Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation
Abstract
Antibodies to the CD4-binding site (CD4bs) of HIV-1 envelope gp120 have been shown to inhibit MHC class II presentation of this antigen, but the mechanism is not fully understood. To define the key determinants contributing to the inhibitory activity of these antibodies, a panel of anti-CD4bs monoclonal antibodies with different affinities was studied and compared to antibodies specific for the chemokine receptor-binding site or other gp120 regions. Anti-CD4bs antibodies that completely obstruct gp120 presentation exhibit three common properties: relatively high affinity for gp120, acid-stable interaction with gp120, and the capacity to slow the kinetics of gp120 proteolytic processing. None of these antibodies prevents gp120 internalization into APC. Notably, the broadly virus-neutralizing anti-CD4bs IgG1b12 does not block gp120 presentation as strongly, because although IgG1b12 has a relatively high affinity, it dissociates from gp120 more readily at acidic pH and only moderately retards gp120 proteolysis. Other anti-gp120 antibodies, regardless of their affinities, do not affect gp120 presentation. Hence, high-affinity anti-CD4bs antibodies that do not dissociate from gp120 at endolysosomal pH obstruct gp120 processing and prevent MHC class II presentation of this antigen. The presence of such antibodies could contribute to the dearth of anti-gp120 T helper responses in chronically HIV-1-infected patients.
Abbreviations:
-
- CD4bs:
-
CD4-binding site
-
- CD4i:
-
CD4-induced chemokine receptor-binding site
-
- Conf B:
-
undefined conformational gp120 epitope distinct from the CD4-binding site or the chemokine receptor-binding site
-
- sCD4:
-
soluble CD4
Introduction
The capacity of Ab to alter antigen uptake and processing by APC resulting in enhanced or suppressed antigen presentation has been demonstrated with a number of antigens, including tetanus toxoid, β-galactoside, apo-cytochrome c, and HIV-1 envelope glycoproteins 1–6. In the case of HIV-1, Ab produced by chronically HIV-1-infected subjects have been shown to inhibit MHC class II presentation of the virus envelope gp120 and, consequently, to prevent CD4 T cell proliferation and cytokine production in response to this antigen 1, 7. Specifically, the inhibitory activity is correlated with the serum Ab titers to the CD4-binding site (CD4bs) of gp120 7. By screening a panel of human anti-gp120 mAb, we ascertained that this inhibitory activity is mediated by Ab to the CD4bs; Ab to V2, V3, C2, or C5 did not exhibit such an effect 1. These findings raise the possibility that anti-CD4bs Ab induced during HIV-1 infection could be a key factor contributing to the lack of anti-gp120 CD4 T cell responses observed in most HIV-1-infected patients 8–10. Consistent with this hypothesis, high titers of serum anti-CD4bs Ab were found associated with faster disease progression, while exceptional HIV-1-infected subjects who were able to maintain virus envelope-specific lymphoproliferation and the majority of long-term non-progressors lacked these Ab 7, 11.
The mechanisms by which anti-CD4bs Ab affect gp120 presentation to MHC class II-restricted CD4 T cells have not been fully investigated. Previous studies demonstrated that these Ab do not affect T cells themselves; the Ab do not block T cell recognition of gp120 peptide epitopes presented by MHC class II and do not affect T cell recognition of other antigens, such as HIV-1 p24, mycobacterial antigens, and cytomegalovirus antigens 1, 2. The Ab also do not affect the overall functioning of APC since the ability of the APC to process unrelated antigens and to present peptide epitopes is unchanged 1, 2. Although anti-CD4bs Ab are known to block CD4-gp120 interaction 12–15 and can potentially inhibit gp120 uptake via CD4 on the surface of APC, gp120 can be taken up by APC independently of CD4 and this process is not affected by the anti-CD4bs Ab 2. Rather, the binding of anti-CD4bs mAb to gp120 was found to alter gp120 proteolysis by lysosomal enzymes from APC 16. On the basis of these data, we postulate that gp120 proteolytic processing is the primary step in the MHC class II antigen presentation pathway affected by anti-CD4bs Ab. In support of this idea, an earlier report by Kwong et al. 17 demonstrated that Ab interaction with the receptor-binding sites of gp120, i.e. CD4bs and the CD4-induced chemokine receptor-binding site (CD4i), induces substantial thermodynamic changes, which render gp120 more rigid and more resistant to degradative enzymes such as endoglycosidase H. Nevertheless, the critical factors and mechanisms underlying the anti-CD4bs Ab capacity to block gp120 processing remain unanswered.
In previous studies, only high-affinity anti-CD4bs mAb were examined; these mAb completely block MHC class II presentation of gp120 antigens 1, 2. However, it is not known if all anti-CD4bs Ab equally mediate such a strong inhibition. Since gp120-mAb complex formation was shown to be critical for anti-CD4bs mAb to block gp120 processing and presentation 1, 2, we reasoned that the Ab affinity could be a key determinant for their suppressive activity. Indeed, early studies pioneered by Watts et al. 4, 5 demonstrate that MHC class II antigen presentation is modulated by high-affinity antibodies that remain bound to the specific antigens during processing in APC. More recently, Brooks and Knight 18 also indicated that the affinity between antigen and B cell receptor is an important factor influencing antigen processing and presentation. Hence, in the present study we selected a panel of six anti-CD4bs mAb with different relative affinities for gp120 and examined their ability to suppress gp120 presentation to CD4 T cells. In addition, we tested anti-CD4i mAb binding to the chemokine receptor-binding site that, similar to anti-CD4bs mAb, were previously reported to render gp120 more resistant to degradative enzymes 17. For comparison, a mAb specific for a conformation-dependent epitope outside the receptor-binding sites (39H10/A11) and a relatively high-affinity anti-V3 mAb (694D/98D) were also tested. The ability of each of these mAb to suppress MHC class II antigen presentation to gp120-specific CD4 T cells was correlated with the mAb affinity for gp120. The uptake of gp120 by APC was also evaluated in the presence of these mAb. Furthermore, we measured the stability of the mAb-gp120 interaction at acidic pH, representing the endolysosomal environment in APC, and quantified the effect of the mAb on the rate of gp120 proteolytic processing by lysosomal enzymes in vitro.
Results
Anti-CD4bs mAb and their effects on gp120 antigen presentation
Six mAb were selected to represent anti-CD4bs Ab with different relative affinities for gp120IIIB. The relative affinities of the mAb were determined in ELISA on the basis of their half-maximal binding, which ranged from 0.043 to >10 µg/mL (Fig. 1A). Hence, the weakly reactive anti-CD4bs mAb 67G6/C4 showed no binding to gp120IIIB, whereas mAb 5145A bound gp120IIIB best, and the remaining mAb had intermediate binding levels. Subsequently, we examined the proliferation of the gp120-specific CD4 T cell line PS01 in response to gp120IIIB (1 µg/mL) when combined with different concentrations of each mAb (Fig. 1B, top graph). Autologous EBV-transformed B cells were used as APC. The T cells proliferated well in response to gp120IIIB alone, but the response was strongly suppressed in the presence of mAb 654D and 5145A, which are the two anti-CD4bs mAb with the highest affinities. In fact, complete suppression was observed with 1 µg/mL of these mAb. However, these anti-CD4bs mAb did not inhibit the PS01 response to its specific gp120 peptide (Fig. 1B, bottom graph), indicating that the mAb did not affect the ability of the APC to present processed gp120 epitopes. Comparable data were observed with another gp120-specific T cell line, PS02 (Fig. 2C). The T cell epitopes recognized by PS01 and PS02 are located in the C1 and C2 regions of gp120, respectively 19; these sites are distinct from the conformation-dependent epitope recognized by anti-CD4bs mAb (Fig. 1C).
(A) Relative affinities of different anti-CD4bs mAb. mAb binding to recombinant gp120IIIB antigen was measured in ELISA as described in Materials and methods. The mAb relative affinities were assessed based on their half-maximal binding (1/2 max). The background OD values taken from wells treated with either an irrelevant human anti-parvovirus mAb or no mAb were 0.268 ± 0.014. The data from one of two characteristic experiments are shown. (B) Proliferative responses of gp120-specific CD4 T cells in the presence of different anti-CD4bs mAb. The gp120-specific CD4 T cell line PS01 was tested in [3H]thymidine incorporation assays for proliferative responses to 1 µg/mL gp120IIIB (top graph) or a 20-mer peptide representing the PS01 epitope (bottom graph) pretreated with 1, 3, or 9 µg/mL anti-CD4bs mAb. The T cell responses are shown relative to the response to gp120 or peptide alone, which was defined as 100%. Averages and standard deviations from three separate experiments are shown. (C) The location of T cell epitopes and the anti-CD4bs mAb epitope in the context of the three-dimensional structure of gp120. The T cell epitopes in the C1 and C2 regions 19 are shown in blue and yellow, respectively. The amino acids critical for the conformation-dependent anti-CD4bs mAb are marked by orange spheres (Ser256, Thr257, Asn262, Asp368, Glu370, and Asp477); changes in these amino acids resulted in >50% reduction of anti-CD4bs mAb binding to gp120 (26, 33, 34 and unpublished data). The gp120 structure shown in green is the core gp120 X-ray crystal structure resolved as bound to CD4 and anti-CD4i mAb 17b 35. The truncated V2 and V3 loops are shown in black.
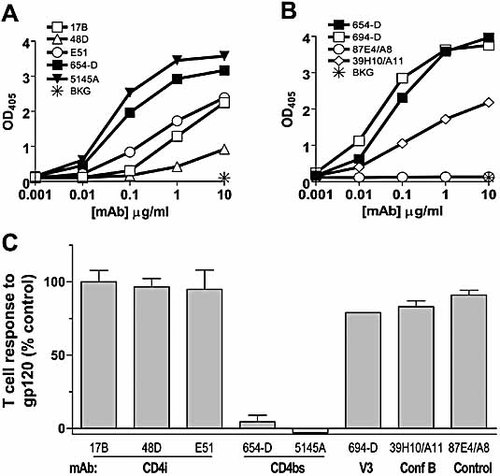
(A, B) Relative affinities of different anti-gp120 mAb as compared to anti-CD4bs mAb 654D and 5145A. The binding of anti-CD4bs mAb (654D or 5145A) was compared to that of anti-CD4i mAb (17b, 48d, and E51) (A), mAb 694/98D specific for the V3 loop and mAb 39H10/A11 specific for an undefined conformation-dependent epitope (Conf B) (B). mAb 87E4/A8, which does not recognize gp120IIIB, was included as a negative control. ELISA was performed with recombinant gp120IIIB as described in Materials and methods. Background OD405 values were taken from wells treated with no mAb. The data from one of three characteristic experiments are shown. (C) Effect of different anti-gp120 mAb on gp120 antigen presentation to CD4 T cells. Proliferation of the gp120-specific CD4 T cell line PS02 was examined in response to gp120IIIB (1 µg/mL) pretreated with 1 µg/mL mAb to the different gp120 epitopes. Similar results were obtained with higher mAb concentrations (data not shown). The T cell responses are presented relative to the response to gp120 alone (defined as 100%). EBV-transformed B cells were used as APC in the assays. The results represent the mean values and standard deviations obtained from up to four separate experiments.
In contrast to mAb 654D and 5145A, only partial or weak suppression was observed in the presence of mAb 38G3/A9, 1125H, and IgG1b12, even when the mAb concentration was increased to 9 µg/mL. mAb 67G6/C4, which did not bind gp120IIIB, had no inhibitory effect at any of the concentrations tested. Notably, IgG1b12 showed high affinity comparable to 654D, but IgG1b12 did not mediate as strong suppression. These data indicate that while high binding affinity is essential, other factors are required for anti-CD4bs mAb to strongly suppress gp120 presentation.
Effect of anti-CD4i mAb and other anti-gp120 mAb on gp120 antigen presentation
The relative affinities of CD4i mAb specific for the chemokine receptor-binding site were low compared to those of anti-CD4bs mAb 654D and 5145A (Fig. 2A). While anti-CD4bs mAb 654D and 5145A exhibited half-maximal binding at 0.06 and 0.04 µg/mL, respectively, anti-CD4i mAb 17b, 48d, and E51 did not plateau even at 10 µg/mL, the highest mAb concentration tested. Addition of soluble CD4 (sCD4) significantly increased anti-CD4i mAb affinities, but the levels were still lower than those of mAb 654D or 5145A (data not shown). We also examined anti-V3 mAb 694/98D and another anti-gp120 mAb recognizing a distinct non-CD4-blocking conformational epitope, 39H10/A11. mAb 694/98D with half-maximal binding at 0.03 µg/mL has an affinity comparable with mAb 654D and 5145A, while mAb 39H10/A11 binds gp120IIIB more weakly (Fig. 2B).
When tested in the T cell proliferation assay, the anti-CD4i mAb 17b, 48d, and E51 had little or no effect on the stimulation of the CD4 T cell response to gp120IIIB, while, as expected, the anti-CD4bs mAb 654D and 5145A strongly suppressed gp120 antigen presentation to the CD4 T cells (Fig. 2C). Both 694/98D and 39H10/A11 also had no suppressive effect on gp120 antigen presentation, similar to the non-binding mAb 87E4/A8 included as a negative control. These results show that human anti-CD4i mAb and other anti-gp120 mAb, including anti-V3 mAb with a relatively high affinity, did not affect gp120 presentation to CD4 T cells. The data demonstrate that only high-affinity anti-CD4bs mAb exhibited the suppressive activity, while mAb to other gp120 regions, regardless of their binding affinities, had no effect on CD4 T cell responses to gp120. Autologous EBV-transformed B cells were used in this study as APC to stimulate the T cells, but comparable results were obtained with unfractionated PBMC or monocyte-derived dendritic cells 1.
Effect of anti-CD4bs and anti-CD4i mAb on gp120 uptake by APC
Next, we examined the uptake of gp120 antigen by APC in the presence of anti-CD4bs or anti-CD4i mAb using FITC-conjugated gp120IIIB (gp120-FITC). Similar to the unlabeled gp120, T cell responses to gp120-FITC were also inhibited by anti-CD4bs mAb (data not shown). Adherent monocytes, used as APC, were incubated with gp120-FITC pretreated with anti-CD4bs mAb 654D, an irrelevant mAb specific for parvovirus B19 (860D), or no mAb. After incubation at 37°C, the cells were washed and the levels of fluorescence associated with the cells determined by a flow cytometer. Fig. 3A shows the histogram of monocytes incubated with gp120-FITC alone, gp120-FITC + mAb 654D, or gp120-FITC + mAb 860D. The mean fluorescence intensity of these cells was comparable (Fig. 3B, left panel). The cells were then treated with an anti-fluorescein mAb, which would quench surface-bound but not internalized gp120-FITC. The data in Fig. 3B (right panel) clearly show that the fluorescence of the cells was not reduced by the anti-FITC mAb, indicating the internalization of gp120-FITC into the cells regardless of the presence or absence of anti-CD4bs mAb 654D. Data from a separate experiment on cells incubated with gp120-FITC and the other anti-CD4bs or anti-CD4i mAb also show that these mAb did not prevent the uptake of gp120 (Fig. 3C). The same experiments were performed with B lymphoblastoid cells, and similar results were observed, albeit the percentages of cells associated with gp120 were much lower than those seen with the adherent monocytes (data not shown). Altogether, these data indicate that anti-CD4bs or anti-CD4i mAb binding to gp120 does not inhibit gp120 uptake into APC.
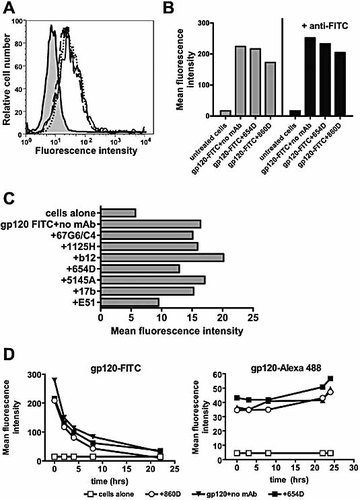
Internalization of gp120-mAb complexes by APC. (A) Flow cytometric analysis was performed on monocytes incubated with gp120-FITC in the presence of anti-CD4bs mAb 654D (dotted line), control mAb 860D (solid line), or no mAb (dashes). The background fluorescence from untreated cells (solid line with gray fill) was also determined. (B) Mean fluorescence intensity of the cells before (left panel) and after treatment (right panel) with a quenching anti-FITC mAb. (C) The levels of fluorescence associated with cells treated with uncomplexed gp120-FITC or gp120-FITC complexed with mAb to CD4bs (67G6/C4, 1125H, b12, 654D, 5145A) or CD4i (17b, E51). (D) Changes in fluorescence intensity were monitored over time after the cells were pulsed with gp120-FITC (left graph) or gp120-Alexa 488 (right graph) in the presence of no mAb, control mAb 860D, or anti-CD4bs mAb 654D. The data shown are from one of two independent experiments.
We subsequently assessed whether gp120 complexed with anti-CD4bs mAb 654D was transported into the endolysosomes. Since FITC is quenched at acidic pH, the uptake of gp120-FITC into the acidic endolysosomes would result in the loss of its fluorescence signals. Adherent monocytes were incubated for 2 h at 37°C with gp120-FITC pretreated with mAb 654D, control mAb 860D, or no mAb. After washing, the cells were further incubated in medium alone and analyzed at different time points. Fig. 3D (left graph) shows that the fluorescence intensity of cells decreased over time to reach almost the background level after 24 h, indicating the exposure of gp120-FITC to an acidic environment inside the cells. Comparable results were observed with cells treated with gp120-FITC alone, gp120-FITC + control mAb 860D or gp120-FITC + mAb 654D. By contrast, the fluorescence intensity of the cells treated with gp120 conjugated with acid-resistant fluorochrome Alexa 488 did not change over time (Fig. 3D, right graph). These results indicate that anti-CD4bs mAb did not inhibit the internalization or transport of gp120 antigen into the endolysosomes of the APC.
Correlation between the acid stability of gp120-mAb interaction and the capacity of the mAb to interfere with gp120 antigen presentation
For Ab to interfere with gp120 antigen processing, the Ab-gp120 complexes must remain stable in the acidic environment. To assess the stability of the mAb-gp120 complexes in acidic pH, we used an ELISA-based Ab-antigen dissociation assay described in the Materials and methods section. Fig. 4A shows changes in the levels of mAb bound to gp120 after a 1.5 h exposure to pH 7.0, 5.0, or 4.0. For comparison among the different mAb, the percentages of mAb-gp120 complexes remaining in acidic pH relative to those in pH 7.0 were calculated and are shown in Fig. 4B. The data show that the complexes made of gp120 and anti-CD4bs mAb 654D or 5145A did not dissociate after 1.5 h of incubation at pH 5.0, while at pH 4.0, as much as 45–47% of mAb 654D and 5145A remained bound to gp120. Notably, these two mAb had the highest binding affinities for gp120 and the most suppressive effect on gp120 antigen presentation (Fig. 1). The complexes of gp120 and IgG1b12, which caused partial suppression of gp120 presentation, were less stable; they were only 52% intact at pH 5.0 and dissociated completely at pH 4.0. The interaction of gp120 with anti-CD4bs mAb 1125H was, to a large extent, disrupted at pH 5.0 and 4.0, such that <9% of these mAb remained bound to gp120. Comparable data were observed whether 1.0 or 0.1 µg/mL of mAb was used (Fig. 4A). gp120 interaction with anti-CD4i mAb (17b, 48d, E51) and sCD4 also dissociated completely at pH 5.0 and pH 4.0. These results suggest that anti-CD4bs mAb 654D and 5145A, which mediate strong suppression of gp120 presentation, would remain stable as immune complexes in the acidic endosomes or lysosomes. In contrast, the mAb that have only partial or no inhibitory effect on gp120 presentation dissociate more readily from gp120 at low pH. Interestingly, the binding of anti-V3 mAb 694/98D was mostly retained at pH 5.0; nevertheless, this mAb did not inhibit gp120 presentation. Similarly, mAb 39H10/A11 against an undefined conformational gp120 epitope distinct from the CD4-binding site or the chemokine receptor-binding site (Conf B) remained ∼50% bound at pH 5.0, but this mAb caused no inhibition at all. These results indicate that Ab binding to V3 and other gp120 regions does not have the same effect as that of anti-CD4bs Ab.
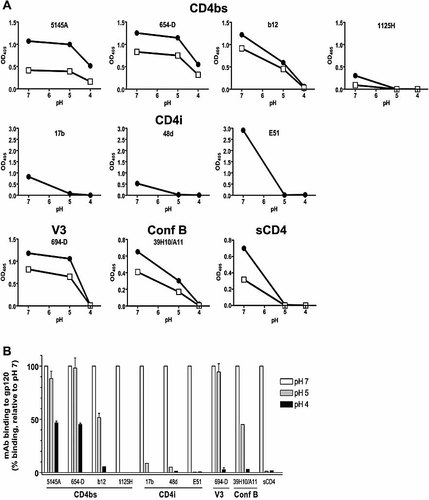
Dissociation of gp120-mAb complexes after treatment with neutral versus acidic buffers. The stability of gp120 interaction with mAb or sCD4 was assessed after 1.5 h of incubation with buffers of pH 7.0, 5.0, or 4.0, using ELISA described in Materials and methods. (A) Changes in OD observed in one representative experiment. mAb were tested at 1 (solid circles) or 0.1 µg/mL (open squares), while sCD4 was used at 10 (solid circles) or 2 µg/mL (open squares). (B) Percentages of mAb or sCD4 remaining bound to gp120 after incubation with acidic buffers, calculated relative to those bound after treatment with the pH 7.0 buffer (defined as 100%). Average values and standard deviations from two experiments are shown.
Proteolytic processing of gp120-Ab complexes by lysosomal enzymes
To measure the effect of anti-CD4bs and other anti-gp120 mAb on gp120 antigen processing, an in vitro proteolysis assay was developed in which gp120 or gp120 complexed with anti-gp120 mAb was digested for 0–22 h at pH 5.0 by lysosomal enzymes derived from EBV-transformed B cells routinely used as APC. The amounts of gp120 remaining over time were measured by ELISA in which gp120 was captured by anti-C terminus Ab and then detected by a biotinylated anti-N terminus mAb. Fig. 5A shows that gp120 alone was digested efficiently such that <5% of intact proteins remained after 22 h. The same kinetics was observed when gp120 was treated with the non-binding mAb 67G6/C4. In contrast, the digestion of gp120 complexed with anti-CD4bs mAb 5145A or 654D was substantially slower; ∼50% of gp120 was still detectable after 22 h. The rate of digestion of gp120 complexed with the other three anti-CD4bs mAb (IgG1b12, 1125H, and 38G3/A9) was intermediate or comparable to that of uncomplexed gp120.
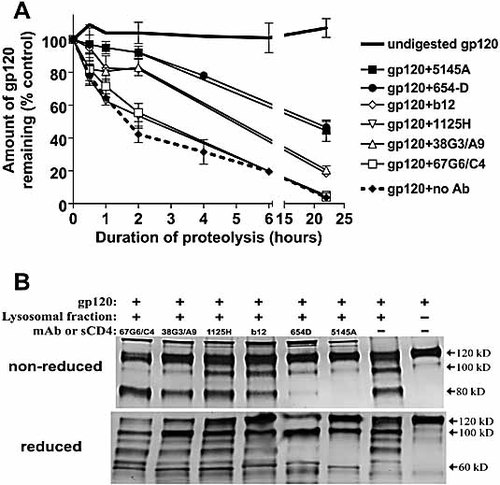
Proteolysis of gp120 or gp120 complexed with different anti-CD4bs mAb by lysosomal enzymes. Recombinant gp120IIIB was treated with anti-CD4bs mAb and then digested with lysosomal enzymes extracted from EBV-transformed B lymphoblastoid cells. (A) The amounts of gp120 remaining at different time points after digestion were measured by ELISA as described in Materials and methods. The data are presented as percentage of intact gp120 remaining relative to the concentration at t = 0 (defined as 100%). Averages and standard deviations from two experiments are shown. (B) The fragmentation patterns of gp120 and gp120-Ab complexes after overnight digestion were analyzed in Western blots. The reaction products were run on SDS-PAGE gels under nonreducing or reducing conditions and stained after Western blotting with sheep anti-gp120 serum. Undigested gp120 and the digestion of gp120 alone were included in each blot. Representative blots from one of three experiments are shown.
We subsequently asked whether there were qualitative differences in the fragmentation of gp120 when bound or not bound by the mAb. The overnight digestion products were subjected to Western blotting and staining with anti-gp120 polyclonal serum (Fig. 5B). For controls, we included in each blot undigested gp120. The digestion of gp120 bound by mAb 654D or 5145A produced a different fragmentation pattern from that of uncomplexed gp120 or gp120 treated with the other anti-CD4bs mAb. Under nonreducing conditions, gp120 complexed with 654D or 5145A appeared to remain mostly intact after digestion. Under reducing conditions, limited cleavage of gp120 with a distinct pattern became apparent; in particular, gp120 fragments between 60 and 100 kD observed in the digestion of uncomplexed gp120 were not visible in the digestion products of gp120 complexed with 654D or 5145A. In comparison, the digestion of gp120 treated with anti-CD4bs mAb 67G6/C4, 38G3/A9, 1125H, or IgG1b12 yielded an 80-kD fragment that was readily detectable in the nonreducing gel. In the reducing gel, more extensive fragmentation was clearly seen, similar to that of uncomplexed gp120.
We also measured the proteolysis of gp120 complexed with anti-CD4i mAb or the other anti-gp120 mAb. Fig. 6A again shows that the anti-CD4bs mAb 5145A substantially retarded gp120 digestion, but the other mAb tested had minimal effect. Minor differences were seen at early time points (<6 h) in the digestion kinetics of gp120 treated with 39H10/A11, 694/98D, the anti-CD4i mAb and control mAb 87E4/A8, but after 22 h, similar amounts of gp120 remained. When reaction products from the overnight digestion were analyzed by Western blot, it was again apparent that anti-CD4bs mAb 654D and 5145A caused alteration of gp120 proteolysis, while the fragmentation of gp120 bound by anti-CD4i mAb 17b, 48d, and E51 was comparable to that of uncomplexed gp120 (Fig. 6B). sCD4 binding to gp120 similarly did not affect gp120 proteolysis. The anti-V3 mAb 694/98D was shown previously not to retard gp120 fragmentation or prevent the generation of gp120 peptides recognized by CD4 T cells 16.
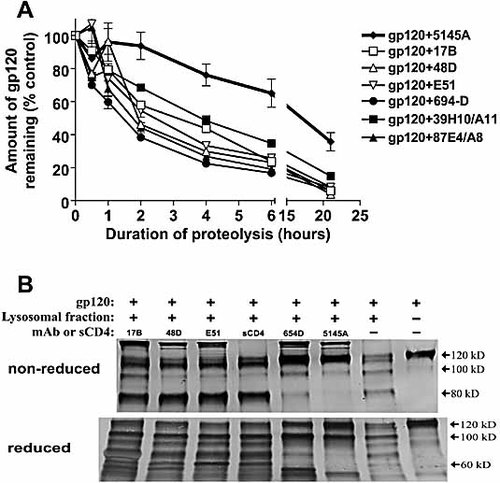
The effect of anti-CD4i mAb and other anti-gp120 mAb as compared to anti-CD4bs mAb on gp120 proteolysis. (A) Recombinant gp120IIIB pretreated with anti-CD4bs mAb (654D or 5145A), anti-CD4i mAb (17b, 48d, E51), mAb to the V3 loop (694/98D), mAb specific for the undefined conformation-dependent epitope Conf B (39H10/A11), or a control mAb (87E4/A8) was digested with lysosomal enzymes. The rate of gp120 digestion was measured on the basis of the amounts of intact gp120 detected in ELISA at the different time points. The data are presented as percentage of intact gp120 remaining relative to the concentration at t = 0 (defined as 100%). Averages and standard deviations of replicate wells from one of three representative experiments are shown. (B) The fragmentation patterns of gp120 complexed with anti-CD4i mAb (17b, 48d, E51), anti-CD4bs mAb (654D or 5145A), or sCD4 after overnight digestion were analyzed by Western blot with polyclonal anti-gp120 serum. Undigested gp120 and the digestion of gp120 alone were included for comparison. The digestion products were run on SDS-PAGE gels under nonreducing or reducing conditions. Representative blots from one of three experiments are shown.
Discussion
This study demonstrates that certain anti-CD4bs mAb have the unique capacity to inhibit gp120 antigen presentation by MHC class II. These mAb affect the presentation of different T cell epitopes located in gp120 regions that are distinct from the binding site of the mAb. The mAb do not interfere with the uptake and intracellular transport of gp120 by APC; rather, they appear to affect the subsequent step(s) in the MHC class II presentation pathway. In an attempt to determine the inhibitory mechanism of these mAb, we defined here three key determinants that distinguish these anti-CD4bs mAb from other mAb: (1) the strongly inhibitory anti-CD4bs mAb have a relatively high affinity for gp120, (2) the mAb-gp120 interaction is stable under acidic conditions, and (3) the binding of these mAb renders gp120 more resistant to proteolysis by lysosomal enzymes from APC. Anti-CD4bs mAb that bind gp120 weakly or dissociate from gp120 readily at acidic pH do not have as strong inhibitory effects on gp120 processing and presentation. Nevertheless, anti-V3 mAb that have a relatively high affinity did not exhibit any inhibitory effects on gp120 processing, indicating that the capacity of anti-CD4bs Ab to suppress gp120 processing and presentation is not simply due to their high binding affinities. Previous studies have shown that gp120 interaction with anti-CD4bs or anti-CD4i mAb, similar to CD4, triggers significant structural rearrangements in gp120 that increase the resistance of gp120 to enzymatic degradation 17. Indeed, the present study shows that high-affinity anti-CD4bs mAb impede the proteolytic processing of gp120. However, the three anti-CD4i mAb tested here do not have the same effect, most likely because these anti-CD4i mAb have relatively low affinities and detach completely from gp120 at acidic pH.
It is of interest to note that although anti-CD4bs mAb 654D and 5145A inhibited gp120 presentation to CD4 T cells, these mAb did not completely block in vitro proteolysis of the bound gp120; as much as 50% of gp120 bound by 654D or 5145A was digested after 22 h of reaction. One explanation is that the digestion of the gp120-Ab complexes may proceed more rapidly in vitro, since various proteases present in the lysosomal fraction work simultaneously at pH 5.0. Inside APC, on the other hand, antigen processing is more gradual as the gp120-Ab complexes move from mildly acidic early endosomes to late endosomes or lysosomes, and the stepwise processing may be more sensitive to the blocking effects of the anti-CD4bs Ab. We also should point out that although gp120 complexed with high-affinity anti-CD4bs Ab seems to be partially digested, such digestion may not produce appropriate peptide epitopes that can be presented to or recognized by the CD4 T cells. This is supported by our previous data showing that the digestion products of gp120-anti-CD4bs Ab complexes, when presented by fixed APC, failed to stimulate gp120-specific CD4 T cells 16. Direct examination of gp120 antigen processing in the APC is in progress, to test these possibilities.
Anti-CD4bs Ab with highly suppressive effects on gp120 antigen presentation, as represented by 654D, 5145A, and six other previously reported anti-CD4bs mAb 1, are relatively high-affinity Ab produced from cells of chronically HIV-infected subjects. mAb to other gp120 regions such as V3, V2, C2, C5, and Conf B did not exhibit the same suppressive activity, irrespective of their affinities. Anti-CD4i Ab examined in this study also caused no suppression, although it remains possible that high-affinity anti-CD4i Ab, if they are generated in response to HIV, may also have suppressive effects on gp120 processing and presentation. Of note, anti-CD4i Ab were frequently found in acute HIV-infected patients who receive highly active anti-retroviral therapy (J. Robinson, unpublished data), whereas anti-CD4bs Ab were not detectable in sera of the acutely treated HIV+ subjects we studied 11. Rather, anti-CD4bs Ab were produced to high titers by asymptomatic chronically HIV-infected patients who eventually progressed to AIDS 7, 11. These observations suggest that the highly suppressive anti-CD4bs Ab may develop only after chronic exposure to gp120 antigens produced during HIV-1 infection.
One question raised from these observations is how high-affinity anti-CD4bs Ab are generated in the first place if such Ab actually suppress the stimulation of helper CD4 T cells necessary for the affinity maturation of the CD4bs-specific B cells. The present findings showing that low-affinity anti-CD4bs Ab cause only minimal suppression indicate that low-affinity anti-CD4bs Ab produced very early after HIV infection should not totally block the initial stimulation of gp120-specific CD4 T cells. These cells may provide sufficient help to CD4bs-specific B cells to undergo somatic hypermutation and maturation in order to produce high-affinity Ab with suppressive activity. In addition, CD4 T cells specific for other HIV antigens may provide inter-molecular help for the development of CD4bs-specific B cells. Moreover, non-classical T cell help may also play a role. The development of B cells producing potent high-affinity Ab is evident in a number of knockout mouse models (e.g., CD4–/–, MHC class II–/–, TCRαβ–/–), despite the lack of any conventional MHC class II-restricted CD4 T cells 20–22. Once high-affinity anti-CD4bs Ab are generated, the ability of the chronically HIV-infected hosts to sustain the existing anti-gp120 CD4 T cell responses and to stimulate new CD4 T cells against emerging gp120 variants would be affected. Studies are currently being performed in the animal model to evaluate the development of CD4 T cell responses in vivo in the presence of high-affinity anti-CD4bs Ab.
Although many anti-CD4bs mAb display high affinities for gp120 of various HIV-1 isolates, these mAb typically have poor or no neutralizing activity against HIV-1 clinical isolates 23–26. One exception is IgG1b12 which has potent and broad virus-neutralizing activity 14. Hence, it is of interest to point out that IgG1b12 is only partially suppressive. The addition of mAb IgG1b12 to gp120, even at a high Ab-to-gp120 ratio, did not cause complete blockage of T cell responses to gp120 (Fig. 1B). The affinity of IgG1b12 for gp120IIIB was only slightly lower than that of the highly suppressive mAb 654D and 5145A, but the interaction of IgG1b12 with gp120 was more sensitive to acidic pH. This latter feature may account for the diminished ability of IgG1b12 to inhibit gp120 presentation. Kwong et al. 17 also reported that the binding of IgG1b12 to gp120 did not cause as high an entropic change as the other anti-CD4bs mAb. More importantly, the data presented here suggest the possibility that potently neutralizing anti-CD4bs Ab, such as IgG1b12, may provide effective anti-viral protection without strong suppressive effects on CD4 T cell-mediated immunity. In contrast, the generation of poorly neutralizing, highly suppressive anti-CD4bs Ab, such as 654D or 5145A, would not offer much protection and may even exacerbate the immune suppression associated with HIV-1 infection.
In summary, the present studies demonstrate that poorly neutralizing anti-CD4bs Ab produced by chronically HIV-1-infected patients prevent the stimulation of gp120-specific CD4 T cell responses. These Ab form relatively stable high-affinity immune complexes which are resistant to proteolytic processing by lysosomal enzymes. The presence of such Ab in sera of HIV-1-infected patients may contribute to the dearth of helper CD4 T cell responses to the virus envelope antigens and consequently weaken the anti-viral immunity necessary to control the chronic HIV infection and disease.
Materials and methods
Antigens and mAb
Recombinant HIV-1 gp120IIIB used in the study was purchased from Progenics (Tarrytown, NY) or ImmunoDiagnostics (Woburn, MA). Recombinant gp120IIIB conjugated with FITC (gp120-FITC) was also obtained from Progenics. Labeling of gp120 with Alexa 488 (Molecular Probes, Eugene, OR) was done according to the manufacturer's protocol. Anti-CD4bs mAb 67G6/C4 and 38G3/A9 were isolated from the gp120SF162-immunized human IgG2κ-transgenic XenoMouse G2 strain 13. mAb qqqq67G6/C4 specific for gp120SF162 and does not react with gp120IIIB. mAb 1125H (IgG1κ), 654D (IgG1λ), and 5145A (IgG1κ) are anti-CD4bs mAb produced by B cells of chronically HIV-1-infected subjects following EBV transformation 12, 13, 15, 27. Recombinant IgG1b12, which is specific for a unique gp120 epitope that overlaps the CD4bs and V2, was derived from a phage Fab library from bone marrow cells of an HIV-infected patient 14. mAb 17b, 48d, E51 (IgG1κ, IgG1λ, IgG1κ, respectively) were generated from HIV-1-infected individuals; these mAb recognize a highly conserved gp120 region that is important for chemokine receptor binding and is exposed better after CD4 binding (CD4i) 28, 29. The relatively high-affinity anti-V3 mAb 694/98D (IgG1λ) was also generated from an HIV-infected patient 30. mAb 39H10/A11 and 87E4/A8 (both IgG2κ) were generated from a gp120-immunized Xeno Mouse G2 strain; mAb 39H10/A11 recognizes an undefined conformational gp120 epitope that was distinct from the CD4-binding site or the chemokine receptor-binding site (designated Conf B) 13, while mAb 87E4/A8 does not recognize gp120IIIB and was included in some experiments as a negative control. mAb specific for human parvovirus B19 [860-55D (IgG1λ) or 1418 (IgG1κ)] were also used as irrelevant mAb control 31. All mAb were purified using protein A or protein G columns (Amersham Pharmacia Biotech, Piscataway, NJ).
Measurement of mAb relative affinities
To compare the relative affinities of the different anti-CD4bs mAb, ELISA were performed as described 7. In brief, recombinant gp120IIIB (1 µg/mL) was coated on the plates and reacted with varying concentrations of the mAb (10–0.01 µg/mL). Bound mAb were detected with alkaline phosphatase-conjugated goat anti-human IgG Ab. To compare the relative affinities of CD4i mAb and other anti-gp120 mAb, the protocol for an indirect ELISA of Xiang et al. 29, which provides more sensitive detection of the anti-CD4i mAb reactivity, was followed. Hence, recombinant gp120IIIB (1 µg/mL) was captured with sheep anti-C5 Ab and reacted with varying mAb concentrations. For anti-CD4i mAb, these assays were performed both in the presence and absence of sCD4. After incubation with alkaline phosphatase-conjugated goat anti-human IgG Fc Ab and addition of substrate for 15 min, OD405 readings were taken. The background OD values were determined from wells treated with either an irrelevant human anti-parvovirus mAb or no mAb. The amount of mAb needed to reach half-maximal binding was calculated using a Microsoft Excel iteration formula.
Measurement of gp120 uptake by APC
Primary adherent monocytes used as APC were prepared from PBMC by overnight adherence to plastic plates. The monocyte population was further enriched by negative selection with mouse anti-human CD19 and CD3 mAb (1 µL each per 1 × 106 cells) and anti-pan mouse IgG-coated Dynabeads (Dynal, Lake Success, NY).
To assess the uptake of gp120, 5 × 105 monocytes/well were seeded in 96-well plates in RPMI 1640 supplemented with 20% human AB serum, 10% FBS, 2 mM L-glutamine, 50 U/mL penicillin and 50 µg/mL streptomycin and incubated overnight at 37°C with 3 µg gp120-FITC pretreated with 10 µg mAb or with no mAb. After washing, the cells were analyzed by a Beckton Dickinson FACScan flow cytometer. To quench gp120-FITC bound on the cell surface, the cells were subsequently treated with anti-FITC mAb (Molecular Probes).
To examine the transport of gp120 to acidic endolysosomes, 3 × 106 cells were incubated at 37°C with 3 µg gp120-FITC or gp120-Alexa 488 pretreated with 10 µg mAb or with no mAb. After a 2-h incubation, the cells were washed and incubated further for up to 26 h at 37°C. Changes in fluorescence intensity were monitored over time by a Beckton Dickinson FACScan flow cytometer.
Measurement of the stability of gp120-mAb complexes at different pH
An ELISA-based assay was developed to measure the dissociation of mAb-gp120 complexes following treatment with buffers of pH 7.0, 5.0, or 4.0. Recombinant gp120 (1 µg/mL) was coated on the wells and reacted with anti-gp120 mAb (1 and 0.1 µg/mL) in PBS pH 7.0. The wells were then incubated with 100 µL PBS pH 7.0 or 0.08 M sodium acetate buffers pH 5.0 or 4.0 for 1.5 h 37°C. After washing to remove dissociated mAb, goat anti-human IgG Fc Ab conjugated with alkaline phosphatase were added, followed by substrate. OD405 readings were taken and the levels of mAb bound to gp120 were calculated relative to the levels observed after treatment with PBS pH 7.0 (defined as 100% binding). A similar protocol was followed to measure the dissociation of sCD4-gp120 complexes, except that sCD4 was reacted with coated gp120 and that mouse anti-CD4 mAb OKT4 and rabbit anti-mouse IgG conjugate were used to detect the bound CD4. For controls, we treated gp120 and mAb separately with the acidic buffers and observed that such treatment did not alter their antigenic reactivity and had no effect on their detection in ELISA (data not shown). Hence, the OD405 reduction after acid treatment was mainly due to the dissociation of anti-gp120 mAb from gp120, and was not caused by loss of gp120 antigenicity or detachment of gp120 from the well surface, or by the loss of mAb reactivity with the anti-human Ig conjugate.
CD4 T cell lines and T cell proliferation assay
Human CD4 T cell lines specific for gp120 were generated from PBMC of asymptomatic chronically HIV-1-infected subjects and maintained as described 1, 19. Two different lines recognizing gp120IIIB were used in the study: PS01 and PS02. The PS01 line is specific for a dominant epitope in C1 (92NFNMWKNNMVEQMHEDIISL111). The PS02 line recognizes multiple peptides representing different epitopes in C2 (206PKISFEPIPIHYCAPAGFAI225) and C1 (92NFNMWKNNMVEQMHEDIISL111). The antigenic specificity and surface phenotype of the lines were examined routinely prior to use in the experiments. We tested both T cell lines for gp120 recognition in the presence of the different mAb and observed comparable results (Figs. 1B and 2C, and data not shown).
Proliferative responses of the T cells were assessed with a standard [3H]thymidine incorporation assay 1. Briefly, recombinant gp120 or synthetic gp120 peptides were incubated alone or with mAb at the designated concentrations for 3 h at 37°C. Autologous EBV-transformed B lymphoblastoid cells that had been treated with mitomycin C (0.1 mg/mL) and/or irradiated (9000 rad) were added (1 × 105 cells/well) and allowed to process the antigens or antigen/mAb complexes overnight. T cell lines (2 × 104 cells/well) were subsequently added and incubated for 2 days. T cells were then pulsed with [3H]thymidine (PerkinElmer, Boston, MA) for 16–20 h and harvested.
Proteolysis assay
The proteolytic digestion of gp120 or gp120-mAb complexes by lysosomal enzymes was examined using the protocol of Chien et al. 16 with some modification. Hence, recombinant gp120 or gp120-mAb complexes formed at a molar mAb-to-gp120 ratio of 2 : 1 were incubated with lysosomal fractions (8.3 × 106 cell equivalent per µg of gp120) at 37°C from 0 to 22 h in 0.08 M sodium acetate buffer at pH 5.0. Lysosomal fractions were prepared from B lymphoblastoid cell lines as described 32. Gp120 (1 µg) was also complexed with sCD4 (2 µg) and digested as above.
To compare the rates of digestion of gp120 complexed with the various mAb or sCD4, a sandwich ELISA was set up that measured the relative amounts of intact gp120 remaining in the digestion reaction at different time points. For this assay, sheep polyclonal antibodies to the C terminus of gp120 (International Enzymes, Fallbrook, CA) were used to capture gp120 onto the wells and then the biotinylated anti-N terminus human mAb EH21 was added to detect the full-length molecules. We also analyzed the digestion products on 10% SDS-PAGE gels under either nonreducing or reducing (with 100 mM DTT) conditions, followed by Western blot analysis. gp120 and its fragments were detected with sheep or goat anti-HIV envelope serum (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) followed by human serum protein-adsorbed alkaline phosphatase-conjugated rabbit anti-sheep IgG (Zymed, South San Francisco, CA) or with conjugated anti-goat IgG (Sigma, St. Louis, MO), and 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium substrate (KPL, Gaithersburg, MD).
Acknowledgements
The authors would like to thank Dr. Susan Zolla-Pazner for reviewing the manuscript. The work was supported in part by a Merit Review Award (C.E.H.) and the Research Enhancement Award Program of the US Department of Veterans Affairs, the New York University Center for AIDS Research Immunology Core (AI-27742), and by NIH Grants AI-24030 (J.R.) and AI-48371 (C.E.H.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




