+CD25+ T reg or both, were determined by ELISA, using rat anti-mouse isotype mAb (Lo-Imex; IgG1 detection: capture antibody loMG1.2, detection antibody loMG1.13; IgG2a detection: capture antibody loMG2a.9, detection antibody loMK1). In some experiments, total CD4+ T cells (90% CD4+CD25– Th cells/10% CD4+CD25+ T reg) or purified CD4+CD25– Th cells were pre-activated as above, in the presence of SB-431542 (10 µM; Sigma-Aldrich), anti-TGF-β (10 µg/mL, clone 1D11; a generous gift from Dr. J. Dasch 30) or control mouse IgG1 (MADNP1; Lo-Imex).
Acknowledgements
We thank P. Veirman for animal care and F. Thielemans for technical support. This work was supported by The Belgian Program in Interuniversity Poles of Attraction Initiated by the Belgian State, Prime Minister's office, Science Policy Programming and by a Research Concerted Action of the Communeauté française de Belgique. F.E. is a Research Fellow at the “Fonds de la Formation à la Recherche dans l'Industrie et l'Agriculture, FRIA», Belgium. F.A. is a Research Associate at the National Fund for Scientific Research, FNRS, Belgium. G.O. is a Research Fellow at the FNRS/Télévie, Belgium.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH