Interleukin-6 is a direct mediator of T cell migration
Abstract
Interleukin (IL)-6 is a pleiotropic cytokine involved in the differentiation and proliferation of hematopoietic cells. Hepatocytes respond to IL-6 with the synthesis and secretion of acute-phase proteins. In addition, IL-6 plays a role as a migration factor in vivo. In the present paper, we studied the potential of IL-6 to mediate migration of human primary T cells and T cell-derived cell lines. IL-6 was found to induce migration only in the presence of extracellular matrix, suggesting a cross-talk between the IL-6- and integrin signal transduction pathways. Furthermore, an IL-6 gradient is required for chemotactic migration. This activity is not due to the release of secondary chemotactic activities, but is a direct response to IL-6. T cell migration could also be observed in response to IL-11, but no migration was found after stimulation with leukemia inhibitory factor or oncostatin M, although these cytokines signal through gp130-containing receptor complexes. Finally, we present evidence that activation of the mitogen-activated protein kinase (MAPK) cascade, the phosphatidylinositol 3-kinase as well as the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is crucial for IL-6-induced migration. Selective activation of the JAK/STAT or the MAPK cascade by mutated receptor proteins shows a crucial role of IL-6-initiated SH2 domain-containing tyrosine phosphatase 2/MAPK activity for migration.
Abbreviations:
-
- EpoR:
-
Erythropoietin receptor
-
- GFP:
-
Green fluorescent protein
-
- JAK:
-
Janus kinase
-
- LIF:
-
Leukemia inhibitory factor
-
- MAPK:
-
Mitogen-activated protein kinase
-
- OSM:
-
Oncostatin M
-
- PI3K:
-
Phosphatidylinositol 3-kinase
-
- PTX:
-
Pertussis toxin
-
- SDF-1:
-
Stromal cell-derived factor 1
-
- SHP2:
-
SH2 domain-containing protein tyrosine phosphatase 2
-
- sIL-6Rα:
-
Soluble IL-6Rα
-
- STAT:
-
Signal transducer and activator of transcription
-
- VLA:
-
Very-late antigen
1 Introduction
Interleukin (IL)-6 is a multifunctional cytokine which has been suggested to be linked to many T cell-dependent immune diseases. A crucial function of IL-6 for the development of autoimmune diseases in mice was described for experimental autoimmune encephalomyelitis (EAE), experimental autoimmune myastenia gravis (EAMG), experimental autoimmune myocarditis (EAM), and antigen-induced arthritis (AIA). Consequently, IL-6-deficient mice resist EAE, EAMG, EAM and AIA 1. The question of how IL-6 deficiency protects against the development of autoimmune diseases has not yet been answered in detail. IL-6 acts on T cells as a growth factor and also as an anti-apoptotic factor 2. The constitutively activated Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway observed in patients suffering from adult T cell leukemia/lymphoma (ATLL) may explain the proliferation of T cells 3. Nevertheless, a simple lack of autoantigenic T cells in IL-6–/– mice could be excluded to cause resistance against experimental autoimmune diseases, since the primary T cell response occurred. Instead, a Th1 to Th2 shift was suggested to contribute to resistance 4. It is likely that IL-6 further influences autoimmune cell activities and that additional steps are required for infiltration and development of inflammatory lesions 5. Treatment of EAE-affected mice with antagonistic anti-IL-6 antibodies and experiments with IL-6-deficient mice demonstrate that IL-6 is required for the entrance of CD4+ T cells into the central nervous system 6. In vitro experiments further show chemotactic activity of IL-6 on PBL, lymphokine-activated killer cells, CD4+ and CD8+ T lymphocytes 7–9. Since IL-6 has also been identified as a migration factor for breast cancer cells 10–12, corneal epithelial cells 13, and keratinocytes 14, 15, we decided to analyze IL-6-induced T cell migration in more detail.
IL-6 exerts its action by binding and activating a receptor complex composed of a specific α subunit, IL-6Rα (gp80, CD126), and a signal-transducing subunit, gp130 (CD130). Receptor complex formation leads to subsequent activation of the JAK/STAT pathway and, through the activation of the adaptor and protein tyrosine phosphatase SHP2, the initiation of the mitogen-activated protein kinase (MAPK) cascade. The molecular mechanism that links gp130 engagement to the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is poorly understood and needs to be elucidated 16, 17.
In the present paper, we investigated the IL-6-dependent migration of primary human T cells and T cell-derived human cell lines and identified IL-6 as a direct mediator of T cell migration. Furthermore, we analyzed migration of native and genetically engineered cell lines that activate either the JAK/STAT or the MAPK cascade in response to activation of the IL-6 receptor complex.
2 Results
2.1 IL-6-induced migration of human primary T cells and T cell-derived cell lines
To clarify whether IL-6 functions as a migration factor, we isolated activated human T cells (CD4+/CD45RO+). The isolated T cell population was monitored by FACS analysis and found to be 98.5% pure (CD4+/CD45RO+) (Fig. 1B).

IL-6-induced migration of primary human T cells and T cell-derived cell lines. (A) Transwells were coated with 5 µg/cm2 fibronectin. Freshly isolated human primary CD4+/CD45RO+ T cells were seeded into the upper reservoir of the chamber. After sedimentation, 20 ng/ml IL-6 was applied to the lower compartment. The number of migrated cells was determined 4 h after stimulation by counting the cells that had penetrated the membrane of the Boyden chamber and were therefore found in the lower compartment of the Boyden chamber. (B) FACS analysis to control the purity of isolated T cells. The isolated human primary CD4+/CD45RO+ cells were analyzed in the absence of antibodies (left panel) or with FITC-coupled anti-CD4 antibodies (FL1-H) and PE-coupled anti-CD45RO antibodies (FL2-H) (right panel). (C) Karpas-299 cells were starved overnight and used for a migration assay. Asterisks indicate significant increases in migration (**p<0.01). Cells (1×105/100 µl) were transferred into a transwell, which was inserted into a well of a 24-well plate containing 800 µl medium. Approximately up to 2% of the cells migrate spontaneously. Up to 8% of the total number of Karpas-299 cells were found to migrate into the lower compartment of the Boyden chamber within the first 4 h post stimulation. Migration was expressed as means ± SEM. The amount of migrating cells is given in relation to that under standard conditions (100%) (for further details see Sect. 4).
IL-6-dependent migration of T cells was measured in a modified Boyden chamber assay. Fig. 1A shows significantly enhanced migration of primary human T cells upon stimulation with IL-6 compared to non-stimulated cells. Human T cell lymphoma-derived Karpas-299 cells were also tested for IL-6-mediated migration. Similar to primary T cells, Karpas-299 cells migrated in response to IL-6 (Fig. 1C). For our ongoing experiments, we used human primary CD4+/CD45RO+ T cells and Karpas-299 cells, which also express CD4 and CD45RO.
To further confirm that stimulation-induced migration is a response to IL-6 signaling and not due to a contamination within the cytokine preparation, antagonistic anti-gp130 antibodies were used to block migration (Fig. 2A). Control antibodies raised against c-Src did not inhibit IL-6-dependent migration (data not shown). We asked whether IL-6 induces chemokinesis or chemotaxis. Fig. 2B shows that efficient migration was only observed when the cytokine was added to the lower compartment of the Boyden chamber, but not when applied to the upper chamber (Fig. 2B, far right bar). Thus, Karpas-299 cells migrate towards IL-6, suggesting that an IL-6 gradient between the two reservoirs is required for IL-6-induced migration. Following this idea, we tested whether migration decreases with the sequential loss of the IL-6 gradient between the upper and lower reservoir of the Boyden chamber. The right part of Fig. 2B confirms that increasing amounts of IL-6 in the upper chamber inhibit migration towards the lower IL-6-containing compartment. We also asked whether it is sufficient to prime the cells with IL-6 to initiate migration. Therefore, the cells were pretreated with IL-6 for 30 min before being transferred to the Boyden chamber. Fig. 2C shows that pretreatment with the cytokine alone neither induces migration nor enhances IL-6-induced migration. These data show that Karpas-299 cell cannot be primed and further support the observation made in Fig. 2B that the cells require an IL-6 gradient to migrate. All these results indicate chemotactic migration in response to IL-6.
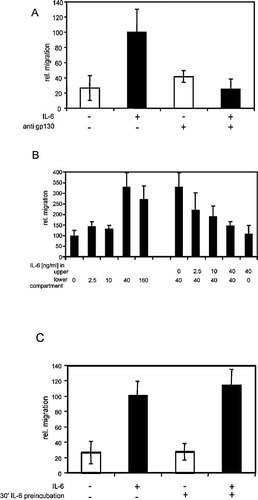
IL-6-induced chemotactic migration. Migration assays were performed with Karpas-299 cells as described for Fig. 1. (A) Where indicated, cells were incubated with 2 µg/ml antagonistic anti-gp130 antibody (B-R3) 30 min before and during the migration assay. The isotype control for the antagonistic anti-gp130 antibody is not shown. (B) Karpas-299 cells were applied to a modified Boyden chamber assay. IL-6 was added either to the lower or the upper or to both compartments, as indicated in the figure. (C) Karpas-299 cells were pre-incubated with IL-6 for 30 min and afterwards subjected to a migration assay as described for Fig. 1.
2.2 IL-6-induced migration depends on the presence of fibronectin
IL-6-initiated migration of Karpas-299 cells depends on the extracellular matrix, since coating the membrane with fibronectin was crucial for IL-6-dependent migration. In contrast, stromal cell-derived factor-1 (SDF-1) acts as a potent chemokine even in the absence of fibronectin (Fig. 3A). This requirement does not seem to be specific for fibronectin, since collagen IV and laminin can substitute for fibronectin. Poly-L-lysine, which does not activate integrins, was not potent to support IL-6-dependent migration (Fig. 3B), suggesting that cell attachment (without integrin activation) is not sufficient for migration. Importantly, we did not observe altered cell attachment in response to IL-6 (data not shown), indicating that IL-6 does not mediate detachment of cells instead of inducing chemotactic migration. To corroborate these observations, we analyzed the surface expression of integrins known to be expressed on T cells and to bind fibronectin. FACS analysis was performed using antibodies specific for the integrin subunits α4, α5, and β1 (Fig. 3C, left panels). Additionally, surface expression prior and after stimulation with IL-6 was compared (Fig. 3C, right panels). FACS analysis demonstrates that the integrins very-late antigen (VLA)-4 and VLA-5 are expressed on Karpas-299 cells and that their expression is not influenced by IL-6 stimulation. To further examine the contribution of VLA-4 and VLA-5 on IL-6-mediated migration, we analyzed migration in the presence of antibodies raised against the integrin subunits α4, α5, and β1. As Fig. 3D shows, IL-6-stimulated Karpas-299 cell migration can specifically be blocked by antibodies against α4 and β1 integrin, but not α5 integrin. These data indicate a requirement of VLA-4 for IL-6-induced migration.
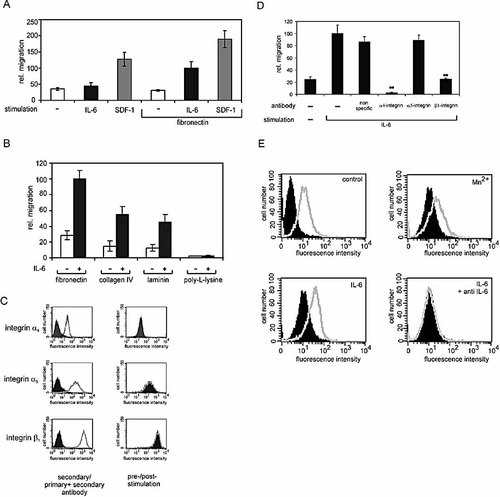
IL-6-induced migration depends on fibronectin. (A) Transwells were coated with fibronectin where indicated. Karpas-299 cells were starved overnight and subjected to a migration assay in the presence of 15 ng/ml IL-6 or 100 ng/ml SDF-1, as described for Fig. 1. (B) Transwells were coated with fibronectin (5 µg/cm2), collagen IV (5 µg/cm2) laminin (2 µg/cm2) or poly-L-lysine (2.7 µg/cm2). IL-6-dependent migration was analyzed as described for Fig. 1. (C) The surface expression of VLA-4 and VLA-5 integrin subunits on Karpas-299 was analyzed by FACS. The diagrams on the left represent FACS histograms from cells incubated with primary and secondary antibodies (open histograms) and those from cells incubated with only the secondary antibody (filled histograms). Diagrams in the right panel exhibit fluorescence intensities of stimulated (open histograms) and unstimulated cells (filled histograms), using the same primary and secondary antibodies as in (A). (D) Karpas-299 cells were incubated for 30 min with the indicated antibodies (10 µg/ml) on ice and afterwards transferred to the transwells to perform migration assays as described for Fig. 1. The antibodies were also present in the medium while the migration assay was performed. For negative controls, none (–) or an nonspecific antibody (anti-IRF-1) was used. ** indicates a significant reduction in migration in comparison to migration in the presence of no or unspecific antibodies (p<0.01). (E) Activation of β1 subunit-containing integrins by IL-6 was monitored by FACS analysis using 9EG7 antibodies specific for the activated β1 integrin subunit [18]. FACS analysis was performed in the absence of any stimulation, in the presence of Mn2+ or after stimulation with IL-6 for 30 minutes. For negative control, blocking anti-IL-6 antibodies were added 30 min prior to stimulation with IL-6. The open histogram from the unstimulated cells (control) was copied into the following plots for reference. The filled histogram from the unstimulated cells (control) represents fluorescence in the absence of the primary antibodies.
Consistently, we proved whether IL-6 affects integrin activation. 9EG7 antibodies recognize the activated β1 integrin subunit and therefore represent a good tool to monitor VLA-4 activation in response to IL-6 (Fig. 3E). Mn2+, known to activate VLA-4, was used as a positive control 18 (upper right panel). In the presence of Mn2+, antibody binding to the β1 subunit was significantly increased [compare closed histogram (without Mn2+) and open histogram (with Mn2+)]. Similarly, IL-6 initiated enhanced 9EG7 antibody binding (open histogram in lower left panel), which could be reverted by addition of IL-6-blocking antibodies (open histogram in the lower right panel). These data suggest that IL-6 activates β1 subunit-containing integrins such as VLA-4, shown above to be crucial for IL-6-induced cell migration.
2.3 Migration in response to IL-6-type cytokines
IL-6-type cytokines signal through gp130 homodimers (IL-6, IL-11), gp130/leukemia inhibitory factor (LIF) receptor heterodimers [LIF, ciliary neurotrophic factor (CNTF), oncostatin M (OSM), cardiotrophin 1 (CT-1), CT-1-like factor (CLC)], the gp130/OSM receptor heterodimer, or a gp130/WSX-1 heterodimer (IL-27). Signaling of IL-6, IL-11, CT-1, CNTF, and CLC depends on the presence of additional α receptors. Stimulation with IL-6 in the presence of the soluble IL-6Rα (sIL-6Rα) allows signaling independently of endogenous IL-6Rα expression. Although addition of sIL-6Rα slightly enhanced the chemotactic activity of IL-6, it is not required (Fig. 4A), since IL-6Rα is expressed on the plasma membrane of Karpas-299 cells (Fig. 4C).
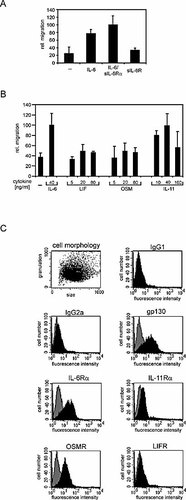
Migration in response to IL-6-type cytokines. (A) For migration assays, Karpas-299 cells were stimulated with 20 ng/ml IL-6 and/or 1 µg/ml sIL-6R (B) or with LIF, OSM or IL-11 at the concentrations indicated. (C) Surface expression of IL-6-type cytokine receptors was monitored by FACS analysis. Gray histograms show FACS analysis with secondary antibodies only. Black histograms show fluorescence after incubation with primary and secondary antibodies.
To elaborate whether migration can only be induced by activating gp130 homodimers or whether it can also be induced by gp130/LIF receptor- or gp130/OSM receptor-utilizing cytokines, Karpas-299 cells were stimulated with IL-6, IL-11, LIF, or OSM. Fig. 4B illustrates that only IL-6 and IL-11 are potent chemotactic factors. Stimulation with LIF did not lead to migration, due to the lack of cell surface expression of the LIF receptor, as monitored by FACS analysis (Fig. 4C). OSM also did not induce migration, although the OSM receptor is expressed on the plasma membrane (Fig. 4C). These observations suggest that cytokines utilizing gp130 homodimers are more potent to stimulate migration of Karpas-299 cells than those binding to gp130-containing heteromeric receptor complexes.
2.4 IL-6-induced migration is a direct response
In order to exclude that IL-6 stimulates migration indirectly by inducing the release of autocrinally acting chemokines, we analyzed whether pertussis toxin (PTX), which inhibits G protein-coupled receptor signaling, affects migration in response to IL-6. As shown in Fig. 5A, IL-6-initiated migration of primary human CD4+/CD45RO+ T cells is insensitive to PTX. SDF-1 is a classical chemokine and signals through the G protein-coupled receptor CXCR4. Fig. 5B shows SDF-1 to be much more potent to induce migration of Karpas-299 cells than IL-6 (compare also with Fig. 2B). SDF-1-dependent migration was significantly inhibited by PTX, but the toxin had no effect on IL-6-induced migration (Fig. 5C). These data suggest that IL-6 does not act via chemokines that signal through PTX-sensitive G protein-coupled receptors.
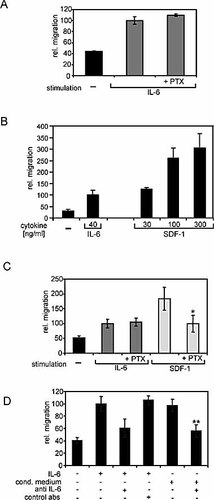
Migration is a direct response to IL-6. (A) Human primary CD4+/CD45RO+ T cells were preincubated with 0.1 µg/ml PTX for 16 h and analyzed for IL-6-induced migration. (B) Migration assays were performed in the presence of increasing concentrations of SDF-1 in the medium. For comparison, migration without cytokine or in the presence of IL-6 is shown. (C) Karpas-299 cells were pre-incubated with 0.1 µg/ml PTX for 16 h. PTX was also present during stimulation with cytokines. Cells were stimulated with 20 ng/ml IL-6 and 100 ng/ml SDF-1. * indicates a significant decrease in migration in comparison to SDF-1-stimulated cells (p<0.05). (D) Karpas-299 cells were stimulated with IL-6 (15 ng/ml). Conditioned medium of these cells was harvested and used for a second migration assay. The IL-6 in the conditioned medium was inactivated by addition of 2 µg/ml antagonistic anti-IL-6 antibody (mAb206) or an isotype control antibody 30 min prior to the migration assay. ** indicates a significant decrease in migration (p<0.01) due to the antagonistic anti-IL-6 antibody.
To further evaluate whether migration is directly mediated by IL-6, we analyzed IL-6-conditioned medium for the presence of secondary chemotactic activities (Fig. 5D). Karpas-299 cells were stimulated with conditioned medium that had been depleted of IL-6 with neutralizing antibodies (Fig. 5D, far right bar). The capability to induce migration was significantly reduced as compared to cells stimulated with IL-6-containing conditioned medium (second bar from the right), indicating that induction of migration is triggered by IL-6 alone.
2.5 Contribution of individual signaling pathways for migration
ERK activation is well known to be important for migration in response to many different stimuli. Thus, we tested whether IL-6-initiated migration of human primary CD4+/CD45RO+ T cells is sensitive to U0126, the low-molecular weight inhibitor of the ERK pathway. Fig. 6A shows a total block of T cell migration in the presence of 10 µM U0126, suggesting a crucial role of ERK also for IL-6-induced migration of T cells.
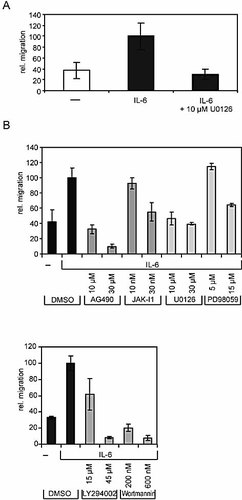
Role of the MAPK and PI3K cascades and the JAK/STAT pathway for IL-6-dependent migration. (A) Human primary CD4+/CD45RO+ T cells were pre-incubated with DMSO or with 10 µM U0126 for 30 min and applied to migration assays as described for Fig. 1. (B) Karpas-299 cells were pre-incubated with low-molecular weight inhibitors AG490, JAK Inhibitor 1, U0126, PD98059, LY294002, and Wortmannin for 30 min.
To further dissect which of the signaling pathways initiated by IL-6 or integrin activation contribute to IL-6-mediated cell migration, we analyzed to which extent well-characterized low-molecular weight inhibitors for JAK (AG490 and JAK Inhibitor 1), PI3K (LY294002 and Wortmannin), or ERK (U0126 and PD98059) affect IL-6-dependent migration of Karpas-299 cells. Fig. 6B demonstrates a crucial role for JAK, which is in line with the upstream function of JAK in IL-6 signaling. Interestingly for our studies on IL-6-induced migration, the PI3K and ERK pathways were found to be crucial for migration. The inhibitors used did not affect the mortality of the cells, as monitored by trypan-blue staining (data not shown).
2.6 Migration through chimeric erythropoietin receptor/gp130 receptors
The above-mentioned experiments carried out with low-molecular weight inhibitors do not allow an analysis of the specific contribution of gp130 in the migration process, but they give a first hint at the general contribution of specific signaling cascades, irrespective of whether they are initiated by integrins or gp130. To focus on the contribution of IL-6-initiated signaling cascades, experiments were designed that allow the specific activation of either the JAK/STAT pathway or the MAPK cascade in response to IL-6. gp130 mutants have been shown to specifically activate either pathway: gp130 mutants lacking Tyr759 are unable to activate the SHP2/MAPK cascade after IL-6 stimulation 19. On the other hand, gp130 mutants lacking the four membrane-distal tyrosine residues (Tyr767, Tyr814, Tyr905 and Tyr915) do not activate STAT1 and STAT3 transcription factors 20, 21. We used these mutants to create chimeric receptors harboring the extracellular part of the erythropoietin receptor (EpoR) and the transmembrane and cytoplasmic parts of gp130. Chimeric receptors are useful and established tools to analyze signaling through exogenous receptor mutants, independently from endogenous receptors.
We infected the cells with a retroviral expression system that does not interfere with the migratory activity triggered by endogenous receptors. The control experiments demonstrate that naive Karpas-299 cells, as well as those infected with recombinant green fluorescent protein (GFP)-encoding retrovirus, migrate in response to IL-6, but not in response to Epo. Furthermore, Epo stimulation does not mediate migration of Karpas-299 cells, a prerequisite for the use of retrovirally expressed EpoR/gp130 chimeric receptors for the following studies (data not shown). Karpas-299 cell clones were generated, stably expressing chimeric EpoR/gp130 constructs containing (a) all six cytoplasmic tyrosine residues [EpoR/gp130(YYYYYY)], (b) a substitution of all six tyrosine residues by phenylalanine [EpoR/gp130(FFFFFF)], as a negative control, (c) a single phenylalanine for tyrosine substitution at position 759 [EpoR/gp130(YFYYYY)], or (d) an exchange of the four membrane-distal tyrosine residues by phenylalanine [EpoR/gp130(YYFFFF)] (Fig. 7A).
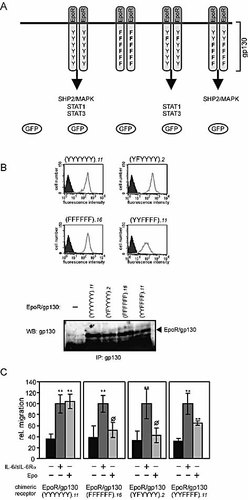
Influence of the SHP2 and STAT binding sites within gp130 for IL-6-dependent migration. (A) The chimeric receptors contain the extracellular part of the EpoR and the transmembrane and intracellular part of gp130. The six tyrosine residues of gp130 are partially or fully substituted by phenylalanine in the different receptors. Infection with the retroviral vectors used leads to expression of GFP as well. (B) Respective cell clones were analyzed for GFP expression by FACS analysis (upper part). Filled histograms represent cells expressing non-transduced cells, whereas open histograms represent GFP expression of the respective cell clones. Chimeric receptors were immunoprecipitated from lysates of the indicated cell clones. Immunocomplexes were subjected to SDS-PAGE. After Western blotting, the membrane was incubated with antibodies recognizing the intracellular part of gp130 (lower part). (C) Karpas-299 cells expressing the indicated chimeric receptors were stimulated with 20 ng/ml IL-6 and 1 µg/ml sIL-6Rα or 7 U/ml Epo, or left untreated. ** indicates a significant increase in migration (p<0.01) in comparison to non-stimulated cells, ø indicates no significant increase in migration (p>0.1) in comparison to non-stimulated cells.
Unfortunately, no antibody against the extracellular domains of the murine EpoR part of the chimeric receptors appropriate for FACS analysis is available. Instead, expression levels of GFP and the chimeric receptors were monitored by FACS and Western blot analysis, respectively (Fig. 7B). Expression of EpoR/gp130(YYFFFF) was slightly reduced in comparison to the other chimeric receptors.
Migration in response to IL-6 and Epo was studied, comparing different clones of these four cell lines (Fig. 7C). The left panel shows that cells expressing the wild-type chimeric receptor, EpoR/gp130(YYYYYY), migrate in response to IL-6 (signaling through the endogenous receptor complex) as well as in response to Epo (signaling through the exogenous chimeric receptor). IL-6- and Epo-induced migration was similarly sensitive to the inhibitors tested in Fig. 6 (data not shown). Expression of a chimeric receptor lacking all cytoplasmic tyrosine motifs [EpoR/gp130(FFFFFF)] led to a specific loss of migration in response to Epo, whereas migration in response to IL-6 was unaffected. These observations indicate that the cytoplasmic tyrosine motifs of gp130 are crucial for the gp130-dependent migration of T cells (Fig. 7C, panel 2).
A single mutation of Tyr759 [EpoR/gp130(YFYYYY)] also specifically impairs Epo-dependent migration, indicating that the tyrosine motif responsible for the activation of SHP2 and the MAPK cascade in response to IL-6 stimulation 19 is essential for gp130-mediated cell migration (panel 3). Finally, cells expressing the receptor mutant EpoR/gp130(YYFFFF) unable to initiate STAT1 and STAT3 activation 20, 21 still migrate in response to Epo. The reduced migration observed results very likely from reduced receptor expression in these cell clones. Our data suggest no essential function of STAT activation for the migration of human T cells.
3 Discussion
T cell migration is part of a complex inflammatory defense induced by infection and injury. The ability of IL-6 in local inflammatory reactions to amplify leukocyte recruitment was deduced from the observation that IL-6-deficient mice show reduced leukocyte accumulation at inflammatory sites. The current view of IL-6-mediated leukocyte migration in vivo is that IL-6, in combination with its sIL-6Rα, acts indirectly through stimulation of endothelial cells to produce chemokines that subsequently induce T cell migration 22.
IL-6 could also directly target activated T cells to sites of inflammation, since gp130 and the IL-6Rα are expressed on leukocytes. To check whether IL-6 has also the potential to directly induce migration of T cells, we had to avoid any interference with chemokines produced by other cell types (e.g. endothelial cells). Consequently, we performed our studies in an in vitro system that allowed us to analyze the migration of activated primary (CD4+/CD45RO+) T cells or T cell-derived cell lines expressing CD4/CD45RO as a direct response to IL-6. Indeed, we found IL-6-dependent migration of primary human T cells as well as of Karpas-299 cells (Fig. 1).
We could exclude that IL-6-induced cytokines act in an autocrine manner to induce migration, since an IL-6 concentration gradient, and not just the presence of the cytokine alone, is required for chemotaxis of Karpas-299 cells (Fig. 2B). Furthermore, conditioned medium of IL-6-stimulated Karpas-299 cells did not contain migratory activities besides IL-6 (Fig. 5D). Also IL-6-induced migration of Karpas-299 and primary human T cells did not depend on the activation of G protein-coupled receptors (Fig. 5A, C). From all these results, we suggest that IL-6 is a direct mediator of T cell migration.
All IL-6-type cytokines signal through receptor complexes containing at least one gp130 subunit, which could explain the redundancy of the biological activities of these cytokines. The different composition of the individual receptor complexes (i.e. the presence of specific α receptors, LIF receptor or OSM receptor) may lead to the specific responses to the individual cytokines. In spite of the fact that the OSM receptor is present on the plasma membrane of these cells (Fig. 4C), OSM concentrations known for maximal stimulation of other cell types (data not shown) do not initiate migration (Fig. 4B). The specificity of signaling through gp130 homodimers or gp130/OSM receptor heterodimers may account for these differential potentials to induce migration.
Our experiments have shown that integrin activation is essential for IL-6-dependent migration (Fig. 3B). Consistent with findings that VLA-4 is more efficient than VLA-5 in mediating mononuclear cell migration, a blocking anti-VLA-4 mAb significantly inhibited IL-6-induced transmigration. Enhanced VLA-4 and VLA-5 expression was reported to mediate increased adhesion of NK cells in response to IL-6 23. Similarly, Otori and co-workers suggested that IL-6-stimulated migration of corneal epithelial cells may also depend on increased expression of fibronectin receptors 13. It should be noted that integrin expression was analyzed after stimulation with IL-6 for 6 days or 24 h, respectively. In contrast, we analyzed migration already 4 h after stimulation with IL-6 and did not observe up-regulation of VLA-4 or VLA-5 integrins. Furthermore, increased integrin expression would rather induce chemokinesis than chemotaxis. From these data, we suggest that a cross-talk between IL-6 signaling and VLA-4 is required for IL-6-stimulated transmigration. This idea was further supported by the observation that IL-6 initiates β1 integrin activation (Fig. 3E). Therefore, studies to understand the molecular link between gp130 and integrin receptors are currently ongoing.
Studies with low-molecular weight inhibitors gave us first clues as to the signaling pathways involved in IL-6-induced migration, irrespective of whether they are initiated by integrin or gp130 (Fig. 6). Since activation of the upstream JAK is essential for all IL-6-induced signal transduction pathways, it was not surprising that JAK inhibitors block migration. The MAPK and PI3K cascades seem to be involved in IL-6-dependent migration. Activated integrin receptors initiate the MAPK and PI3K cascades as well 24, 25. Therefore, the low-molecular weight inhibitors also targeted integrin signaling. At this point the relevance of gp130-dependent activation of both pathways remained unresolved. In contrast to the known studies, we did not consider the role of individual pathways per se (independent of their origin) but rather tried to analyze the role of IL-6-induced pathways. We analyzed migration induced by a panel of chimeric gp130 receptors resembling the gp130 wild-type protein or mutants specifically activating either the SHP2/MAPK or the JAK/STAT pathway (Fig. 7A) 21. As expected, cell migration initiated by activating chimeric receptors lacking all cytoplasmic tyrosine motifs was impaired (Fig. 7C, panel 2). Interestingly, a single point mutation of the tyrosine that mediates gp130-dependent SHP2 recruitment and activation of the MAPK cascade [EpoR/gp130(YFYYYY)] specifically abolishes migration in response to Epo (Fig. 7C, panel 3). These observations suggest that gp130-dependent activation of SHP2 is crucial for IL-6-induced migration.
A central role of SHP2 for migration has already been deduced from studies with mice expressing a truncated SHP2. These animals are embryonically lethal and have a similar phenotype as focal adhesion kinase (FAK)- or fibronectin-deficient mice 26. Embryonic fibroblasts from these mice show altered migration, adhesion and spreading 27, 28. Unfortunately, most of the studies dealing with SHP2 and migration have been performed with adherent cells rather than with cells in suspension, and not in the context of IL-6. Since migration of adherent and non-adherent cells differs, it needs to be investigated whether these studies are applicable to T cell migration.
Mutation of the four STAT-recruiting tyrosine motifs within gp130 did not completely inhibit migration after stimulation of the chimeric receptor with Epo (Fig. 7C, panel 4), indicating no essential role of activated STAT for migration. The reduced migration observed is likely due to reduced receptor expression in the respective cell clones. Nevertheless, a modulating function of STAT cannot be excluded. A contribution of STAT3 has already been described for the migration of keratinocytes 14, 15 and myeloid cells 29. In contrast, IL-6-induced migration of breast carcinoma cells does not depend on STAT3 12.
All the data summarized and discussed above suggest that interleukins, besides chemokines and growth factors, should also be considered as cytokines with chemotactic activity. Although the function of IL-6 (in complex with its sIL-6Rα) to stimulate endothelial chemokine secretion is likely to be the more efficient mechanism for inducing T cell migration, the direct action of IL-6 on T cells should not be underestimated. The current study might help to understand the molecular mechanisms of the cross-talk between integrin and cytokine signaling.
4 Materials and methods
4.1 Materials and cell lines
Recombinant human (h)IL-6 and sIL-6R were prepared as described 30. hIL-11, hOSM and hSDF-1 were purchased from TEBU (Frankfurt/Main, Germany); hLIF was from Sigma (Taufkirchen, Germany). Recombinant Epo was a generous gift of Drs. B. Hilger and K. H. Sellinger (Roche, Mannheim, Germany).
For FACS analysis, PE-conjugated F(ab′)2 fragments recognizing mouse IgG or rat IgG, respectively, were used (Dianova, Hamburg, Germany). Anti-LIF receptor antibodies (10.B2) were kindly provided by Y. Jacques (Nantes, France); anti-OSM receptor (AN-E2) antibodies were a gift of H. Gascan (Angers, France). Anti-IL-6Rα (B-N12), anti-gp130 (B-P8, B-R3), and isotype control (B-Z2) antibodies were donated by J. Wijdenes (Diaclone, Besançon, France). Antagonistic anti-IL-6 antibodies (mAb206) were from R&D Systems (Minneapolis, MN). Anti-IL-11R antibodies were kindly donated by F. A. Montero-Julian (Marseille, France); antibodies raised against the cytoplasmic part of gp130 were from Upstate Laboratories (Lake Placid, NY). Anti-integrin α4 (HP1/2) and α5 (A5-PUJ1) antibodies were kindly provided by M. E. Hemler (Boston, MA). Anti-fibronectin, -integrin β1, -CD4-, and -CD45RO antibodies were purchased from BD Biosciences PharMingen (Heidelberg, Germany). Blocking antibodies to integrin α4, α5 and β1 were from Chemicon (Hofheim, Germany). The anti-IRF-1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Collagen IV, poly-L-lysine, and pertussis toxin were from Sigma (Taufkirchen, Germany). U0126 was purchased from Promega (Mannheim, Germany). All other inhibitors were from Calbiochem (Bad Soden, Germany). Laminin was from Harbor Bio-Products (Norwood, MA). The Karpas-299 cells were from DSMZ in Braunschweig, Germany.
4.2 Isolation of primary human T cells
Human PBMC were isolated from fresh blood (200 ml). Activated (CD4+/CD45RO+) T cells were isolated with a MACS cell negative isolation kit (indirect magnetic labeling system for CD4+ and CD45RO+ cells) (Miltenyi Biotec, Bergisch Gladbach, Germany), as described by the manufacturer. Purity of the cells was monitored by FACS analysis using FITC-coupled anti-CD4 antibodies and PE-coupled anti-CD45RO antibodies.
4.3 Cell culture
Karpas-299 cells were grown in RPMI supplemented with 10% FCS, streptomycin (100 mg/l), and penicillin (60 mg/l). For migration assays, FCS and antibiotics were omitted from the medium. HEK293T cells were grown in DMEM supplemented as described above.
4.4 Construction of expression vectors
The retroviral vector pM5-EpoR/gp130-NeoGFP is based on the myeloproliferative Sarcoma virus. cDNA were cloned in a bicistronic configuration into the multiple cloning site: the cDNA encoding an EpoR/gp130 chimeric receptor is followed by the IRES of the human NRF gene and the cDNA for an enhanced-GFP/neomycin resistance fusion protein. Four different retroviral vectors were generated to express EpoR/gp130(YYYYYY), EpoR/gp130(YFYYYY), EpoR/gp130(YYFFFF), and EpoR/gp130(FFFFFF) 21. These chimeric receptors contain tyrosine to phenylalanine substitutions within the cytoplasmic part of the receptor, as indicated. Additionally, a retroviral vector for the expression of GFP without any chimeric receptor has been used as a control.
4.5 Modified Boyden chamber assay
Cells (7.5×105/ml) were incubated overnight in RPMI containing 2% FCS. Transwell inserts (6.5-mm diameter, 8-µm pore size; Corning Costar, Bodenheim, Germany) were coated with human fibronectin. Into a transwell, 1×105 cells/100 µl were transferred. The transwell was inserted into a 24-well plate containing 800 µl medium. Cell migration to the bottom chamber was assessed 4 h later. For each experiment, the cells from four different fields were enumerated. Every migration assay was performed three times in triplicate. Approximately up to 2% of the cells migrate spontaneously. Migration was induced by addition of 20 ng/ml IL-6 (and 1 µg/ml sIL-6Rα where indicated) into the lower compartment. Up to 8% of the total number of Karpas-299 cells were found to migrate into the lower compartment of the Boyden chamber within the first 4 h post stimulation. The migration was expressed as means ± SEM. The amount of migrating cells is given in relation to that under standard conditions (100%) to indicate how specific treatment of the cells affects their chemotactic activities. For statistical analysis of significance, we applied one-way analysis of variance (one-way ANOVA).
4.6 FACS analysis
Cells (5×105) were harvested and washed with FACS buffer (PBS containing 5% FCS, 0.1% NaN3). The cells were resuspended in 100 µl ice-cold FACS buffer and incubated with 1 µg of antibodies raised against the extracellular part of the respective receptors. After washing, mouse IgG-specific PE-conjugated goat antibodies were added to monitor receptor expression, using a FACSCalibur (Becton Dickinson, Heidelberg, Germany). GFP expression was analyzed in the absence of antibodies. For monitoring activated β1 integrin, Karpas-299 cells were grown overnight in RPMI supplemented with 2% FCS and starved in 25 mM Hepes buffer supplemented with 150 mM NaCl, 500 µg/ml soluble fibronectin, and 1 mM MgCl2 (or 5 mM MnCl2 for positive control of activated integrins 18) for 30 min. Stimulation was performed with 15 ng/ml IL-6 for 30 min. The cells were incubated at 4°C for 30 min with 9EG7 antibodies, and after washing, rat IgG-specific PE-conjugated goat antibodies were added. After washing with FACS buffer, cells were monitored for activated β1 integrin subunits. Negative control was performed by adding blocking anti-IL-6 antibodies (2 µg/ml) 30 min prior to stimulation with IL-6.
4.7 Immunoprecipitation and immunoblot analysis
For immunoprecipitation, 6×107 cells were lysed in 500 µl RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Igepal, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 15% glycerol). Buffers were supplemented with 10 µg/ml of each aprotinin, pepstatin, and leupeptin. After precipitation with antibodies against gp130, immune complexes were separated by SDS-PAGE and transferred to a poly(vinylidene difluoride) (PVDF) membrane. Antigens were detected by incubation with primary antibodies specific for the cytoplasmic part of the EpoR/gp130 chimeric receptor and horseradish peroxidase (HRP)-coupled secondary antibodies (1:5,000) (Dako, Hamburg, Germany). The membranes were developed with an enhanced chemiluminescence kit (Amersham-Pharmacia, Uppsala, Sweden).
4.8 Retroviral gene transfer
Production of retroviruses was performed with pV-Pack-Vectors from Stratagene (La Jolla, CA), as described in the manufacturer's protocol. HEK293T cells were transfected using FuGene (Roche Diagnostics GmbH, Mannheim, Germany) with pVPackGP, pVPack-10A1 and pM5-EpoR/gp130-NeoGFP. The retrovirus-containing supernatant was added to Karpas-299 cells two days after transfection, together with 10 µg/ml polybrene (Sigma, Taufkirchen, Germany). After infection (1 day), the cells were selected in RPMI containing 10% FCS and 2 mg/ml neomycin. Single clones were isolated, the expression of GFP was examined using FACS analysis, and the expression of the EpoR/gp130 chimeric receptors was monitored by immunoprecipitation and subsequent Western blot analyses. A representative of at least two clones for each receptor mutant is shown.
Acknowledgements
We thank Sonja Linnemann for her excellent technical assistance and Christoph Roderburg for help in isolating primary human T cells. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Bonn, Germany) and the Fonds der Chemischen Industrie (Frankfurt a.M.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




