Serum and mucosal antibody responses to pneumococcal protein antigens in children:relationships with carriage status
Abstract
Streptococcus pneumoniae causes significant morbidity and mortality especially in children. Some pneumococcal protein antigens can protect mice against infection. Little information is available concerning the nature of naturally acquired protective immunity to pneumococci in humans induced by these antigens. This study investigates the relationships between systemic and local antibody production and carriage in children. Children undergoing adenoidectomy (n=112, ages 2–12 years) were studied. Nasopharyngeal swabs were collected for pneumococcal culture. Serum and saliva were assayed for antibodies to several pneumococcal proteins: choline binding protein A (CbpA), pneumolysin (Ply), pneumococcal surface adhesin A (PsaA) and pneumococcal surface protein A (PspA). Adenoidal mononuclear cells (MNC) were cultured with pneumococcal culture supernatants or recombinant proteins. Cell culture supernatants were analyzed for antigen-specific antibodies. Carriage rates fell with age and serum levels of anti-CbpA, Ply and PspA rose. Anti-CbpA and -Ply serum and salivary IgG antibody levels were higher in children who were culture negative than those who were colonized. Antigen stimulation increased respective antigen-specific IgG production by adenoidal MNC and these responses were greater in those who were colonized than in culture-negative children. Antibodies to CbpA and Ply may protect children aged 2 years and older against pneumococcal colonization. Adenoids may be important local induction and effector sites for both mucosal and systemic antibody production to pneumococcal proteins in children.
Abbreviations:
-
- CbpA:
-
choline binding protein A
-
- CCS:
-
concentrated culture supernatant of pneumococci
-
- MNC:
-
mononuclear cells
-
- OD:
-
optical density
-
- Ply:
-
pneumolysin
-
- PNPP:
-
p-nitrophenyl phosphate
-
- PS:
-
polysaccharide
-
- PsaA:
-
pneumococcal surface adhesin A
-
- PspA:
-
pneumococcal surface protein A
Introduction
Streptococcus pneumoniae is an encapsulated bacterium associated with significant global morbidity and mortality 1. Although pneumococcal colonization is largely asymptomatic, it can progress to invasive disease, and in children the bacterium is a leading cause of bacterial meningitis and septicemia. Currently, there are two types of vaccine available against pneumococcal infection, capsular polysaccharide (PS) and protein-PS conjugate vaccines. However, the efficacies of these PS-based vaccines are limited in the former case by poor immunogenicity in high-risk populations, especially young children, and in both, but particularly the latter, by limited serotype coverage. Although carriage rates of conjugate vaccine serotypes fall in immunized populations, carriage rates of other serotypes rise, partially offsetting this effect 2.
Efforts have been made recently to develop effective pneumococcal protein vaccines that are likely to cover most serotypes of pneumococci. The protein antigens currently under study include pneumococcal surface protein A (PspA), pneumolysin (Ply), pneumococcal surface adhesin A (PsaA) and choline-binding protein A (CbpA, also known as PspC, Hic and SpsA) 3–8. Studies in mice have shown that immunization with these proteins can protect mice against infection with multiple serotypes of pneumococci and/or prevent nasopharyngeal carriage 9–11. Data regarding the immunogenicity and effectiveness of pneumococcal protein antigens in humans are limited. Antibodies to PsaA, PspA and Ply have been found in human serum and mucosal secretions 12, 13, emerging with age and exposure to pneumococci. Children with invasive pneumococcal infection had lower acute phase serum anti-PspA titers than controls in one study, implying a protective role for these antibodies 14. To explore these pneumococcal protein antigens further as human vaccines, it is important to characterize naturally acquired immune responses particularly in relation to nasopharyngeal carriage of pneumococci and to investigate whether they are protective.
Pneumococcal carriage is common in infants and young children, the age groups also at high risk of invasive disease. Almost half of children in industrialized countries are colonized with pneumococci at least once by the age of 1 year 15, 16, and in developing countries, carriage rates are even higher 17. There is evidence that pneumococcal disease does not occur without being preceded by nasopharyngeal colonization with the homologous strain 16, 18. Pneumococcal carriage is also considered to be the main source for horizontal spread of this pathogen within the community. Therefore, an optimally effective pneumococcal vaccine would be one that protects comprehensively against invasive disease as well as colonization, and thus results in herd immunity.
Data are scarce as to whether antigen-specific antibodies to pneumococcal proteins modulate carriage of pneumococci. In an adult volunteer carriage study, McCool et al. 19 provided evidence that serum IgG and/or secretory IgA antibodies to an N-terminal region of PspA, which varies between strains, are protective against carriage, while homotypic serum anti-capsular IgG was not protective. They went on to show that experimentally induced nasal colonization in adults induces serum IgG antibodies both to PspA and CbpA, but not several other pneumococcal antigens 20. Rapola et al. 21 showed that high serum anti-PsaA predicted low risk of progression to otitis media in colonized children aged 9–24 months. However, they went on to show that higher serum anti-PsaA in children aged less than 9 months predicted a greater likelihood of subsequent otitis 22, suggesting complex age-dependent effects, perhaps reflecting changes in antibody function. They also provided data suggesting that, although previous pneumococcal otitis is itself a risk factor for subsequent episodes, asymptomatic carriage protects against otitis later 22. There are no data available regarding the relationship between carriage and anti-Ply, anti-CbpA and anti-PspA serum or mucosal antibodies in children, or between antibodies to any pneumococcal protein antigens in children over the age of 2, a period during which rates of nasopharyngeal carriage of all serotypes normally fall. It seems unlikely that this observation can be entirely explained by some combination of anticapsular 23 and herd immunity effects. Accordingly, studying the acquisition of mucosal immune responses to pneumococcal proteins in children in this age group alongside their carriage status may provide important information.
As pneumococci are primarily a mucosal commensal colonizing the nasopharynx, intranasal mucosal immunization is a promising preventative strategy. Immunization via the nasal route can induce not only mucosal immunity but also antibody responses in serum 24–26. Adenoids (nasopharyngeal tonsils) which are located in the anatomical area of pneumococcal carriage, are known to be important immune inductive sites for oropharyngeal immunity 27. It is therefore likely that adenoids play a significant role in the development of natural immunity to pneumococci.
We have previously shown that antibody-secreting cells to pneumococcal protein antigens are present among adenoidal mononuclear cells (MNC) isolated from children undergoing adenoidectomy, suggesting that these pneumococcal proteins prime specific B cells for production of specific IgA, IgG and IgM antibodies in these children 28. Here we report antibody responses to pneumococcal protein antigens in serum, saliva and adenoidal MNC after in vitro antigen stimulation in relation to carriage status in children.
Results
Demographic data and carriage rates
The demographic data for the patients recruited into the study (n=112) and the pneumococcal carriage rates in nasopharyngeal swabs are presented in Table 1. No difference was found in carriage rates between males and females. As expected, younger children undergoing adenoidectomy had higher carriage rates than older children among whom rates were roughly stable between 20% and 30% (Table 1).
|
Patients’ age group(years) |
Number studied (% culture positive) |
|
|---|---|---|
|
|
Male |
Female |
|
2 |
3 (66.7) |
3 (66.7) |
|
3 |
7 (42.8) |
9 (44.4) |
|
4 |
7 (28.5) |
8 (25.0) |
|
5 |
14 (28.6) |
16 (25.0) |
|
6–7 |
12 (25.0) |
13 (30.8) |
|
8–12 |
10 (20.0) |
10 (20.0) |
Serum antibodies in relation to age in study subjects and controls
In study subjects mean serum antibody levels to CbpA, Ply and PspA (but not PsaA) tended to rise with age from 2 to 3 years, and changed little thereafter (Fig. 1a). In healthy children (controls) serum anti-CbpA, -Ply and-PspA likewise showed rises occurring after 13 months of age, while mean anti-PsaA titers were already elevated at 5 months and did not change over the period of observation (Fig. 1b). Direct comparison of serum antibody levels in the 3-year olds in the two groups showed closely similar titers for all four antigens (Fig. 1a, b; boxes).
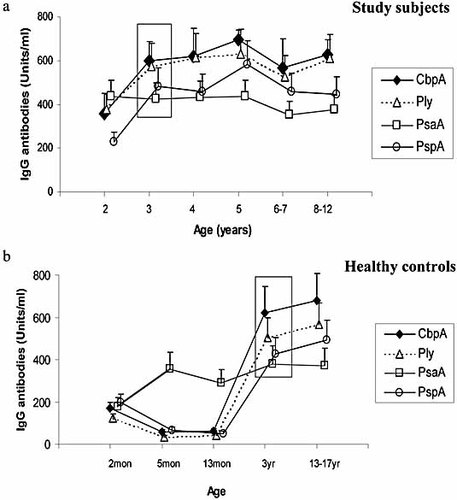
Serum IgG antibodies to CbpA, Ply, PsaA and PspA in 112 children undergoing adenoidectomy grouped by age (a). Data from five groups of 30 healthy children from whom sera were available are shown in (b) for comparison. Data from the same age group (age 3 years) in both study children (a) and controls (b) are boxed for comparison. Geometric mean titers and 95% confidence intervals (CI; error bars) are shown.
Serum and salivary antibodies in relation to nasopharyngeal carriage
Serum IgG antibody concentrations to CbpA and Ply were significantly higher in culture negative than in colonized children (Fig. 2a, p=0.03 and p=0.02, respectively). Using a general linear model of analysis of variance, it was found that the effect of carriage status on the antibody levels to CbpA and Ply was independent of age, i.e., both carriage status and age were determinant factors of the antibody levels. There was no statistically significant differences between the two groups (culture negative and colonized) in antibody levels to PsaA and PspA (p>0.05).
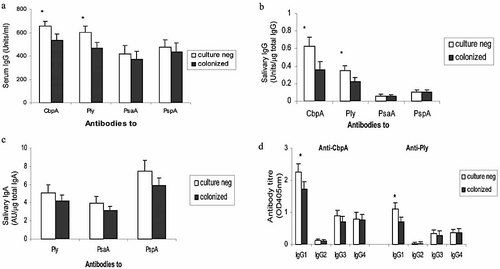
Serum IgG (a), salivary IgG (b) and IgA (c) antibodies to CbpA, Ply, PsaA and PspA, and serum IgG 1–4 subclasses (d) of anti-CbpA and Ply IgG antibodies in patients colonized with and with negative nasopharyngeal cultures for S. pneumoniae. *p<0.05 compared with colonized. Results represent geometric mean titers and 95% CI (error bars) of antibodies in 112 (a) and 64 (b, c, d) children of whom 34 and 20 were colonized and of whom 78 and 44 had nasopharyngeal swabs negative for pneumococci in (a) and (b–d), respectively. Anti-CbpA IgA antibody was not measured as CbpA binds secretory component, and thus anti-CbpA IgA in mucosal secretions cannot be detected reliably by immunoassay 28.
Salivary IgG antibody levels to CbpA and Ply were also higher in culture negative children (Fig. 2b, p=0.039 and p=0.048, respectively). There was a positive correlation between salivary and serum IgG antibody concentrations to CbpA, Ply and PspA (r=0.51, 0.56 and 0.42, respectively, all p<0.05), while salivary IgG antibodies to PsaA were generally low. For salivary IgA antibodies (expressed either as absolute concentrations or as the concentration ratio of antigen-specific to total IgA), apparent small differences between colonized and culture negative subjects were not statistically significant (Fig. 2c).
To assess whether unexpectedly high salivary anti-pneumococcal IgG concentrations in some individuals were due to increased transepithelial transudation of serum proteins following abrasion of the oral mucosa by the sponge collection swab, the concentration of albumin was assayed in 30 saliva and serum sample pairs for which anti-pneumococcal protein antigen antibody concentrations had previously been measured. The albumin concentrations were generally very low in the saliva (mean ± SD: 0.19 ± 0.04 g/100 mL) compared to serum samples (3.62 ± 0.95). No discernable correlation between the ratio of saliva to serum antibody concentration and saliva to serum albumin concentration was found (r values 0.04, 0.02, –0.04 and 0.13 for the four antigens, i.e., CbpA, Ply, PsaA and PspA).
Antigen-specific serum IgG subclass analysis to CbpA and Ply showed that IgG1 was the predominant subclass in both cases, followed by IgG3 and IgG4. Little or no IgG2 was detected (Fig. 2d). The IgG1 antibody titers in the culture negative subjects were higher than colonized patients with mean IgG1 optical density (OD) ratios of 1.44 and 1.61 for anti-CbpA and anti-Ply, respectively (p<0.05). No significant differences were observed between culture negative and colonized subjects in antigen-specific IgG3 or IgG4 levels (p>0.05).
Pneumococcal proteins induce Ag-specific antibody responses in adenoidal MNC culture
Concentrated culture supernatant of pneumococci (CCS) derived from encapsulated type 2 pneumococci (D39) induced significant antigen-specific IgG antibody responses to pneumococcal proteins CbpA, Ply, PsaA and PspA in adenoidal MNC cultures (Fig. 3a, all p<0.001). As in serum, the predominant subclass was IgG1, with substantial detectable IgG3 and IgG4, but IgG2 was almost undetectable (data not shown). These responses were abolished by both heat and by proteinase K (0.2 mg/mL) but not altered by DNase treatment of the CCS (data not shown). There was no significant increase in total IgG, IgA or IgM in the culture supernatants after CCS stimulation (Fig. 3b), which was in contrast to significant increases in all three isotypes of antibodies induced by a positive control stimulus (polyclonal B cell activator CpG-ODN) (Fig. 3b). Low levels of antigen-specific IgA, but no IgM were detected in some cultures after stimulation (data not shown). CCS derived from isogenic mutants of pneumococci deficient for CbpA (CbpA-) or for pneumolysin (Ply-), induced no significant antigen-specific antibody to the respective proteins (Fig. 3c). Co-incubation of recombinant CbpA or pneumolysin (1 and 0.1 μg/mL, respectively) with CCS derived from the respective deficient strain with adenoidal MNC partially restored antigen-specific antibody responses, while medium containing the same concentrations of the recombinant proteins alone induced no detectable antigen-specific antibodies (Fig. 3c). Adding appropriately diluted pneumococcal culture medium to cell culture medium alone or with recombinant antigens did not induce any antibody response (data not shown).
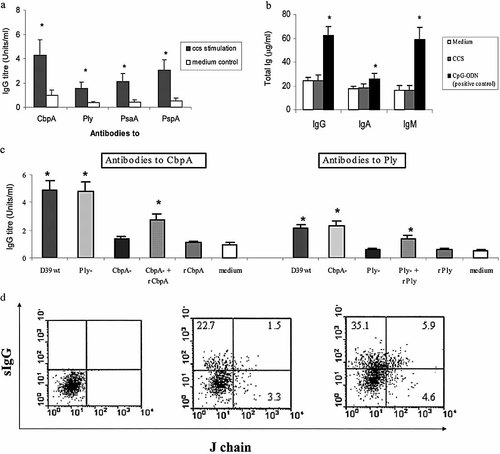
(a) IgG antibodies to pneumococcal proteins CbpA, Ply, PsaA and PspA in adenoidal MNC cultures in 30 patients after stimulation with a CCS derived from type 2 pneumococci (D39). Results are geometric mean titers with 95% CI (error bars). *p<0.001 compared to unstimulated control. (b) Total IgA, IgG and IgM concentrations in adenoidal MNC culture after stimulation for 8 days with CCS or CpG-ODN. *p<0.05, compared with medium control. (c) Anti-CbpA and -Ply antibodies after stimulation with CCS derived from D39, isogenic mutant strains (CbpA- or Ply-), the same mutant CCS with recombinant (r) CbpA or Ply (final concentrations 1 and 0.1 μg/mL), respectively, or recombinant proteins only (1 and 0.1 μg/mL, respectively); medium: unstimulated control (containing pneumococcal culture medium only). Data represent means + SD of four experiments. (d) FACS dot-plots showing J chain expression in sIgG+ cultured adenoidal immunocytes. (Left) isotype control, (middle) unstimulated MNC, (right) MNC stimulated with CCS for 8 days. Figures denote percentages of lymphocytes (gating typical events using forward/side scatter) in each quadrant. Data from one of three representative experiments are shown.
Flow cytometric analysis after dual staining for surface IgG and intracellular J chain showed that 23 ± 3.1% (mean ± SD) of the adenoidal MNC were surface IgG+ immunocytes, among which a small percentage co-express J chain before stimulation. Both J chain-expressing and non-expressing IgG+ cells were significantly increased in number compared to unstimulated controls, after stimulation with pneumococcal culture supernatant (day 7–8 of culture) (Fig. 3d). Staining IgG+ immunocytes in peripheral blood mononuclear cells (PBMC) from the same patients did not show J chain co-expression (data not shown).
Pneumococcal colonization is associated with increased in vitro antibody production in adenoidal MNC
Concentrations of antigen-specific IgG antibodies to all four protein antigens in adenoidal MNC cultures from children with positive nasopharyngeal cultures for S. pneumoniae after in vitro stimulation with type 2 pneumococcal (D39) CCS were significantly higher than those from children who were culture negative (Fig. 4, p<0.01). No significant differences were found between the total IgA, IgG and IgM concentrations produced in vitro by adenoidal MNC cultures from colonized and uncolonized children (data not shown). There were no age-related trends in antibody concentrations detected in cell cultures after CCS stimulation (data not shown).
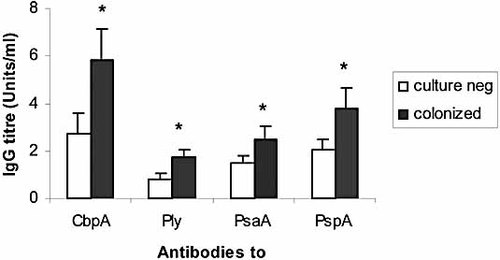
Anti-CbpA, Ply, PsaA and PspA IgG antibodies in adenoidal MNC cultures after stimulation with CCS derived from wild type (D39) in 12 colonized and 20 uncolonized subjects. Data represent geometric means and 95% CI (error bar) of antibody levels. *p<0.05 compared with those who were culture negative.
Discussion
This study provides several important new insights into local immunity to pneumococci in children and its relationships with systemic immunity and carriage status. Pneumococcal colonization of the nose is common in young children, and becomes rarer as they approach school age 29, 30. We provide the first data on naturally acquired human mucosal immunity to several pneumococcal antigens in this age group. We show that secreted pneumococcal proteins induce significant antigen-specific antibody responses in adenoidal cells in vitro to CbpA, Ply, PsaA and PspA, especially in those children who were colonized with pneumococci. We also show that the serum and salivary antibody levels to both CbpA and Ply in children who were culture negative for pneumococci were significantly higher than those carrying the organism. These results suggest that adenoids may be important induction sites for pneumococcal protein antigen-specific antibody responses and that antibodies to CbpA and Ply in serum and at the local mucosal level (saliva) may be protective against carriage of pneumococci.
In this study, we studied children aged over 2 years, spanning the age range in which decline in carriage rates occur when, presumably, natural immunity is acquired 23. The study group comprised children undergoing adenotonsillectomy, but the rates and downward trend with age of nasal colonization were similar to those reported previously in healthy children by us and others 29–31. Further, the trends with age in levels of serum IgG antibodies to the pneumococcal protein antigens in this group resembled trends we observed in several sets of sera from healthy UK children (Fig. 1). Mean serum antibody levels to all four proteins in 3-year olds (the only age group for which sera were available both for study children and controls) were closely similar in both groups (Fig. 1, boxes). Taken together, these observations suggest that our study group is representative of children in the general population for the parameters we studied, and that antibodies to all four antigens emerge with age.
In both study subjects and healthy controls, there was a noticeable difference between age of acquisition of serum IgG anti-PsaA antibodies and antibodies to the other three antigens tested. In healthy children, sera taken at the age of 5 months already had higher levels of anti-PsaA antibodies than at 2 months (while levels for the other antigens fell, presumably reflecting loss of transplacentally acquired antibodies). Unlike the others, no further rise in anti-PsaA antibodies was seen after the age of 2 years. Studies in Finnish children have also shown early acquisition of detectable serum anti-PsaA antibodies and progressive rises in anti-Ply and -PspA antibodies over the first 24 months of life 13 and a closely similar pattern was also observed in a seroprevalence study in Kenya 32. Both those studies and ours suggest either that in pneumococcal colonization, PsaA is more immunogenic than other antigens in the first year of life, or that anti-PsaA antibodies are induced by another non-pneumococcal cross-reacting antigenic stimulus.
Detectable levels of serum antibody to all four antigens were found in nearly all samples from the studied children. This presumably reflects the near-universality and high immunogenicity of previous pneumococcal colonization by the time children reach adenotonsillectomy. However, there were differences between children colonized with pneumococci and those who were culture-negative at the time of surgery. For all four antigens tested, for serum and salivary IgG and salivary IgA antibodies, the observed trend was for culture negative children to have higher antibody levels. These apparent differences were statistically significant for anti-CbpA and anti-Ply IgG antibodies in both blood and saliva. The significance was independent of age differences between culture-positive and –negative groups, and appears to be principally due to differences in levels of antigen-specific IgG1 (Fig. 2d). These suggest that anti-CbpA and/or anti-Ply antibodies are protective against carriage in children aged 2 and older. Investigators in Finland, studying children aged up to 2 years, found higher serum anti-PspA, anti-Ply and anti-PsaA levels in individuals who had previously had pneumococci isolated 13. Unlike ours, their analysis was by previous colonization, not colonization at the time that serum was collected. In subsequent detailed studies of anti-PsaA antibodies, the same group also showed that higher concentrations of serum anti-PsaA antibodies were associated with lower risk of pneumococcal carriage subsequently progressing to otitis media after the age of 9 months, although in younger infants the higher titers were associated with higher risk of colonization 21. The only other published data on serum anti-pneumococcal protein antibodies and carriage in children are from West African infants aged 3–5 months and showed lower titers of anti-PsaA antibodies in colonized children 33. As in our study, sera and nasal swabs were all taken at the same time. McCool et al. 19 produced evidence for a protective role for anti-PspA antibodies against colonization following experimental nasal inoculation of adults with pneumococci, but also demonstrated that the immunogenic region of PspA shows significant strain to strain variation and instability of expression. These factors, or age differences between studies may explain the lack of significant differences in our study between anti-PspA antibody levels in colonized and culture-negative children. McCool et al. 20 also demonstrated induction of both anti-PspA and anti-CbpA serum antibodies by experimental colonization. Notwithstanding the obvious differences in age, population and design among these several studies, it is possible to conclude that infection of the upper respiratory tract by pneumococci induces anti-protein antibody responses some of which are protective. It also appears that there may be important differences between antigens and changes in the induction and function of this immunity with age, as well as exposure.
The moderate correlation between individual serum and salivary concentrations of antibody, as seen for three of the four antigens tested in this study, is often taken as supportive evidence that the salivary IgG antibodies are derived, to some extent from serum 34. However, variations that are commonly observed, with for example some individuals with unexpectedly high salivary antibody concentrations to one or more antigens but low serum concentrations, raise the possibility that the antibody levels detected in the saliva samples are at least partly locally produced. Since IgG-producing plasma cells are scarce in salivary glands, and it has been shown that that immunocytes, especially IgG-secreting cells, are present in significant numbers in surface secretion on adenoids) 35, 36, this may represent an additional source of immunoglobulin in local mucosal secretions including saliva. We have previously reported that adenoids commonly contain significant numbers of antibody-secreting cells to the pneumococcal protein antigens studied here 28. Another possibility is that contact between the sponge swab and the oral mucosa increased passive transudation from serum to a variable extent, thus explaining the unexpectedly high concentrations of IgG to some antigens in some individuals’ saliva. However, measurement of albumin concentrations in saliva and serum samples and comparing these values to antibody concentrations did not support this hypothesis.
To explore whether local mucosal lymphoid tissues play a role in the induction of pneumococcal protein-specific antibodies, MNC from adenoids obtained from these children have been studied in vitro to examine the relationships between carriage, antigen stimulation and antibody production. Exposure of these cells to pneumococcal antigens induced antibody production to all four pneumococcal antigens which varied in quantity substantially between subjects but, on average, increased threefold or more under these experimental conditions (Fig. 3a). Again, this induction was not simply a reflection of polyclonal B cell stimulation (Fig. 3b) but of strictly antigen-specific responses (Fig. 3c), which showed a similar IgG subclass distribution to that seen in serum (Fig. 2d), suggesting that responses in this model do resemble those induced by in vivo interactions between colonizing pneumococci and nasopharyngeal immune cells. The predominance of IgG1 and IgG3 with paucity of IgG2 is typical of anti-protein responses. Both IgG1 and IgG3 are likely to be functional against pneumococcal infection as both subclasses can opsonize bacteria and activate complement, thereby resulting in clearance. The relative abundance of IgG4 responses are of note, given the relatively low numbers of IgG4-secreting cells in adenoids 37, but are in accordance with reports of clinical significance of IgG4 deficiency 38, raising the possibility that this may relate not only to anti-capsular 39 responses, but also anti-protein responses. Studies using CbpA and Ply mutants showed that, although the specific antigen was necessary to induce the response, it was itself not sufficient, since using purified recombinant protein alone (rCbpA or rPly) at a range of different concentrations did not induce significant antibody responses. It is possible that the native pneumococcal proteins in the CCS may be complexed, which may affect their uptake, processing and presentation. Nevertheless, our results indicate that one or more additional components of pneumococcal culture supernatants may be necessary to induce antibody production in this model. This putative adjuvant activity could have implications for the immunogenicity of future vaccines containing one or more of these pneumococcal protein antigens. Preliminary results in our laboratory show that pneumococcal culture supernatant, but not pneumococcal culture medium, can enhance the production in vitro of antibody to tetanus toxoid in adenoidal cells (data not shown). Further characterization of this activity is the subject of ongoing studies by our group.
Adenoids generate B cells of both mucosal and systemic phenotype 40, and thus may contribute to both local mucosal and systemic immunity. Mucosal B cells from adenoids home preferentially to mucosae and glands of the upper airways 41. Although the phenotype of the antibody-secreting cells that generated the IgG detected in our cultures was not determined, dual staining of immunocytes for IgG and immunoglobulin J chain showed that some IgG+ immunocytes, from adenoids but not from PBMC, co-express J chain (Fig. 3d), a presumed marker of mucosal phenotype of antibody-secreting cells 40, and that this dual expression was up-regulated by pneumococcal CCS stimulation. The fact that both J chain-expressing and non-expressing IgG+ cells were significantly increased in number after stimulation provides supporting evidence that adenoids may be important local induction and effector sites for both mucosal and systemic antibody production to pneumococcal proteins.
Given our initial general findings of higher serum antibody levels and significantly higher anti-CbpA and -Ply IgG in culture-negative subjects, it appears at first surprising to find significantly higher mean in vitro production of IgG antibodies to all four antigens in adenoidal MNC from colonized subjects than culture-negative subjects (Fig. 4). Again, this was not a generalized B cell polyclonal stimulation, nor just a reflection of age. However, both systemic and local mucosal-specific immune responses may regulate acquisition, duration and elimination of mucosal carriage of pneumococci and may exert their effects independently. High antecedent levels of antibodies may help prevent colonization, and enhanced memory-type local immune responses following re-exposure may help terminate it. The association between higher serum anti-CbpA and anti-Ply IgG levels and lower nasopharyngeal carriage supports the former; the ability of adenoidal MNC to produce higher levels of antibodies in vitro after antigen stimulation in colonized patients supports the latter, and the two are not mutually exclusive. The results of these in vitro experiments confirm that adenoids can be both important induction and effector sites for pneumococcal protein antigen-specific antibody production.
To date, studies in children have relied upon close observations of events that occur in association with natural colonization, since experiments involving deliberate exposure of children to pneumococci have not been undertaken. Since carriage in young children is common, this approach is feasible. Additional studies, including investigation of the regulatory mechanisms of local mucosal responses, determination of serological markers of B and T cell-mediated protection against carriage in longitudinal studies, and quantitative measurements of the density of nasal colonization over time, will be required to elucidate further the functional importance of these antibodies.
Materials and methods
Subjects and samples
Adenoids were obtained from children aged 2–12 years (median age 5) suffering from adenoidal hypertrophy who underwent adenoidectomy at the Royal Hospital for Sick Children, Bristol, UK. Patients who were taking antibiotics within 2 weeks prior to the operation were excluded from the study. Before operation, samples of saliva were collected by soaking a sponge in the mouth as described previously 34. Immediately before operation, peripheral venous blood and a nasopharyngeal swab (under general anesthesia) were taken for microbiological and immunological studies. The study was approved by the South Bristol local research ethics committee (LREC number E5142) and written informed consent was obtained in all cases.
Serum samples from healthy controls at 2, 5 and 13 months, 3–4 years, 13–17 years (30 samples for each age group) were used to establish reference ranges for serum antibodies to pneumococcal proteins. These samples were from individuals involved in recent, unrelated vaccine trials. Ethics approval and appropriate consent for this use of samples was obtained in all cases. All subjects and controls had had no previous immunization with any pneumococcal vaccine.
Bacterial culture of S. pneumoniae
Nasopharyngeal swabs (with calcium alginate tip) (Medical Wire & Equipment Co. Corsham, UK) were placed in tubes containing 1 mL skim milk-trypone-glucose-glycerin (STGG) broth 42, which was then immediately transported to the laboratory in a cold box (4–8°C) and stored at –70°C until bacterial culture. After vortex mixing, 50 μL of the inoculated broth was added onto a blood agar plate, using a standard inoculation technique. After overnight incubation at 37°C, 5% CO2, colonies of pneumococci were identified by typical morphology and α-hemolysis and subculture with standard optochin disc testing.
Isolation of MNC from adenoids
Adenoids were transported in minimum essential medium (Sigma, Dorset, UK) supplemented with glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL) and amphotericin B (1 μg/mL) to the laboratory and processed within 1 h after operation following the methods described previously 28. Briefly, adenoid tissue was minced using a sterile scalpel and teased with the help of a sterile steel mesh to release cells into the medium. The cell suspension was passed through a Nylon mesh (30 μm). MNC were isolated using Ficoll (Ficoll-Paque Plus, Pharmacia Biotech, UK) gradient centrifugation (400 × g for 30 min). Cells were washed in PBS and resuspended in RPMI medium supplemented with HEPES, penicillin, streptomycin and 10% fetal bovine serum (FBS) (Sigma). The viability of the cells was assessed by trypan blue dye exclusion and was consistently >99% viable.
Pneumococcal culture supernatants and recombinant proteins
Pneumococcal strains used in this study included a standard encapsulated type 2 (D39), an isogenic CbpA-deficient mutant (kindly provided by Dr Jeff Weiser, Philadelphia, PA), and an isogenic Ply-deficient mutant strain 43. Each strain was cultured in Todd-Hewitt broth (Oxoid, Basingstoke, UK) supplemented with 0.5% yeast extract and grown in 5% CO2 at 37°C to exponential phase (OD 0.4 at 620 nm, approx 108 cfu/mL). After centrifugation (3000 × g for 30 min), the culture supernatant was removed and passed through a 0.2-mm sterile filter and concentrated (tenfold) using a Vivaspin concentrator (Vivascience, Hannover, Germany). The protein concentrations of the CCS were determined using the Bio-Rad protein assay following the manufacturer's instructions (Bio-Rad, Hemel Hempstead, UK). Western blots of the CCS using specific antisera to CbpA, Ply, PsaA and PspA confirming the presence of these proteins, and further blots confirming the absence of CbpA or Ply in the CCS derived from the respective mutant strains were performed (Fig. 5) following methods described previously 28. These CCS were subsequently used at a predetermined protein concentration of 1 μg/mL in cell stimulation experiments.
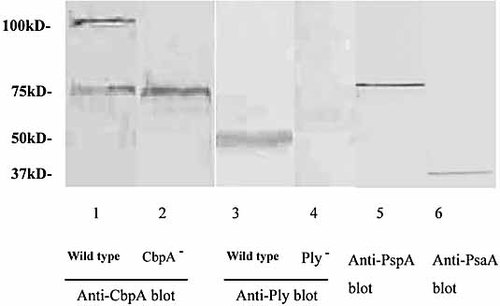
Western blots of CCS derived from wild-type 2 (D39) pneumococci visualized using mouse antiserum to CbpA (lane 1), Ply (lane 3), PspA (lane 5) and PsaA (lane 6). Lanes 2 and 4 showed the absence of CbpA and Ply, respectively, in CCS derived from isogenic CbpA- and Ply-deficient mutant strains. CbpA:∼110 kDa, Ply: ∼53 kD,. PspA:∼75 kDa, PsaA:37 kDa. Anti-CbpA serum cross-reacts with PspA at 75 kDa.
Recombinant proteins used for cell stimulation in this study include recombinant Ply 44, 45 and CbpA 46. The endotoxin levels of these recombinant proteins were <0.01 ng/μg of protein as determined by limulus assay (BioWhittaker, Walkersville, MD). A range of different concentrations of each protein (rCbpA: 0.1–10 μg/mL, and rPly: 0.01–1 μg/mL) was tested to find out the optimal dosage of stimulation. In some experiments, the pneumococcal CCS was incubated with DNase I (0.1 mg/mL) for 1 h at 4 C, or treated with proteinase K (200 μg/mL) for 1 h at 37°C followed by heat-treatment at 98°C for 30 min before cell stimulation.
Antigens used for assaying pneumococcal protein-specific antibodies by ELISA were also recombinant proteins including PspA, PsaA, CbpA and Ply toxoid 46–49. Ply toxoid has a Trp433→Phe mutation reducing cytotoxicity, but it retains full immunogenicity 49. The recombinant CbpA was the N-terminal fragment corresponding to the amino acids 1–445 of the mature CbpA (PspC) polypeptide 3, 46. CbpA (PspC) has been reported to induce protective antibodies cross-reacting with PspA 3. These recombinant proteins were expressed and purified from recombinant Escherichia coli expressing the respective cloned gene 46–49. The original source for each gene was the encapsulated type 2 pneumococcal strain, D39.
Cell culture
MNC isolated from adenoid tissue were resuspended at 4 × 106/mL in RPMI medium containing glutamine, penicillin, streptomycin and 10% FBS. Cells were cultured either in 96-well culture plates or 24-well plates (Corning Inc, Corning, USA) in the presence/absence of different stimulants (pneumococcal CCS, recombinant protein antigens). Cells were cultured for up to 8 days. Cell culture supernatant was collected and stored at –70°C until assay.
Flow cytometry
Adenoidal lymphocytes were analyzed for intracellular expression of immunoglobulin J chain in IgG+ immunocytes using dual staining flow cytometry. Briefly, adenoidal MNC were first incubated with cell membrane-permeabilizing solution (BD Bioscience, Oxford UK) for 10 min at room temperature, followed by incubation with mouse monoclonal anti-human J chain antibody (Serotec, Oxford, UK) or mouse IgG1control antibody (BD Bioscience) for 30 min at 4°C. Cells were subsequently stained with FITC-conjugated anti-mouse antibody (Sigma). The cells were then washed three times before staining of surface IgG by incubation with PE-labeled anti-human IgG (Serotec) for 30 min at 4°C. Finally, cells were analyzed by flow cytometry (FACScan, Becton Dickinson, UK).
ELISA for anti-pneumococcal protein antibodies
Cell culture supernatants collected on day 8 were assayed for anti-PspA, Ply, PsaA and CbpA antibodies following methods described previously 28. Briefly, 96-well Costar plates were coated with each pneumococcal protein (2 μg/mL). After blocking with 10% FBS in PBS (FBS-PBS) at 37°C for 1 h, cell culture supernatants diluted in 10% FBS-PBS were added and incubated at room temperature for 2 h with rocking on a horizontal shaker at 150 rpm. After washing, alkaline phosphatase-conjugated goat anti-human IgA, IgG or IgM was added with incubation for 2 h at room temperature. p-Nitrophenyl phosphate (PNPP) was added, followed by incubation at room temperature for 30 min. OD at 405 nm was measured immediately using a microtiter reader (Bio-Rad, UK). Human immunoglobulin (Sandoglobulin, Sandoz, UK), which contains high IgG antibody titers to CbpA, Ply, PsaA and PspA, was used as a reference IgG standard for measurement of IgG antibodies and was assigned an antibody titer of 1000 U/mL.
The IgG subclasses (IgG1, IgG2, IgG3 and IgG4) of antibodies to CbpA, Ply, PsaA and PspA were also analyzed by immunoassay. Briefly, microtiter plates were coated overnight with individual antigens as described above. Antigen-coated plates were blocked with 10% FBS-PBS, followed by incubation of diluted serum samples (1:200 in 10% FBS-PBS) at room temperature for 1.5 h. After washing, murine monoclonal anti-human IgG1, IgG2, IgG3 or IgG4 (SkyBio, Wyboston, UK) (all 1:1000 in 10% FBS-PBS) were added, respectively, to designated plates and incubated for 1.5 h at room temperature. Alkaline phosphatase-conjugated goat anti-mouse IgG (Jackson Laboratories, West Grove, USA) was subsequently added and incubated for 1.5 h at room temperatrue. After washing, substrate PNPP was incubated for 30 min at room temperature and OD were read at 405 nm using the microtiter reader.
Anti-PspA, -Ply, -PsaA and -CbpA antibodies in saliva (for IgA and IgG) and serum (for IgG) were measured similarly, except that the dilutions for saliva and serum were 1:30 and 1:200–400, respectively. For antigen-specific salivary IgA antibody level, arbitrary units (AU;OD 405 nm ×1000) were used.
The specificity of the ELISA measuring anti-pneumococcal protein antibodies was confirmed by antigen-specific inhibition assays following methods described previously 34. Briefly, each recombinant protein (rCbpA, rPly, rPsaA, rPspA) was added at 0.1–10 μg/mL to the sample to be assayed (i.e., adenoidal MNC culture supernatant or serum) to adsorb the specific antibody present in each sample for 30 min at room temperature, followed by a standard ELISA procedure. The percentages of inhibition of antibody activity after adsorption with specific antigen (10 μg/mL) for all four antigen-specific antibodies were all greater than 95% (data not shown).
There was no cross inhibition of detection of IgG antibody to each of the four antigens after adsorption with different recombinant proteins except that anti-CbpA cross-reacted with rPspA, as shown by Western blotting (Fig. 5) and detection was inhibited by 10–28% after rPspA adsorption. However, there was no inhibition of anti-PspA IgG antibody detection following adsorption with rCbpA.
Measurement of total IgA, IgG and IgM
Production of total IgG, IgA and IgM by adenoidal MNC was measured after stimulation by pneumococcal CCS or an unmethylated CpG-ODN (PS2006, TCGTCGTTTTGTCGTTTTGTCGT) 50 (Qiagen-Operon, Crawley, UK) used as a positive control. Cells were cultured in the presence of CCS (1 μg/mL) or CPG-ODN (1 μg/mL) for 8 days and culture supernatant was collected and stored at –70°C until immunoassay. Briefly, microtiter plates were coated with goat anti-human IgA or IgG or IgM (Dako, Glostrup, Denmark) in PBS and incubated overnight at 4°C. After blocking with 10% FBS-PBS, diluted cell culture supernatant (1:200) along with IgA, IgG and IgM standard (Sigma) were added and incubated at room temperature for 2 h. Alkaline phosphatase-conjugated anti-human IgA, IgG and IgM (Sigma) (1:2000) was added after washes and incubated for 2 h at room temperature. After further washes, PNPP substrate was added and the plates incubated at room temperature for 15 min. OD were read at 405 nm, and the concentration of each sample was calculated against the standard curve.
As salivary flow rates are known to vary between different individuals and in the same individual under different conditions 51, total salivary IgA and IgG were measured as described previously 34, so that the ratio of protein-specific IgA or IgG to total IgA or IgG could be calculated for each individual subject as a method of compensating for dilution.
Measurement of albumin concentration in saliva and serum
Albumin concentration were measured both in paired serum and saliva samples using a QuantiChrom albumin assay kit (BioAssay Systems, Hayward, CA) following the manufacturer's instructions.
Statistical analysis
Log transformation of data was used before analysis by parametric statistical methods. Differences between two groups (i.e., colonized and uncolonized) were analyzed by Student's t-test. The general linear model of analysis of variance (ANOVA) was used to analyze the effects of age and carriage status on the antibody levels. Differences between stimulated and unstimulated cell samples in a specified group of subjects were analyzed by paired sample Student's t-test. A p value of <0.05 was taken to indicate statistical significance. Analysis was performed using SPSS software version 11.
Acknowledgements
We are grateful to members of the ENT department of Bristol Royal Hospital for Children for assisting in the collection of samples and to the patients who took part in the study and their families. We also thank Prof. Jeffrey Weiser at the University of Pennsylvania of Philadelphia, USA for kindly providing the pneumococcal strains D39 and the CbpA-deficient mutant, Dr. John Leeming in the Department of Microbiology, Bristol Royal Infirmary for technical advice and help, Dr. Linda Hunt in the institute of Child Health of University of Bristol for advice on statistical analysis, and Prof. Howard Jenkinson in the Department of Oral and Dental Medicine and Prof. Rob Heyderman in the Department of Pathology and Microbiology of the University of Bristol for critical reading of the manuscript. We acknowledge the financial support from the David Telling Charitable Trust, the Special Trustees of the United Bristol Healthcare Trust and the North Bristol NHS Trust Research Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




