Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro
Abstract
Tolerogenic activity of myeloid dendritic cells (DC) has so far been attributed mostly to immature or semi-mature differentiation stages but never to their precursor cells. Although myeloid suppressor cells (MSC) have been isolated ex vivo, their developmental relationship to DC and their precise phenotype remained elusive. Here, we describe the generation of MSC as myeloid DC precursors with potent suppressive activity on allogeneic and OVA-specific CD4+ and CD8+ T cell responses in vitro. These MSC appear transiently in DC cultures of bone marrow (BM) cells after 8–10 days under low GM-CSF conditions or after 3–4 days under high GM-CSF conditions. They represent CD11c– myeloid precursor cells with ring-shaped nuclei and are Gr-1low (i.e. Ly-6C+, Ly-6Glow), CD11b+, CD31+, ER-MP58+, asialoGM1+ and F4/80+. Sorted MSC develop into CD11c+ DC within 6 days. Their suppressor activity partially depends on IFN-γ stimulation. Suppression is mediated through mechanisms requiring cell contact and nitric oxide but is independent of TNF, CD1d and TGF-β. Together, our data describe the generation of MSC with distinct suppressor mechanisms in vitro preceding their development into immature DC.
Abbreviations:
-
- highGM:
-
high doses of GM-CSF
-
- IDO:
-
2,3-indoleamine dioxigenase
-
- L-MMA:
-
L-monomethylarginine
-
- lowGM:
-
low doses of GM-CSF
-
- MSC:
-
myeloid suppressor cell
-
- NO:
-
nitric oxide
Introduction
DC are not only potent inducers of adaptive immune responses but also mediators of tolerance. Initial work suggested that certain subsets of exclusively tolerogenic DC might exist, while other subsets are immunogenic. Meanwhile, it is becoming more evident that all subsets identified so far can act immunogenic or tolerogenic depending on the signals derived from self or foreign antigens influencing their maturation state and the expression of cell surface (e.g. ILT-3) or secreted (e.g. IL-10 or 2,3-indoleamine dioxigenase, IDO) tolerogenic molecules 1–4. Resting or steady-state DC exert tolerogenic activities, and similar types of DC can be generated in vitro for successful application in animal models. These have been termed immature, modified or semi-mature DC 5. In contrast, little information is available about the functional capacities of DC precursor cells.
Myeloid precursor cells have been isolated from mice and could be shown to be involved in the suppression of T cell immune responses. A population of Gr-1+CD11b+ myeloid suppressor cells (MSC) appeared in the spleens of mice injected with tumor cells that were transfected with the GM-CSF gene 6. Depletion of Gr-1+ cells recovered the CTL function 7. Isolation of Gr-1+ cells from the spleen and culture under different conditions resulted in their differentiation into inhibitory macrophages or stimulatory DC 8. Others have shown that Gr-1+CD11b+ cells can be induced by IL-13 to secrete TGF-β and thereby suppress CTL activity in a murine tumor model 9. Finally, such human GM-CSF-dependent suppressor cells might exist and seem to be induced by certain human tumors secreting GM-CSF 10. So far, MSC have been analyzed only ex vivo, and their phenotype, origin and function were not well characterized.
Previously, we have shown that immature DC can induce MHC II-restricted T cell anergy and, when injected prior to an allogeneic heart transplantation, can prolong the allograft survival from 8 days (in untreated mice) to more than 100 days 11. Others have shown a prolongation from only 8 days to 24 days in the same model 12. The difference between the experimental settings responsible for the extended tolerogenic potential of our immature DC has been attributed to their maturation-resistant state, in contrast to the cells used by others, which ensures their continued immaturity after injection. This resistance of DC to maturation following LPS, TNF or CD40 ligation occurs in cultures given low doses of GM-CSF (lowGM) in the absence of IL-4 13, but the precise mechanisms of the lowGM immaturity and the enhanced tolerogenic potential of the DC remained unclear.
We therefore investigated the non-DC fraction of such lowGM BM cultures. Our data revealed that in day 8–10 cultures under lowGM conditions, both immature DC and MSC are present. The same MSC phenotype appears in cultures treated with high doses of GM-CSF (highGM) after 3–4 days. MSC that develop under both conditions are able to suppress CD4+ and CD8+ T cell responses in vitro. Their suppressive activity is partially induced by IFN-γ and is mediated by cell contact and the secretion of NO.
Results
Day 10 lowGM and day 4 highGM DC cultures suppress CD4+ and CD8+ allogeneic and OVA-specific T cell responses in vitro
To test whether BM cell cultures can develop a suppressive activity, we added fresh BM cells or BM cells taken at different time points during low or high GM-CSF culture to an allogeneic MLR. The proliferation of T cells was completely suppressed when day 10 lowGM cells or day 4 highGM cells were added to the MLR, but cells cultivated for other time periods under the respective highGM and lowGM conditions and fresh BM cells were not suppressive (Fig. 1A). This indicates that the suppressor cell population is not present at all, is not present in sufficient numbers or is not functional in the fresh BM but needs to be generated or activated in culture by GM-CSF.
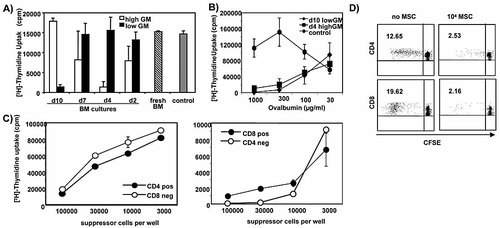
Suppression of allogeneic and antigen-specific T cell responses by day 4 highGM and day 8–10 lowGM BM DC cultures. A) BM cells were prepared from C57BL/6 mice and used freshly (hatched bar) or after culture under highGM (white bars) or lowGM (black bars) conditions for the indicated periods of time and then added as potential suppressor cells in triplicates (1 × 105/well) to an allo-MLR consisting of mature DC from C57BL/6 mice (1 × 104/well) as stimulators and lymph node cells from BALB/c mice (2 × 105/well) as responders. The control MLR shows the proliferation without adding a third potentially suppressive population (grey bar). Standard deviations of triplicate cultures are shown. Two experiments with similar results were performed. B) Day 4 highGM- and day 10 lowGM-cultured cells from C57BL/6 mice (1 × 105/well) were added to cultures of OT-II lymph node cells (2 × 105/well) with titrated amounts of OVA protein. Standard deviations of triplicate cultures are shown. Two experiments with similar results were performed. C) Separated CD8+ and CD4+ T cells from lymph nodes and spleens (BALB/c) were cultured with mature DC (C57BL/6) and titrated amounts of day 4 highGM cells. The proliferation of CD4+ and CD4– or CD8+ and CD8– cells from two separate experiments are shown within the same graphs. D) Suppression of CD4+ and CD8+ T cell responses in bulk culture. The lymph node responder cells were labeled with CFSE before the MLR. Separate CD4 and CD8 stainings at day 6 of cultures are shown. Two experiments with similar results were performed.
Similarly, the proliferation of OVA-specific TCR-transgenic OT-II T cells induced by mature DC in vitro could be suppressed with both day 10 lowGM and day 4 highGM cells (Fig. 1B). At low concentrations of OVA, the T cell proliferation was not suppressed, although the same number of day 10 lowGM or day 4 highGM cells was present in the cultures, excluding a nonspecific suppressive effect. The suppression improved with increasing doses of OVA in the culture, indicating an antigen-dependent mechanism of suppression.
An allo-MLR is a clearly Th1/CTL-polarized T cell response resulting in the release of IFN-γ but not IL-4 into the culture supernatants (not shown). Therefore, we addressed the question of whether IFN-γ-producing allogeneic CD4+ Th1 and CD8+ CTL can be suppressed equally well. Allogeneic CD4+ and CD8+ T cells were positively enriched by MACS technology and the positively and negatively selected cells stimulated with mature allogeneic DC in the presence of allogeneic day 4 highGM cells. While 1 × 105 suppressor cells were needed for complete suppression of proliferation by the CD4-enriched T cell population, the CD8-enriched fraction could be blocked by the addition of only 1 × 104 suppressor cells (Fig. 1C). The suppressive capacity on CD4+ and CD8+ T cells was further confirmed when CFSE-labeled bulk lymph node cells were used as responders and analyzed separately after 6 days (Fig. 1D). Here, the suppression of CD8+ T cells was only about 2× better than that of the CD4+ T cells, calculated as the ratio of the percentage of CFSE-diluted cells without/with MSC (ratio CD4+ cells, 5; ratio CD8+ cells, 9.08). It is of note that the T cells remain as CFSE+ undivided CD4+ cells (Fig. 1D) which do not bind Annexin V (not shown), indicating that they are not dying from apoptosis during suppression.
A subpopulation of Ly-6Glow cells with ring-shaped nuclei is suppressive
From our previous experiments, we knew that day 10 lowGM cultures contain MHC IIlow immature DC that are able to induce T cell anergy through their MHC II molecules in the absence of costimulation and MHC II– myeloid precursor cells 11. Now we wanted to dissect which cell type is responsible for the suppressive activity in the day 10 lowGM and day 4 highGM cultures, in contrast to the non-suppressive fresh BM cells. When a surface double staining for MHC II and the early myeloid and granulocyte marker Gr-1 was performed on the differentially cultured cells, two major populations could be detected after culture in GM-CSF. In the day 4 highGM cultures, predominantly Gr-1+ cells that were MHC II– appeared (Fig. 2A). Under lowGM conditions we found that in addition to the Gr-1+ cells, Gr-1–MHC IIlow immature DC also grew out (Fig. 2A), as we described before 11.
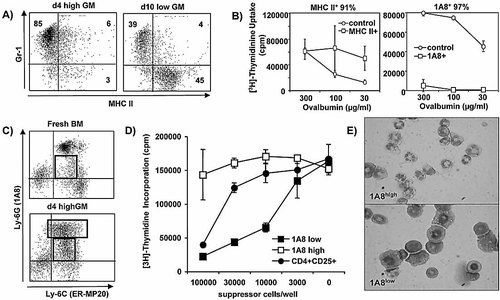
Within the day 4 highGM and day 10 lowGM BM cell cultures, only the Ly-6Glow Ly-6C+ cells are suppressive and show a ring-shaped morphology. A) FACS analysis of day 4 highGM and day 10 lowGM cells showing Gr-1 and MHC II surface expression. Six experiments with similar results were performed. B) Day 10 lowGM cells were separated by MACS into MHC II+ and 1A8+ cells and tested for their suppression of OT-II cell proliferation. Three experiments with similar results were performed. C) Further subfractionation of the suppressive day 4 highGM cells into Ly-6C+ (ER-MP20) Ly-6Glow and Ly-6Ghigh (1A8) cells by FACS sorting. Fresh BM cells and day-4 highGM cultures were stained for the indicated markers. Ly-6Glow cells only appear after culture. The rectangles in the lower dot plot show the gates used in (D). Three experiments with similar results were performed. D) Sorted 1A8low and 1A8high cells were compared with MACS-enriched CD4+CD25+ regulatory T cells in their suppressive capacity in an allo-MLR. The suppressive activity in day 4 highGM cells could be attributed to the 1A8low fraction and was about ten times higher than that of the regulatory T cells. E) May-Grünwald-Giemsa staining of cytospin preparations of the sorted subfractions from (C) revealed 1A8high granulocytes and 1A8low ring-shaped cells.
The Gr-1 antibody recognizes the two molecules Ly-6G and Ly-6C. Therefore, we also used antibodies recognizing the Ly-6G (1A8) and Ly-6C (ER-MP20) markers separately. Inhibition of 1A8 FACS surface staining by the Gr-1 antibody indicated that both antibodies are directed against the same overlapping Ly-6G epitope (not shown). Therefore, both antibodies were used equivalently in the following analyses. To functionally distinguish whether the immature DC or the myeloid precursors were responsible for the suppressive effect, the populations were separated with MACS beads directed against MHC II or Ly-6G (1A8). When titrated into the OVA-restricted presentation to OT-II T cells, only the 1A8+ cells were suppressive, while the immature DC, which have been shown to induce T cell anergy in other types of assays 11, 14, rather increased proliferation at suboptimal concentrations of antigen in this type of suppressor assay (Fig. 2B).
The Gr-1 staining always appeared with Gr-1high- and Gr-1low-expressing cells. To investigate which subset contributed to the suppressor activity, the day 4 highGM cultures were double stained for the markers Ly-6G (1A8) and Ly-6C (ER-MP20). The staining pattern revealed that 1A8high and 1A8low cells expressed equivalent levels of Ly-6C, and therefore the 1A8 and Gr-1 antibodies can be used equivalently and are sufficient to distinguish Ly-6Ghigh- or Ly-6Glow-expressing cells (Fig. 2C). When the two populations were sorted and tested for suppression of an allo-MLR, only the 1A8low cells were suppressive (Fig. 2D); the 1A8high population was not suppressive, even when added at different numbers to the allo-MLR. This lack of any effect of the 1A8high population on the MLR also excludes simple sterical hindrance or competition for the stimulator DC within the cultures. To further evaluate the suppressive strength of 1A8low cells, we magnetically sorted the subset of CD4+CD25+ regulatory T cells and titrated them into the allo-MLR. The results indicate that the 1A8high cells are about ten times superior to the CD4+CD25+ regulatory T cells in their suppressive potential. Morphological evaluation of cytospin H&E stainings identified the 1A8high cells as typical granulocytes, whereas the 1A8low cells resemble the myeloid “ring cells” described by others 15. These data indicate that MHC II–Gr-1/Ly-6Glow myeloid precursor cells with ring-shaped nuclei (and not immature DC or granulocytes) represent the suppressive cell population, further referred to as MSC.
MSC surface marker phenotype
To achieve a more precise description of the phenotype of the MSC, day 4 highGM cells were double stained for Gr-1 and a large panel of other markers and gated on the Gr-1low population (Fig. 3A). The analysis indicates that the MSC are early myeloid cells (CD31+, Ly-6C+, ER-MP58+, CD11b+) that have lost the very early hematopoietic marker CD34 and do not yet express further differentiation markers for monocytes (CD14), differentiated macrophages or their subsets (MOMA-1, CD169, FA11) or myeloid DC (CD11c, CD13, 33D1, CD205). Also, markers for T cells (CD4, CD8α), B cells/plasmacytoid DC (B220) or NK/NKT cells (NK1.1, DX5) were not expressed. Surprisingly, the typical NK/NKT cell marker asialoGM1 is expressed on MSC. Interestingly, only the macrophage marker F4/80, which has previously been associated with tolerogenic APC 16 is expressed on the MSC. The cells express FcγRII/III (2.4G2) but not CD14/TLR4 for antigen recognition and MHC I and CD1d but not MHC II for antigen presentation. Only the costimulatory molecules CD80 and CD54 are present on the cell surface (Fig. 3B). Expression of the homing receptor CD62L indicates that they might have the potential to enter lymph nodes from the blood through high endothelial venules. These data show that our in vitro-generated MSC might be identical to some MSC described in earlier reports 6–8, 17 but are different from suppressive cells reported by others 9, 18, the latter potentially representing other subsets of natural suppressor cells.
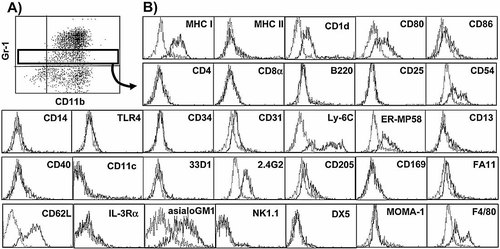
Surface marker expression on day 4 MSC reveal an early myeloid phenotype. A) Day 4 highGM cells (C57BL/6) were double stained for Gr-1 and CD11b. B) Histograms shown for the indicated markers (straight lines) and isotype controls (dotted lines) represent cells gated on Gr-1low expression. Six experiments with similar results were performed.
MSC are precursors of DC with transient suppressor activity
MSC seem to represent myeloid precursor cells, but the question remained whether they are terminally differentiated suppressive effector cells or they still bear the capacity to differentiate into other myeloid cells such as DC. Therefore, day 4 highGM cells were FACS sorted into 1A8low and 1A8high cells and further cultured under highGM conditions for 6 days. While unsorted BM cultures readily expanded (367% of cellular input), the 1A8low cells remained in the same range (78% of cellular input) (Fig. 4A). Sorted 1A8high cells could not differentiate into DC and rather declined in numbers (21% of cellular input). The FACS profile for surface B7-2 and CD11c double staining of unseparated and 1A8low cells after culture indicates that DC development occurred similarly well (Fig. 4A). The surface phenotype correlated with the morphology of DC clusters and single stellate cells appearing in the cultures (Fig. 4B). To test whether 1A8low MSC can also develop functionally into DC, the sorted and for 6 days-cultured 1A8low cells were used directly or after LPS maturation as stimulators of an allo-MLR. The results show that the T cell priming potential was comparable with that of normal BM DC (Fig. 4C). When the same sorted and cultured 1A8low cells were tested for their suppression of an allo-MLR, the data clearly indicate that the cultured cells lost their suppressive potential and the capacity to release nitric oxide (NO) (Fig. 4D). Taken together, 1A8low MSC can act suppressive but are not fully differentiated, as they still have the capacity to develop into fully functional mature DC. The suppressive state of MSC is also transient, as continued culture in GM-CSF leads to a loss of their suppressive activity, as already indicated in Fig. 1A.
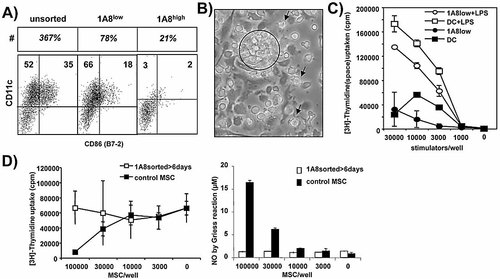
MSC represent precursors of DC with transient suppressor function. Day 3 highGM cells were FACS sorted into 1A8high and 1A8low cells as in Fig. 2C and cultured in GM-CSF for another 6 days. As a control the unseparated day 4 cells were cultured in parallel. A) At day 9 cell yields were counted (expressed as the percentage of input, #) and surface stained for CD11c and CD86. B) Morphology of typical DC cultures as observed by phase contrast microscopy with typical adherent DC clusters (circle) and individual spontaneously mature DC in suspension (arrows). C) 1A8low cells develop into functional DC. Sorted 1A8low cells cultured for 6 days or standard day 8 DC with and without LPS stimulation overnight were used as stimulators of an allo-MLR for 3 days. D) The suppressive potential of 1A8low cells is transient. Sorted 1A8low cells cultured for 6 days or normal day 4 MSC were titrated into an allo-MLR. At day 3 supernatants were tested for NO content or [3H]-thymidine was added overnight to measure proliferation. At least two experiments of each type with similar results were performed.
The MSC suppressive activity requires cell contact and NO but not TNF, CD1d or TGF-β
Several mechanisms of suppression have been attributed to distinct populations of MSC. TNF has been described to act as a suppressive mediator of MSC 19. However, MSC generated from TNF–/– mice fully suppressed an allo-MLR, as did their wild-type counterparts (not shown). MSC have also been described to release the suppressor cytokine TGF-β, which in turn can activate CD1d-restricted T cells further involved in the suppressive process 9. We found that suppression mediated by our MSC requires neither TGF-β, as shown by blocking antibodies in the culture (Fig. 5A), nor CD1d-restricted T cells, as MSC from CD1d–/– mice and their wild-type counterparts were equally potent suppressors (Fig. 5B). The suppression of C57BL/6 MSC that were allogeneic to the BALB/c responder T cells was slightly superior to that of BALB/c MSC (Fig. 5B). As MSC do not express MHC II molecules at the surface (Fig. 3), this may indicate that CD4+ T cells are suppressed antigen-unspecifically, while CD8+ T cells within these bulk lymph node cultures may partially recognize the MHC I molecules on MSC, indicating additional cell contact-dependent mechanisms of suppression.
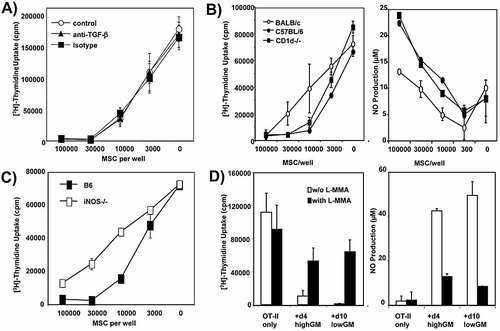
The suppression by MSC is mediated by NO production but not by TGF-β or CD1d. Day 4 highGM MSC were used. A) Blocking anti-TGF-β had no effect on the suppressive potential of MSC. Titrated amounts of MSC were added to an allo-MLR in the presence or absence of anti-TGF-β or an isotype control mAb (20 μg/mL each). Two experiments with similar results were performed. B) The higher suppression potential of allogeneic compared to syngeneic MSC correlates with NO production in the cultures. No altered suppression or NO production was observed for MSC from CD1d–/– mice. MSC derived from the indicted mouse strains were titrated into an allo-MLR mediated by BALB/c responder lymph node cells (4 × 105/well) and mature C57BL/6 DC (1 × 104/well). After 3 days proliferation was measured and culture supernatants tested for NO content. Three experiments with similar results were performed. C) MSC from iNOS–/– mice show impaired suppressor activity. MSC (1 × 105/well) from wild-type C57BL/6 or iNOS–/– mice were added to an allo-MLR mediated by BALB/c responder lymph node cells (4 × 105/well) and titrated amounts of mature DC (C57BL/6). The control MLR remained without suppressors. Two experiments with similar results were performed. D) Day 4 highGM and day 10 lowGM MSC both employ NO as a suppression mechanism. Reversion of suppression can be achieved by addition of the iNOS inhibitor L-MMA. OT-II lymph node cells (2 × 105/well) were cultured with 1 mg/mL OVA protein and cultured alone or in the presence of 1 × 105 MSC generated under the two indicated conditions. After 3 days proliferation was measured and culture supernatants tested for NO content. Four experiments with similar results were performed.
When MSC were generated from iNOS–/– mice, their suppressor activity was heavily impaired (Fig. 5C). Analysis of the MLR supernatants revealed that NO was absent in a control MLR without MSC, while supernatants with either day 4 highGM (Fig. 5B, D) or day 10 lowGM MSC contained NO with increasing numbers of MSC added to the cultures. The presence of NO correlated with the suppression of proliferation in the MLR and could be reverted by the NO inhibitor L-monomethylarginine (L-MMA) to the same extent (Fig. 5D). Thus, NO production is a major component of the suppressive activity mediated by MSC.
The suppressive activity of the soluble mediators requires cell contact
To evaluate whether the production of suppressive NO and TGF-β can act over a distance to the MLR or requires close contact of MSC to the DC and T cells, transwell experiments were performed. When MSC were added into transwell inserts with 5 μm pores on top of the MLR, still allowing active or random MSC transmigration through the pores, optimal suppression was observed (Fig. 6A) and correlated with the NO production in the cultures (not shown). However, when the transwell pores were only 0.4 μm wide, where cells could no longer transmigrate, the suppressive effect (Fig. 6B) as well as the NO production (not shown) was completely abrogated. This indicates that cell contact, most likely to the allogeneic T cells, is required to initiate the release of NO and perform the suppressive activity. These data indicate that MSC can actively or randomly migrate towards the allo-MLR, but this topic of MSC migration needs further investigation.
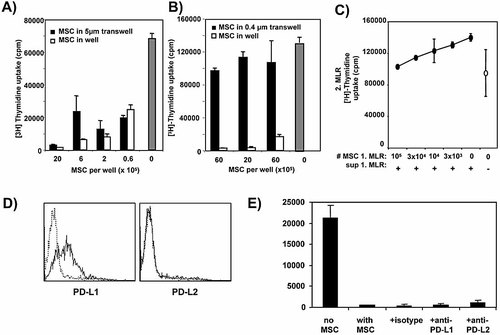
Suppression by MSC requires cell contact. MSC (C57BL/6) were added at titrated amounts into the upper chamber of a transwell system with 5 μm (A) or 0.4 μm (B) pore size and an allo-MLR performed in the lower chamber. Only the large (5 μm) pore system that allows cell penetration still showed suppression. Two experiments with similar results were performed. C) MLR supernatant alone does not suppress. The day 3 supernatants from a first allo-MLR suppressed by MSC were transferred into second allo-MLR (50% of final volume) or left without supernatant. After another 3 days, the proliferation of the second MLR was measured. Two experiments with similar results were performed. D) Surface staining of day 4 highGM MSC revealed the expression of the negative costimulator PD-L1 but not PD-L2. E) Suppression is independent of PL-L1 signals. An allo-MLR was performed and suppressed by addition of MSC. Further addition of the indicated antibodies to the suppressed MLR could not relieve the suppression.
To prove that soluble mediators are insufficient to block the allo-MLR, supernatants derived from a first day 3 MLR suppressed by MSC were immediately added to a second day 0 MLR without adding further MSC. The result indicates that the supernatant containing NO is not sufficient to inhibit proliferation (Fig. 6C).
As the negative costimulator PD-1, expressed on T cells, has been shown to be involved in immunosuppressive settings 20, we tested the expression of both PD-L1 and PD-L2 on MSC. While PD-L1 was expressed, PD-L2 could not be detected on MSC (Fig. 6D). Blocking of either ligand with antibodies during the suppression of an allo-MLR did not revert the suppressive effect of MSC (Fig. 6E), indicating that PD-1-mediated suppression is not involved.
Activation of the suppressor function of MSC is partially mediated by IFN-γ
Reports by others suggested that IL-13 or IFN-γ can activate MSC to release NO and/or TGF-β 21–23. Our findings that especially allogeneic Th1/CTL responses might be targets of the suppressor activity of the MSC indicate that IFN-γ might be involved in the activation of the MSC suppressor function. When we added IL-13 or IFN-γ or their combination to MSC, we could not detect any NO production, indicating that these cytokines alone might not be sufficient to activate MSC function (not shown). However, when an IFN-γ-blocking antibody was added to an MLR suppressed by MSC, we clearly found that IFN-γ contributes to MSC activation, and this also correlated with the NO released during the suppressed MLR (Fig. 7). Addition of 1-methyl-tryptophan to block potential IDO activity, which has been described to be activated by IFN-γ, had no effect on the MLR (not shown), excluding a role for IDO production by MSC interfering with tryptophane metabolism, as described before 24. Thus, the release of IFN-γ together with a cell contact-dependent signal can activate MSC.
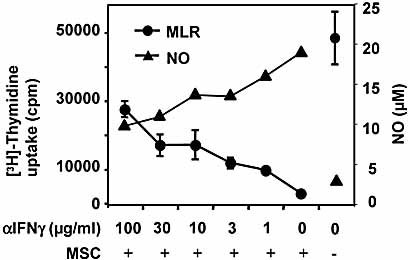
Induction of suppressive NO partially depends on IFN-γ release during the allo-MLR. An allo-MLR was fully suppressed by addition of MSC (1 × 105/well) or left without suppression. Titrated amounts of blocking anti-IFN-γ were given to the cultures and proliferation and NO measured after 3 days. Anti-IFN-γ reverted the suppression and reduced the amount of NO produced in the cultures. Two experiments with similar results were performed.
Discussion
In this study we describe for the first time the in vitro generation of MSC by a standard BM DC culture method. MSC have so far only been isolated and characterized ex vivo from mice and humans (reviewed in 25). Our findings here enabled us to unravel the MSC origin and DC lineage relationship, growth requirements, morphology, surface markers, activation and effector mechanisms. Our data indicate that a defined stage of myeloid DC precursors transiently acquires the capacity to act as suppressor cells of IFN-γ-dependent Th1 or CTL cell responses by NO production and close cell contact.
In the literature MSC have been mostly characterized by their expression of the β2-integrin CD11b and the early myeloid marker Gr-1, which recognizes the two molecules Ly-6C and Ly-6G. While CD11b is widely recognized as a marker for macrophages, Gr-1 is the prototype marker for murine granulocytes. However, both markers are also expressed on developing early myeloid cells 26. The overall phenotype of our in vitro-generated MSC is highly similar to MSC analyzed ex vivo by others 8, 17, 27 but less similar to CD11b–F4/80– suppressor NK cells, although they also express the alialoGM1 marker 28. Injection of anti-Gr-1 antibody into mice depletes not only granulocytes but also MSC as well as memory CD8+ T cells 29. Nevertheless, the application of anti-Gr-1 antibody has been associated mainly with ablation of the MSC suppressor activity on CD8+ T cells rather than the loss of CTL by depletion. Isolation of MSC and their maintenance under different culture conditions revealed that they can act as suppressors but can also stimulate CTL responses 8.
Currently, there is an enormous research focus on the potent suppressor activity of regulatory T cells 30, 31. Here we used the same type of in vitro suppressor assay for MSC that is commonly applied to test CD4+CD25+ regulatory T activity and was also used by our group previously 32. Regulatory T cells optimally suppress an allo-MLR in vitro at a 1:1 ratio to the effector T cell population. Typically, 105 cells of each T cell population is then stimulated by 5 × 103 mature allogeneic DC per well 32. Here we stimulated 4 × 105 bulk lymph node cells with 104 allogeneic mature DC and could fully suppress the T cell proliferation with only 1 × 104 to 3 × 104 MSC (Fig. 5A–C). These data indicate a much higher suppressive potential of MSC than observed for regulatory T cells in the in vitro allo-MLR suppression assay.
Our in vitro-generated MSC clearly employ the release of NO as one suppressive mechanism. As MSC do not express MHC II molecules but nevertheless suppress CD4+ T cells, the release of suppressive NO is not initiated by antigen-specific mechanisms. In vivo the NO release by MSC has been associated with GM-CSF production leading to MCS expansion, as demonstrated in tumor models 6, 33. This is in line with our data showing that GM-CSF is required for the generation of MSC from BM precursors. IFN-γ from activated T cells or other cellular sources then initiates their suppressor function, partially by NO release. NO has been shown in vivo to counteract CTL responses in tumor or transplantation models 22, 33–36.
Cell contact is a second requirement for suppression, as isolation of MSC by transwells abrogated the inhibition of proliferation and NO release, indicating that IFN-γ alone is not sufficient to activate the suppressive activity. In addition, the allo-MLR suppression of bulk lymph node cells by MSC is more prominent when allogeneic interactions with T cells can occur (Fig. 5B). This may be explained by MHC I-dependent interactions of CD8+ T cells within the bulk cultures with MSC, as observed in Fig. 1D. This potential MHC I-dependent mechanism of suppression needs further investigation. Interestingly, transwells that allow MSC transmigration seem to mobilize them to the site of the allo-reaction. However, in our assays a directed or random migration of MSC through the transwells cannot be distinguished. The additional expression of CD62L as a homing receptor for the lymph nodes will make further investigations of the chemokine response and migration of MSC in vivo very interesting.
We did not find that MSC can be activated by IL-13 to release TGF-β as a suppressive mechanism, as described by others 9, 23, indicating that IL-13 alone might not be sufficient for MSC activation, similar to the findings for IFN-γ in our studies. It is also possible that there are different MSC subsets with different activation requirements and effector mechanisms.
An increased occurrence of MSC in spleens of mice was initially found after injection of GM-CSF-transfected tumor cells 6, 33. Our data indicate that in vitro long-term low or short-term high doses of GM-CSF support the generation of MSC. Long-term cultures also give rise to immature DC in parallel after 8–10 days. At this time point, granulocytes have disappeared from the cultures. DC generated under lowGM conditions are also resistant to different maturation stimuli such as TNF, LPS or anti-CD40 11. The maturation resistance of DC is regulated by the absence or presence of IL-4 13. MSC appear under highGM conditions at day 3–4 in parallel with granulocytes but before reasonable numbers of CD11c+ DC develop. To generate MSC, isolated BM cells have to develop in the presence of GM-CSF; fresh BM cells are not suppressive (Fig. 1A). Indeed, after 3–4 days of culture under highGM conditions, a Gr-1/1A8lowLy-6C+ population that is responsible for the suppressive effect emerges; the 1A8high granulocytes and 1A8–CD11c+ immature DC are not suppressive. The suppressive activity of MSC is transient, disappearing after continued culture under highGM conditions (Fig. 1A and 4). Thus, GM-CSF first drives the generation of tolerogenic DC precursors (i.e. MSC) before immature or mature DC can develop. High doses of GM-CSF and IFN-γ are also produced by activated Th1, CTL, NK and NKT cells in vivo, which might lead to the generation of MSC and subsequent termination of T cell responses when concentrations of certain cytokines are exceeded. Such a phenomenon has also been observed with high-dose GM-CSF-transfected tumor cells in vivo 33.
Morphologically, our MSC show high similarities with “ring cells”, a distinct myeloid precursor cell population in mice 15. The increased appearance of “ring cells” in human blood has been observed in patients with myeloproliferative disorders 37–39, infectious mononucleosis 40 or severe alcoholism 41. A human correlate to the murine MSC described by Bronte et al. 6 might exist in patients with GM-CSF-secreting tumors as described for head and neck cancer, where the tumor progression is associated with the appearance of early suppressor cell populations 10, 42. It is tempting to speculate whether human MSC, similar to the MSC described here, are increased in certain diseases and may act there as suppressors, e.g. of anti-tumor immune responses mediated by CTL. However, a human counterpart to the murine MSC remains to be identified.
Taken together, we described the GM-CSF-dependent generation of MSC from BM precursors and found that IFN-γ is partially involved in the initiation of their transient suppressor function, while cell contact and NO mediate the suppression. So far, MSC have only been isolated from spleen, which made their detailed characterization difficult. Here we provide two methods to generate large numbers of MSC that can be transferred into mouse models of transplantation, autoimmunity and tumors.
Materials and methods
BM cell culture
The preparation and culture of BM cells from C57BL/6 and BALB/c mice (Charles River, Sulzfeld, Germany), TNF–/– mice (C57BL/6 background, kindly provided by H. Körner, Erlangen), CD1d–/– mice (kindly provided by L. van Kaer, Nashville, TN) and iNOS–/– mice (C57BL/6 background, kindly provided by A. Gessner, Erlangen) to generate DC has been described in detail before 43. MSC were generated using the same method as for generating BM DC 43 but were harvested at day 3 or 4 after highGM conditions and at days 8–10 under lowGM conditions 11. MSC were harvested as non-adherent cells from the cultures. Mature DC used as stimulators of allogeneic and transgenic T cells were generated by adding TNF or LPS overnight to day 8 BM cultures. As a source of GM-CSF, 10% of culture supernatant (highGM) from a murine GM-CSF-transfected cell line 44 or recombinant GM-CSF was used at 200 U/mL (i.e. 40 ng/mL, highGM) or 5 U/mL (lowGM) (Peprotech/Tebu, Frankfurt, Germany).
FACS analysis and sorting
FACS analysis was performed as described before 43. Cells were stained with FITC- or PE-conjugated mAb directed against Gr-1 or Ly-6G (1A8); FITC-conjugated CD1d, CD4, CD8α, CD11b, CD11c, CD14, CD25, CD34, CD40 (3/23), CD80, CD86 (GL1), IL-3Rα (CD123), Gr-1, DX-5, NK1.1, asialoGM1 (all BD Pharmingen, Hamburg, Germany), F4/80 (Serotec, Oxford, UK), TLR4, PD-L1 or PD-L2 (e-bioscience.com); hybridoma supernatants from the clones ER-MP12 (CD31), ER-MP20 (Ly-6C), ER-MP58, ER-BMDM1 (CD13) (kindly provided by P. Leenen, Rotterdam), FA11, Mel-14 (CD62L), 2.4G2, NLDC145 (CD205), MOMA-1, 33D1, B220, M1/42 (MHC I), J11d (CD24), ICAM-1 (CD54), N22 (MHC II) (all ATCC); or the appropriate fluorochome-conjugated mAb or supernatants as isotype controls at 2–5 μg/mL in PBS containing 0.1% sodium azide and 5% fetal calf serum for 30 min on ice in the dark. Secondary anti-rat or anti-hamster mAb were purchased from BD Pharmingen. Samples were washed once in staining buffer, measured and analyzed with a FACScan (Becton Dickinson, Heidelberg, Germany).
For some experiments BM cells were cultured under highGM conditions for 3 days and then stained for Ly-6G (1A8). 1A8low and 1A8high cells were sorted with a MoFlo high-speed sorter (Cytomation Bioinstruments, Freiburg, Germany). Sorted cells were then cultured as indicated.
Cytospins
Cells (2 × 105) were centrifuged in 200 μL medium onto a microscope slide using a Cytospin-3 (Shandon, Life Sciences International, Astmoor, UK) and stained with May-Grünwald-Giemsa dye.
T cell proliferation and suppression by MSC
Triplicates of 2 × 105 to 4 × 105 lymph node cells from BALB/c or OVA-specific TCR-transgenic OT-II mice (kindly provided by F. Carbone, Melbourne) as a source of responder T cells were seeded into a 96-well flat-bottom plate (Falcon) together with titrated numbers of mitomycin C-treated (50 μg/mL, Sigma; for 20 min at 37°C) spleen cells from C57BL/6 mice for the allo-MLR or titrated amounts of OVA protein (grade V, Sigma). Cell cultures were pulsed with 1 μCi/well [3H]-methyl-thymidine (Amersham) overnight for 16 h. The plates were harvested onto glassfiber filter mats with an IH-110 harvester (Inotech, Dottikon, Switzerland) and the filters counted in a 1450 Microplate Counter Wallac (Turku, Finland).
For some experiments CD4+ and CD8+ responder T cells were positively separated by MACS technology (Miltenyi, Bergisch-Gladbach, Germany), and both CD4 or CD8 positively and negatively selected cell subsets were used. For suppression of allogeneic or OVA-specific proliferation assays, 1 × 105 MSC were added if not indicated otherwise. For suppression with enriched MHC II+ or 1A8+ cells from day 4 highGM BM cultures, the purified antibodies were bound with a secondary anti-rat bead-conjugated antibody (MACS). In some experiments CD4+CD25+ regulatory T cells were used. Their separation was performed with the MACS separation kit. Both the regulatory T cells and the MSC were used directly without preactivation. NO production by MSC was inhibited by addition of L-MMA during the proliferation 45. At day 3 of proliferation, supernatants were tested for NO content by the Griess reaction as described 45. The blocking antibody against IFN-γ (BD Pharmingen) was added at the indicated doses simultaneously with the cells; anti-TGF-β (R&D Systems) was given at 20 μg/mL. Transwell inserts were used with 0.4 or 5 μm diameter pores (Costar) to allow diffusion of solutes only or additional cell migration, respectively.
Acknowledgements
This work was supported by the Marohn Foundation (S. R.) and the ELAN program (C. V.), both of the University of Erlangen, a grant (DFG LU 851/2–1) from the Deutsche Forschungsgemeinschaft (M. B. L. and C. W.) as well as the Graduiertenkolleg GK592 (J. H.). We thank G. Schuler for generous support and he as well as E. Kämpgen for critical comments on the manuscript. We are grateful to H. Körner, A. Gessner and L. van Kaer for providing us with gene-deficient mice and Peter Rohwer for cell sorting.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH