The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals
Abstract
Ginsan, a polysaccharide extracted from Panax ginseng, has multiple immunomodulatory effects. In this study, we show that pretreatment of ginsan (25 μg/kg) protected mice from lethality induced by Staphylococcus aureus challenge. This survival benefit was associated with enhanced bacterial clearance from circulation, spleen and kidney. The phagocytic activity of macrophages treated with ginsan was significantly enhanced against S. aureus. However, the production of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, IFN-γ, IL-12, and IL-18, was markedly down-regulated in ginsan-treated mice compared with those of control-infected mice. The expression of Toll-like receptor (TLR) 2 and the adaptor molecule MyD88, which was greatly increased in septic macrophages, was significantly reduced by ginsan treatment in vitro. Similarly, the expression of phospho-JNK1/2, phospho-p38 MAPK, and NF-κB was decreased in the same culture system. These results illustrate that the antiseptic activity of ginsan can be attributed to enhanced bacterial clearance, and reduced proinflammatory cytokines via the TLR signaling pathway.
Abbreviations:
-
- BLP:
-
bacterial lipoprotein
-
- CLP:
-
cecal ligation and puncture
-
- ERK:
-
extracellular signal-regulated kinase
-
- JNK:
-
c-Jun N-terminal kinase
-
- PM:
-
peritoneal macrophages
Introduction
Sepsis is a severe, often fatal systemic illness caused by excessive inflammatory response to microbial infections in the blood. Although antibiotics are used to prevent and cure bacterial infections, the incidence of fatal cases of sepsis continues to increase with the emergence of new antibiotic-resistant bacteria and inevitable nosocomial infections 1, 2. According to a recent report, sepsis occurs annually in about 750 000 people in the United States and results in approximately 200 000 deaths 3.
Both Gram-negative and Gram-positive bacterial infections are capable of causing sepsis. LPS is a major sepsis-inducing component of Gram-negative bacteria, such as Escherichia coli. In Gram-positive bacteria, peptidoglycan and lipoteichoic acid in the cell wall are possible sepsis-inducing molecules 4. Staphylococcus aureus, a Gram-positive bacterium, often causes toxic shock syndrome by producing toxic shock syndrome toxin-1 (TSST-1) 5 or by inducing proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 in infected animals. Therefore, S. aureus is considered an appropriate Gram-positive bacterium for the study of sepsis 6. TNF-α is an important indicator of septic shock in animal models. Together with IFN-γ, TNF-α produces a synergistic effect that brings about lethal results in LPS-induced septic shock 7, 8. As IL-12 and IL-18 ultimately induce the production of IFN-γ by the immune system, the reduction in IL-12 or IL-18 lowers sepsis-induced death rates in animal models 9, 10. Clinical trials in septic patients aimed at down-regulating proinflammatory cytokines using antibodies against TNF-α, antagonists of IL-1, or platelet-derived growth factor have proved to be uniformly disappointing 11, because sepsis is a dynamic process in which the host is challenged with a series of infectious insults and progresses through a variety of cytokine changes. Therefore, the precise regulation of the balance between pro- and anti-inflammatory cytokines is required at all stages in septic animals.
Ginsan is a soluble polysaccharide extracted from the roots of Panax ginseng. We have previously reported that ginsan functions as an effective biological response modifier; ginsan stimulates NK and T cells, produces a variety of cytokines, and induces tumoricidal and antimicrobial activities in macrophages 12. Furthermore, we have demonstrated that ginsan possesses potent anti-septicemic activity by producing nitric oxide via proinflammatory cytokine production in stimulated macrophages in vitro 13. Paradoxically, these mediators are also well known to drastically exaggerate the septic symptoms: therefore, we asked how ginsan modulates the plasma cytokine profiles in septic animals, and whether there are other mechanisms that provide protection against sepsis-induced lethality. We used an S. aureus-infected animal model and attempted to elucidate the molecular mechanism of ginsan action, focusing on TLR signaling. The results showed that ginsan induced an anti-inflammatory response and reduced infection-related mortality by blocking TLR2-mediated NF-κB activation in animals with sepsis.
Results
Ginsan protects mice from the acute sepsis induced by S. aureus infection
We have previously demonstrated that ginsan increased the survival rate of septic C57BL/6 mice challenged with live S. aureus 13. In the present study, we first determined optimal efficacy dose of ginsan. Mice were i.v. injected with 0.012, 0.025, 0.5, 25, and 250 mg/kg ginsan 24 h before the S. aureus challenge (1.5 × 108 CFU/mouse). Pretreatment with ginsan significantly increased survival in the mice infected with S. aureus, from 10% in control mice to 88% (p<0.0001; Fig. 1A). The mice given 25 μg/kg ginsan at 3, 24, or 48 h before the S. aureus challenge had survival rates of 58%, 83% (p=0.011), and 75% (p=0.034), respectively (data not shown). We also examined whether ginsan treatment could provide protection in other septic states, such as Gram-negative bacterial infection induced by live E. coli, and polymicrobial sepsis induced by cecal ligation and puncture (CLP), which closely mimics human acute peritonitis and is regarded as the most clinically relevant animal model of sepsis. The mortality induced by i.p. injection of 1.4 × 107 live E. coli cells was almost completely inhibited, with the survival rate improving from 13.3% without ginsan to 100% with ginsan treatment (100 mg/kg, i.v., 24 h before E. coli injection; p<0.0001; Fig. 1B). Ginsan-conferred protection was also observed in mice challenged with CLP-induced polymicrobial sepsis in which the survival was significantly improved from 0% in control mice to 60% in ginsan pretreated mice on day 5 (100 mg/kg, i.v., 24 h before CLP; p=0.048; Figure 1C). The effective doses of ginsan in the E. coli infection and CLP models were very different from that in the S. aureus challenge experiment, and the biological reasons underlying the dose-response effects are not yet known.
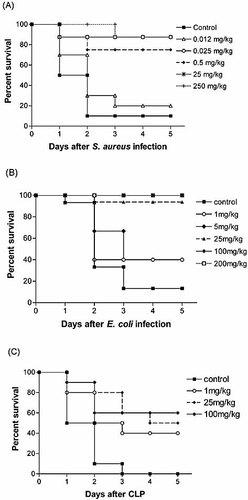
Ginsan protects mice against lethality from different septic challenge. S. aureus-infected mice were given various doses of ginsan i.v. 24 h before challenge (A). The pretreatment of mice with ginsan protected the animals from lethality induced by i.p. injection of E. coli (B) and from CLP-induced polymicrobial sepsis (C). n=10–16 mice per group.
Ginsan enhances in vivo bacterial clearance
To verify that the survival benefit of ginsan treatment is related to enhanced bacterial clearance, mice were culled 2 days after S. aureus infection. Samples of blood and homogenates of the spleen and kidney were cultured at 37°C for 24 h after which bacterial CFU were enumerated and compared (Fig. 2). Pretreatment of ginsan dramatically reduced the bacterial CFU counts in the bloodstream, spleen and kidney of the mice, compared with those of their control counterparts (p<0.001).
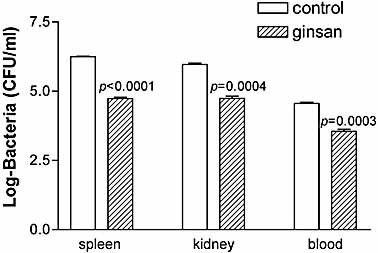
Ginsan enhances bacterial clearance in S. aureus-infected mice. Mice were treated with ginsan (25 μg/kg, i.v.) 24 h before S. aureus infection (1.5 × 108 CFU, i.p.). Spleen, kidney, and blood isolated 2 days after infection were homogenized and used to determine the total bacterial load by dilution plating on blood agar (n=5). Data are expressed on a log scale as the mean ± SEM.
Ginsan increases bactericidal activity of macrophages
To identify possible mechanisms involved in the enhanced bacterial clearance observed in ginsan-treated mice, we assessed whether ginsan increased the bactericidal activity of macrophages isolated from intact or S. aureus-infected mice. Fig. 3A shows a typical FACS analysis of peritoneal macrophages (PM) incubated with ginsan for 3 h, following stimulation with heat-killed S. aureus for 30 min at 37°C. Phagocytosis of S. aureus by PM was significantly increased after ginsan treatment (by approximately 43% compared with the non-treated macrophages). Moreover, the PM isolated from ginsan-treated, S. aureus-infected mice also exhibited enhanced phagocytosis against S. aureus, although the magnitude of phagocytosis was not as great as that in the in vitro assays (Fig. 3B). Given our previous finding of increased nitric oxide production and intracellular killing of bacteria in PM isolated from ginsan-treated S. aureus-infected mice 13, ginsan appears to protect mice against S. aureus-induced sepsis via macrophages activation.
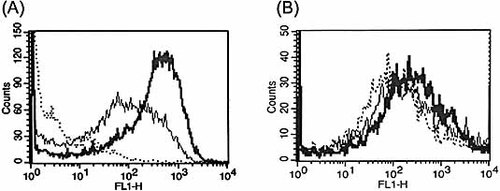
Ginsan enhances phagocytosis in S. aureus-infected macrophages. (A) Phagocytic activity was evaluated in PM isolated from intact mice and incubated with ginsan for 3 h. After washing with PBS, PM were incubated with heat-inactivated, FITC-labeled S. aureus for 30 min at 37°C: non-phagocytosed macrophages used as control (dotted line), macrophages incubated with S. aureus (solid line), and macrophages incubated with ginsan before S. aureus infection (bold line). (B) PM were obtained from non-treated (dotted line) and S. aureus-infected mice treated with or without ginsan (25 μg/kg, bold and solid lines, respectively), and were then stimulated with heat-killed S. aureus for 30 min at 37°C. The phagocytic activities of the cells were determined using FACS analysis. A representative of three separate assays is shown.
To further confirm the crucial role of macrophages in the ginsan-mediated protection of mice infected with S. aureus, we depleted mice of monocyte/macrophages by s.c. etoposide injection once a day for 3 consecutive days (71% mean monocyte reduction as determined by blood cell counts). A suboptimal dose of S. aureus (1 × 107 CFU) was used in this experiment, because lethal bacterial dose would itself result in low survival, making it difficult to differentiate effect of monocyte depletion. The monocytopenic mice displayed higher mortality than the controls (50% vs. 20%), whereas pretreatment of ginsan demonstrated a still much higher survival benefit over the controls (Fig. 4A). In addition, pretreatment of the monocytopenic mice with ginsan led to a statistically significant increase in bacterial clearance from the blood, spleen and kidney at 48 h after the S. aureus challenges (Fig. 4B). Although ginsan augmented the phagocytosis of S. aureus by PM, ginsan still increased survival in macrophage-depleted animals and reduced the S. aureus burden in these animals, suggesting that macrophages may be partly involved in the phagocytosis and antimicrobial activity induced by ginsan in vivo.
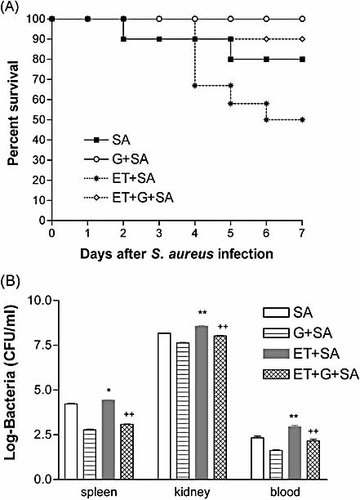
Pretreatment with ginsan increases survival and bacterial clearance in monocyte/macrophage-depleted mice following S. aureus challenge. The mice were depleted of their monocyte/macrophage population using s.c. etoposide (12.5 mg/kg) injection once a day for 3 consecutive days (ET). The mice were subsequently challenged with S. aureus (SA, 1.0 × 107 CFU, i.p.). Survival was monitored for 7 days (A). Spleen, kidney, and blood were obtained from each group 2 days after infection, and bacterial CFU were counted. The letter G indicates the groups treated with 25 μg/kg ginsan 24 h before the bacterial challenge (B). *p<0.01, **p<0.001 vs. infected mice; ++p<0.001 vs. infected and monocyte-depleted mice. n=10–12 mice in each group.
S. aureus-associated release of inflammatory cytokines is attenuated by ginsan
It is generally accepted that poorly regulated inflammatory cytokines play an important role in the pathogenesis of sepsis. Therefore, the production of inflammatory cytokines in septic mice was examined at different times after ginsan treatment (Fig. 5). The highest levels of production of almost all the cytokines examined in this study were observed 8–12 h after S. aureus infection. The typical inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, were robustly produced in S. aureus-infected mice, whereas ginsan pretreatment significantly decreased their production.
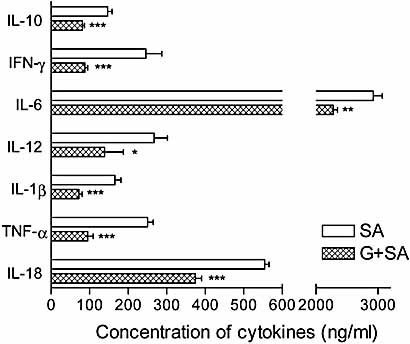
Ginsan attenuates pro- and anti-inflammatory cytokine production in S. aureus-infected mice. Serum cytokine levels were determined at 0, 2, 4, 8, 11, and 24 h after the challenge with 1.5 × 108 CFU S. aureus, and the results for 11 h are illustrated here. The cytokines in the control and ginsan only groups were barely detectable, and there was no difference between the two groups (data not shown). *p<0.05, **p<0.01, ***p<0.001 vs. infected mice.
IFN-γ and TNF-α are known to aggravate sepsis synergistically, and IL-12 and IL-18 stimulate IFN-γ induction. Therefore, we determined the serum levels of these cytokines in septic mice and observed significant decreases in the levels of all of these cytokines with ginsan treatment. In contrast to this down-regulated synthesis of proinflammatory cytokines, ginsan treatment did not affect IL-2 and IL-4 (data not shown).
Ginsan suppresses TLR signaling pathway in macrophages
Given that TLR plays prominent roles in stimulating the protective immune and inflammatory response to infectious challenges, we next examined whether ginsan can modulate the expression of TLR2 and the adaptor molecule MyD88 in response to Gram-positive bacterial infections. PM treated with S. aureus had very high levels of TLR2 and MyD88, and moderate expression of TLR4 and TLR9 (Fig. 6). In striking contrast, PM pretreated with ginsan before S. aureus infection almost completely down-regulated the expression of TLR2 and MyD88. Moreover, the expression of TLR4 and TLR9 was blocked under the same experimental conditions.
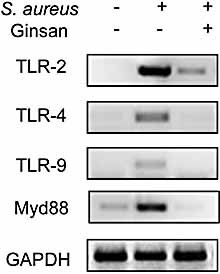
Ginsan suppresses the expression of TLR and the adaptor MyD88 molecule in PM activated by S. aureus. PM (4 × 106 cells/well) were isolated and pretreated with ginsan (0.1 μg/mL) for 6 h. After washing with PBS, the cells were stimulated with heat-killed S. aureus (8 × 107 CFU/well) for 6 h at 37°C. RT-PCR was conducted to evaluate the expression levels of TLR2, TLR4, TLR9, and MyD88. GAPDH was used as a control gene. The results show one representative of three independent experiments.
Ginsan inhibits S. aureus-induced NF-κB and MAPK activation
To more critically evaluate whether the reduction in TLR and MyD88 was required for the suppression of inflammatory cytokines conferred by ginsan, we further investigated the downstream molecules connected to TLR signaling, including extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), p38 mitogen-activated protein kinases (MAPK), and NF-κB. The transduction of TLR signals in monocytes and macrophages activates MAPK, which subsequently leads to the activation of various transcription factors and the production of inflammatory cytokines 14. S. aureus stimulation induced phosphorylation of JNK1/2 and p38 kinases in PM, whereas a marked down-regulation of p38 and JNK1/2 phosphorylation were observed when PM were pretreated with 0.1 μg/mL ginsan (Fig. 7A). However, the expression of phospho-ERK1/2 in PM did not change significantly in response to S. aureus stimulation or ginsan pretreatment, indicating that ERK activation is not required during that period. The phosphorylation of MAPK is associated with the subsequent activation of transcription factors, including NF-κB, which plays a critical role in monocyte inflammatory cytokine production after exposure to bacterial stimuli 14–16. Therefore, we further examined the effect of ginsan on NF-κB activation. Fig. 7B shows that the nuclear extracts of PM that were pretreated with ginsan and subsequently co-cultured with S. aureus showed a remarkable reduction of free NF-κB. As expected, S. aureus infection per se increased NF-κB activation in PM, which was correlated with the findings for both cytokine production and MAPK activation.
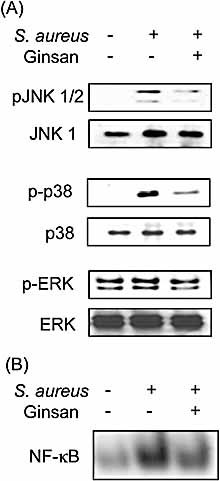
Ginsan inhibits S. aureus-induced MAPK and NF-κB activation in PM. PM were pretreated with ginsan (0.1 μg/mL) for 6 h and were subsequently treated with heat-killed S. aureus for 40 min. (A) MAPK phosphorylation was detected using Western blot analysis with antibodies specific for JNK 1/2, p38, and ERK1/2. (B) The concentration of NF-κB in nuclear extracts was determined using electrophoretic mobility shift assay.
Discussion
In recent years, the emergence of numerous antibiotic-resistant bacterial pathogens has led to an urgent need for new antibacterial agents 17. Augmentation of the host response by immunomodulators is an alternative to the use of antibiotics in the prevention and/or treatment of infections caused by antibiotic-resistant bacteria. For instance, glucan, which has been studied extensively because of its ability to enhance host innate immunity, decreased septic complications and improved survival in septic hosts 18–20.
Here, we demonstrate that ginsan pretreatment could inhibit the lethality induced by broad spectrum of bacteria, including Gram-positive S. aureus, Gram-negative E. coli, and polymicrobial CLP. Ginsan was found to be a potent, efficacious antiseptic agent with a minimal effective dose of 25 μg/kg. However, the optimal doses of ginsan differed depending on the animal model, and the mechanism causing this difference is currently being explored.
Ginsan had potent bactericidal activity that included enhanced phagocytosis in vitro and a dramatic increase in bacterial clearance in vivo (Fig. 2, 3). The depletion of macrophages did not abolish the beneficial effect of ginsan with regard to both animal survival and bacterial clearance, indicating that the other immune cells, especially neutrophils, which also phagocytose invading microbes within the circulation, contribute to the observed results.
In models of severe systemic infection or inflammation produced by the i.v. administration of high doses of bacteria or bacterial products, such as endotoxin, excessive production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, leads to serious systemic disorders with fatal consequences 21, 22. In this respect, ginsan induced significant changes in the levels of proinflammatory cytokines at early stages in S. aureus-infected mice (Fig. 5). Ginsan treatment significantly decreased the proinflammatory cytokines in septic mice, including TNF-α, IL-1β, IL-6, IFN-γ, IL-12, and IL-18, whereas Th2 type cytokines (IL-2 and IL-4) were not affected. To our surprise, ginsan treatment also decreased the anti-inflammatory cytokine IL-10 in S. aureus-infected mice. The role of IL-10 in experimental sepsis remains controversial. The exogenous application of IL-10 can prevent septic shock in mice by inhibiting the synthesis of proinflammatory cytokines, while neutralizing IL-10 inhibited bacterial outgrowth in lungs and improved survival during murine pneumonitis 23, 24. Furthermore, recent studies demonstrated that sepsis is characterized by a biphasic immunological response: an initial hyperinflammatory phase, followed by a hypoinflammatory phase 25, 26. Therefore, it is quite likely that these contradictory observations were attributable to the time of the intervention. The level of IL-6, which also functions as an important and sensitive indicator of inflammation within the body, also proved interesting: it remained high until 24 h, although ginsan administration decreased the level of IL-6. As with IL-10, the controversy on IL-6 revolves around its role in sepsis: is IL-6 a marker of inflammation, an inducer of altered physiology, or a mediator of organ injury 27? According to supportive evidence in which different doses of LPS induced different profiles of serum IL-18 expression inter-related to disparate outcomes in septic mice 28, an optimal range of serum IL-6 appears to be required for efficient antibacterial host defense. Thus, ginsan treatment eventually restored the balance between the pro- and anti-inflammatory arms of the cytokine network in sepsis.
It is well established that in the mammalian innate immune system two pattern recognition receptors, CD14 and TLR, are involved in the recognition of bacteria and their cell wall components. They induce proinflammatory mediators by promoting MAPK, such as ERK, JNK, and p38 MAPK, and NF-κB 29–33. Unfortunately, we experienced difficulties in measuring the protein levels in several visceral compartments in septic mice; therefore, we used an in vitro setting to evaluate the efficacy of ginsan to act on TLR and MAPK signaling transduction pathways. The levels of TLR2 and MyD88 mRNA in S. aureus-infected macrophages were highly augmented, whereas ginsan pretreatment dramatically suppressed the expression of TLR2, TLR4, TLR9, and MyD88, ultimately leading to a reduction in inflammatory cytokines (Fig. 6). In addition to TLR, ginsan itself could activate other receptors, including LPS receptors, receptors for complement, integrins, and scavenger receptors in PM. Therefore, the described results are not the only mechanism of action of ginsan. Interestingly, the fact that ginsan alone stimulates TLR in normal macrophages (unpublished data), but down-regulates them in septic macrophages, leads us to speculate that ginsan can induce tolerance against a variety of septic challenges. In support of this hypothesis, endotoxin or LPS tolerance is a well-established phenomenon in which pre-exposure to a sublethal dose of LPS blunts subsequent lethal LPS-induced mortality, and this effect was closely associated with diminished production of proinflammatory cytokines 34, 35. In addition to LPS tolerance, several non-LPS bacterial cell wall components, including bacterial lipoprotein (BLP), also induce tolerance 36, 37. Unlike LPS or BLP, however, ginsan exhibited a wide spectrum of bacterial tolerance in Gram-positive, Gram-negative, and polymicrobial septic models and did not induce lethal shock, even at high concentrations.
The intracellular signal transduction pathways involved in sepsis have been investigated extensively. Several studies demonstrated that the phosphorylation of JNK1/2 and p38 MAPK, and the translocation of NF-κB induce inflammatory cytokines in sepsis 38, 39. We found that pretreatment of PM with ginsan strongly inhibited the phosphorylation of JNK and p38 MAP kinase and reduced NF-κB activation (Fig. 7), similar to that seen in LPS- or BLP-tolerized cells. Interestingly, the activation of ERK1/2 was not affected by bacterial challenge or ginsan treatment. ERK plays a critical role in regulating cell growth and differentiation. Recently, Song et al. 40 reported that the signaling transduction pathway in phagocytosis and cytokine production was regulated differently by phosphatidylinositol 3-kinase and ERK in primary human microglia. In addition, because the role of MAPK members in phagocytosis appears to be diverse, depending on cell types and stimuli 41, 42, phagocytosis and cytokine production conferred by ginsan may be regulated via different signal pathways. Further studies will include attempts to show that ginsan can also diminish the proinflammatory response and protect from septic lethality in TLR-deficient mice and elucidate the ginsan receptor.
In conclusion, possible cellular and molecular mechanisms of the anti-septic/infective activity of ginsan were studied using experimental septic animals. The results showed that ginsan modulates monocyte/macrophage-mediated innate immunity largely by up-regulating phagocytosis and enhancing bacterial clearance and down-regulating inflammatory cytokine synthesis. The reduced inflammatory responses are the consequence of decreased signals transmitted via TLR, JNK, p38 MAPK, and NF-κB. These well-balanced and finely adjusted actions of ginsan ultimately rescue the animal from lethal sepsis. Therefore, the use of ginsan is a potential treatment modality for sepsis and its application might be expanded when combined with antibiotics.
Materials and methods
Bacteria
S. aureus 25923 or E. coli 018ac:K1:H7, acquired from ATCC, was cultured at 37°C in Trypticase soy broth (Difco, Detroit, MI), harvested at the mid-logarithmic growth phase, washed twice, and resuspended in PBS. The concentration of resuspended bacteria was determined spectrophotometrically at 620 nm.
Preparation of ginsan
Ginsan, a polysaccharide, was purified from the ethanol-insoluble fraction of the aqueous Panax ginseng extract, as described previously 12. Further purification was carried out successively using size exclusion and ion exchange column chromatography, and NMR analysis revealed that ginsan was composed of α(1→6)glucopyranoside and β(2→6) fructofuranoside in a 5:2 molar ratio. The endotoxin level in the purified ginsan preparation was less than 0.03 EU/mg as measured using the Limulus amebocytes lysates assay (Endosafe®, Charles River Laboratories, USA) according to the manufacturer's instructions. Moreover, the treatment of polymyxin B did not inhibit lymphocyte proliferation, excluding possible endotoxin contamination in ginsan. Ginsan was dissolved in PBS (pH 7.4) and filtered through a 0.25-μm Millipore membrane (Millipore, USA).
Sepsis model
Pyrogen-free BALB/c male mice, aged 5–7 weeks (18–22 g), were purchased from the Charles River Breeding Laboratory (Charles River Japan, Atsugi Breeding Center, Yokohama, Japan) and kept for 6 days in our animal quarters for acclimatization. Sterile standard mouse chow (NIH-7 open formula) and water were given ad libitum, and the mice were housed randomly at 60% humidity and 22 ± 2°C on a 12-h light-dark cycle. All animal experiments were performed according to the National Institutes of Health guidelines, based on the protocols approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences (KIRAMS).
For the sepsis model, mice were injected i.p. with bacterial suspension containing either 1.5 × 108 live S. aureus or 1.4 × 107 live E. coli cells. For CLP, the cecum was exposed through a 1.0- to 1.5-cm abdominal midline incision in anesthetized mice, ligated at its base with 3–0 silk suture, punctured once with a 21-gauge needle, and gently squeezed to express fecal materials into the peritoneal cavity. The cecum was then returned to the peritoneal cavity, and the abdominal incision was closed. Survival was monitored for at least 7 days.
Reagents
Etoposide (Sigma, St. Louis, MO) was used to selectively depopulate monocytes/macrophages in the mice, as described elsewhere 43. Mice were injected s.c. for 3 consecutive days with 12.5 mg/kg etoposide in 1% DMSO-PBS before the S. aureus challenge, based on practices established in earlier studies 43, 44. Antibodies specific for ERK1/2, phospho-ERK1/2, JNK1, and phospho-JNK1/2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and antibodies to p38 MAPK and phospho-p38 MAPK were from Cell Signaling Technology (Beverly, MA).
Analysis of cytokines
To measure the S. aureus-induced plasma cytokines, mice were given ginsan 24 h before the S. aureus challenge, and plasma was collected at various times after S. aureus infection. The concentrations of cytokines were measured with ELISA kits for detecting IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, and TNF-α (R&D Systems, USA).
Enumeration of bacteria in blood and organs
Control and ginsan-treated mice were culled 48 h after i.p. injection of live S. aureus. Blood samples were taken, and the dissected spleen and kidney were homogenized. Serial tenfold dilutions of whole blood and organ homogenates in PBS were plated on blood agar and cultured for 24 h at 37°C to determine bacterial CFU.
Determination of phagocytic activity of macrophages
FITC-labeled S. aureus was prepared as described previously 45, 46. Briefly, S. aureus was heat-killed at 70°C for 90 min and labeled with 0.25% FITC at 4°C for 12 h. After washing out unlabeled S. aureus, the ten heat-killed FITC-labeled S. aureus per cell were incubated with PM (5 × 106cells) for 30 min at 37°C. PM was treated with 0.1 μg/mL ginsan 3 h before the S. aureus administration. Bacterial uptake by the cells was assessed using FACScan analysis (Becton Dickinson, USA) with CellQuest software.
RT-PCR
Expression levels of RNA transcripts were quantified using RT-PCR. Total RNA from peritoneal macrophages was isolated using TRIzol reagent (Gibco BRL, USA). The RNA (1 μg/tube) was reverse transcribed into cDNA for 1 h at 37°C using 15 U MMLV RT (Promega, USA) in the presence of 1 μL pd(N)6 random hexamer (Amersham Biotechnology, USA). The cDNA was used as a template for amplifying TLR-2, TLR-4, TLR-9, and MyD88 by PCR (Applied Biosystems, USA). The reaction mixture consisted of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 2.5 mM dNTP (Takara, Japan), 1.25 U Taq DNA polymerase (Takara), and 0.05 mM of each primer. The primers were as follows, with the expected fragment size in parentheses: mTLR2 (375 bp), forward 5′-CAAACTGGAGACTCTGGAAG-3′ and reverse 5′-CTGTAGGAAACAAAGGCATC-3′; mTLR4 (266 bp), forward 5′-GACACCCTCCATAGACTTCA-3′ and reverse 5′-TGTTCA ACATTCACCAAGAA-3′; mTLR9 (297 bp), forward 5′-CCTGTCTCAAAATAACCTGC-3′ and reverse 5′-CTGAGGTTGACCTCTTTCAG-3′; mMyD88 (303 bp), forward 5′-CGATGCCTTTATCTGCTACT-3′ and reverse 5′-TTCTTCATCGCCTTGTATTT-3′; mGAPDH (285 bp), forward 5′-GAGTCTACTGGCGTCTTCAC-3′ and reverse 5′-CCATCCACAGTC TTCTGAGT-3′. The thermal cycle conditions were 94°C for 1 min, 52°–58°C for 30 s, and 72°C for 30 s for 32 cycles. The PCR products were electrophoresed on 1.5% agarose gels and quantified using an image analyzer (Bio-Rad, Hercules, CA).
Electrophoretic mobility shift assay
Nuclear extracts of PM that had been pretreated with ginsan (0.1 μg/mL) for 6 h and then co-cultured with heat-killed S. aureus for 40 min were prepared as described previously 29. The double-stranded oligonucleotide probes containing NF-κB binding sequences (5′-GGGGACTTTCCC-3′) were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase. The nuclear extract (15 μg) was incubated at room temperature for 20 min with 32P-labeled probe in a binding buffer (10 mM Tris, pH 7.8, 420 mM KCl, 1.5 mM MgCl2, 20% glycerol). DNA/nuclear protein complexes were electrophoresed on a 4% non-denaturing acrylamide gel. The gel was dried and exposed overnight to an X-ray film (Agfa, Belgium) in a cassette at –80°C. The film was developed for analysis.
Immunoblot analysis
To investigate the expression of ERK, JNK, and p38 MAPK, 2 × 106 PM were pretreated with ginsan (0.1 μg/mL) for 6 h and further co-cultured with heat-killed S. aureus for 40 min. The cell lysates were prepared by extracting proteins with cell lysis buffer (Cell Signaling) supplemented with protease inhibitors. The protein samples (30 μg/well) were separated by 9% SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schnell Bioscience, Germany). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and then incubated with primary antibodies for 1 h at room temperature. The blots were developed using peroxidase-conjugated secondary antibody, and the proteins were visualized using enhanced chemiluminescence (ECL) procedures (NEN) according to the manufacturer's recommendations.
Statistical analysis
The results are expressed as the mean ± SEM of data obtained from at least three replicate experiments. Survival data were analyzed using the log-rank test. Statistical significance and differences among groups were assessed by one-way analysis of variance and the Newman-Keuls test using GraphPad Prism v3.02 software (San Diego, CA).
Acknowledgements
This work was supported by the Nuclear R&D Program of the Ministry of Science and Technology in Korea.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




