CD4-induced down-regulation of T cell adhesion to B cells is associated with localization of phosphatidyl inositol 3-kinase and LFA-1 in distinct membrane domains
Abstract
We have previously shown that binding of anti-CD4 antibody inhibit LFA-1-dependent adhesion between CD4+ T cells and B cells in a p56lck and a PI3-kinase-dependent manner. In this work, we investigated with two different T cell lines (Jurkat and A201) whether CD4 binding could alter interactions of the proteins putatively involved in this adhesion regulatory pathway. Anti-CD4 binding was shown to induce a transient association between PI3-kinase and LFA-1, which took place in different regions of the plasma membrane. It was detected in detergent soluble membrane but also in detergent insoluble membrane consisting in raft microdomains, composed of GM1 and/or GM3 gangliosides. These results show that anti-CD4 Ab could modify the interaction between LFA-1 and signaling molecules, such as PI3-kinase and induce, in part, their recruitment in raft domains. By using specific inhibitors, raft integrity and CD4 association with GM3 were found necessary for observing the CD4-dependent inhibition of LFA-1-mediated adhesion. These results strongly suggest that these molecular rearrangements in the membrane are necessary to induce down-regulation of LFA-1-mediated adhesion.
Abbreviations:
-
- PI3-kinase:
-
Phosphatidylinositol-3 kinase
-
- MBCD:
-
Methyl β cyclodextrin
-
- RTrf:
-
Transferrin receptor
-
- CT:
-
Cholera toxin
1 Introduction
Adhesion is a fundamental process in leukocyte physiology. It is strictly regulated and involves a number of interactions between different adhesion protein 1. These adhesion processes are required for T cell activation and effector functions 2. We have previously shown that, in the absence of antigen or following anti-CD3 activation, the LFA-1-dependent adhesion between CD4+ T cells (both resting and activated T cells) and EBV-B cell lines, was down-regulated by CD4 ligands 3, 4. This down-regulation involves distinct mechanisms such as activation and phosphorylation of two kinases i.e. tyrosine kinase p56lck associated to CD4 and phosphatidyl inositol-3 kinase (PI3-kinase). This CD4 signaling also involves modification of transient associations between PI3-kinase, cytohesin-1, and other phosphatases which are necessary for the down-regulation of adhesion 5, 6. However, it has not been clearly shown how these interactions mediate the down-regulation of LFA-1-dependent adhesion induced by CD4.
Regulation of LFA-1-mediated adhesion is dependent on an outside/inside activation process leading to both increases in affinity and avidity of LFA-1 7. Associations between LFA-1 and cytoplasmic proteins can modify the affinity/avidity of LFA-1 for its ligand and thereby affect adhesion. More recently, it has been shown that LFA-1 can be recruited in separate membrane microdomains called rafts, where it is clustered 8. Rafts are cholesterol-enriched lipid domains particularly important in the regulation of cell activation 9. It has been proposed that this migration involves an association between LFA-1 and specific proteins localized in these rafts 10. This translocation was correlated to a modification of adhesion 11. Following activation, it has been shown that lipid rafts in T cells recruit several protein receptors like CD4, CD2, CD3, together with protein kinases i.e. p56lck, p59fyn and PI3-kinase 12, 13. PI3-kinase is localized into non-raft membrane regions and can migrate to raft microdomains following an activation event 14. Kolanus et al. 15 have shown that D-3 phospholipids generated by PI3-kinase activation, are one of the intermediary messengers involved in the regulation of LFA-1-dependent adhesion. Indeed, they induce association of LFA-1 with a cytoplasmic protein, cytohesin-1 16, leading to an increased affinity of LFA-1 for its ligand ICAM-1. The role of PI3-kinase in the polar redistribution of LFA-1 induced by chemokines has also been demonstrated 17.
As described above, CD4-dependent down-regulation of LFA-1-dependent adhesion requires the activity of PI3-kinase associated with the CD4-p56lck complex. Because of the relationships between LFA-1 and PI3-kinase, of their raft localization following different activation 11, and of the recruitment into raft of CD4 and p56lck 18, 19, we have addressed the possible role of raft microdomains in the induction of CD4-mediated down-regulation of LFA-1 mediated adhesion.
2 Results
2.1 Anti-CD4 Ab induces a transient association and membrane colocalization of PI3-kinase and LFA-1
It has been previously shown that different CD4 ligands such as an anti-CD4 Ab, can inhibit LFA-1-mediated CD4+ T cell adhesion to HLA class II+ B cells 4. PI3-kinase activity is one of the main components of this inhibition 5. We have investigated whether an interaction between LFA-1 and PI3 K occurs and whether it could be induced by CD4 ligation. By using co-immunoprecipitation experiments, we have detected a basal interaction between LFA-1 and PI3-kinase in the absence of exogenous activation of Jurkat cells (Fig. 1A). It was not observed in a control immunoprecipitation with a mouse anti-Ig. Incubation with an anti-CD4 Ab induced a transient treefold increase of this association, which is maximal after 2 to 5 min incubation and decreased thereafter. Same results were obtained with another T cell line, A201-CD4 (See Fig. S1 of Supporting Information). This association increase is not correlated to an increase of LFA-1 immunoprecipitated (Fig. 1A, blot anti-LFA-1) and is not observed when an anti-HLA class I is used as control Ab (data not shown). This association was also visualized by confocal microscopy analysis (Fig. 1B). Analysis by immunofluorescence imaging showed that p85-PI3-kinase was cytoplasmic in unstimulated Jurkat T cells. Following a 5-min incubation with an anti-CD4 Ab, a significant translocation of PI3-kinase from the cytosol to the membrane was observed, associated with a colocalization between LFA-1 and PI3-kinase. Patches of LFA-1 and PI3-kinase became visible on cell membrane. These patches were not observed following incubation with a control Ab specific to transferrin receptor (Fig. 1B, RTrf).
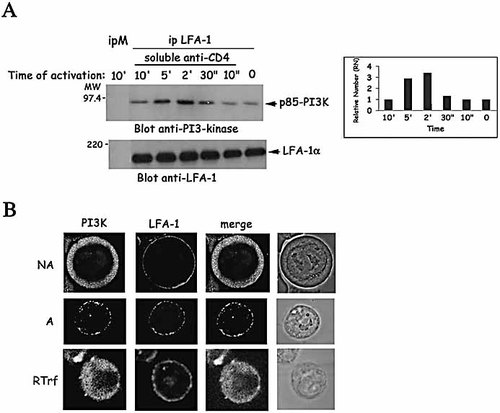
Transient association and membrane colocalization between PI3-kinase and LFA-1 are induced by soluble anti-CD4 Ab. (A) Jurkat T cells (2×107) were incubated for the indicated time with soluble anti-CD4 Ab then washed and lysed as described in Sect. 4. Total cell lysate (40 μg) and equivalent amount of proteins (1 mg) immunoprecipitated with an anti-LFA1β Ab (ip LFA-1), or an anti-mouse Ig (ipM) as control, were resolved on 8% polyacrylamide gel. Western blotting was performed by using an anti-p85-PI3K Ab (blot anti-PI3-kinase) then an anti-LFA-1α Ab (blot anti-LFA-1). Histogram represents the ratio of PI3K coprecipitated with an anti-LFA-1 Ab in activated cells over co-immunoprecipitation in unstimulated cells. Molecular weight markers are indicated (MW). Data depict one representative experiment out of five. (B) Jurkat T cells were untreated (NA), or incubated with an anti-CD4 Ab for 5 min (A), or with an anti-transferrin receptor (RTrf), washed, fixed and labeled as described in Sect. 4 with an anti-LFA-1α Ab or an anti-p85-PI3K Ab. Cells were examined with confocal microscopy and images are derived from one representative experiment out of five.
2.2 Anti-CD4 Ab induces a colocalization of LFA-1 and PI3-kinase in lipid rafts
Colocalization of LFA-1 and PI3-kinase was shown to be not uniformly distributed on T cell membranes following CD4 ligation with an anti-CD4 Ab (Fig. 1B). This suggested that these molecules translocate to specific membrane domains following CD4 ligand binding. The cholesterol-enriched microdomains called rafts have been described to be important for the formation of protein complexes in different signal transduction 9. Therefore, we have investigated whether the LFA-1/PI3-kinase association was localized in raft microdomains. As described in Sect. 4, Jurkat T cells were incubated with an anti-CD4 Ab and then cross-linked with an F(ab′)2 goat-anti-mouse Ig (GAMIg) to improve sensitivity. Cells were then lysed with Brij58 20 to optimally isolate the low-density, detergent-resistant membrane complexes that fulfilled all known structural features of rafts. As previously described by Montixi et al. 12, the detergent was added to the cell sonicate, to prevent during detergent extraction, an artificial trapping of soluble material with rafts. The rafts isolation was validated by dot blot detection with the cholera toxin (CT) raft marker (which binds GM1, one of the raft component). As shown in Fig. 2, we have observed, that independently of the activation, detergent-insoluble microdomains were found mainly in low-density raft fraction F2 and to a lower magnitude in fractions F4 and F3 after ultracentrifugation (Fig. 2A, upper panel). The HPTLC analysis showed that GM3, another raft marker, was mainly found in the F4 fraction (Fig. 2A, lower panel). Detergent-soluble membranes were found in the high-density fractions (F6 and F7) (Fig. 2A). Neither GM1 nor GM3 were detected in these fractions. We have then investigated whether LFA-1 and PI3-kinase were detected in the raft fractions following CD4 ligation. It was observed that LFA-1 was effectively detected in the raft fractions isolated from total lysates (Fig. 2B). Following a short anti-CD4 Ab incubation (2 min), LFA-1 was mainly detected in the fraction F4 where GM3 ganglioside was more detected. Following a 20-min anti-CD4 Ab incubation, LFA-1 was also detected in GM1 containing fractions (F2 and F3). These increases were not observed following a 20-min incubation with an anti-HLA class I control antibody or with the GAMIg used for the CD4 cross-linking. PI3 K was also detected in raft fractions (Fig. 2B). As previously observed for LFA-1, PI3-kinase was mainly detected in fraction F4, following a short incubation with anti-CD4 Ab and the amount increased also in GM1containing fractions following a 20-min incubation with anti-CD4 Ab, not with the control antibodies. In contrast to LFA-1, following a longer cross-linking of CD4 (30 min), PI3 K was always strongly detected in the different raft fractions (F3 and F4). Since both LFA-1 and PI3-kinase were detected in raft fractions, we have investigated whether an association between LFA-1 and PI3-kinase was taking place in the raft fractions. As previously observed in Fig. 1A, with other technical condition used to detect proteins in soluble membrane compartments, a faint basal LFA-1 / PI3-kinase association, was detected in detergent-soluble membrane (fractions F6-F7) after a 30-min GAMIg incubation and this association was transiently increased following CD4 cross-linking (Fig. 2C). This association was also detected to a lower magnitude in some raft fractions (F3 and F4) in absence of CD4 cross-linking. Following incubation with a cross-linked anti-CD4 Ab, an increase of LFA-1/PI3-kinase association became clearly detectable (following a 10 and 20-min cross-linking of CD4) in one of the raft fractions: F2 (Fig. 2C). A modification of repartition of LFA-1 or of PI3 K isolated from total lysates, were not detected in this fraction (Fig. 2B), suggesting that the increase of this association was not related to a recruitment of these proteins in this fraction. This association was not modified in the F3 and F4 fractions. Following a longer CD4 cross-linking exposure (30 min), the LFA-1/PI3-kinase association became identically detected in all the detergent-insoluble fractions. These results indicate that the CD4-mediated signal led in one way to an increased partition of both proteins in different membrane microdomains (mainly F3 and F4) and in another way modify association between these two proteins in another raft compartment (F2) without an increased recruitment of LFA-1 or PI3-kinase. Same results were obtained with A201-CD4 (Fig. S2, Supporting Information).
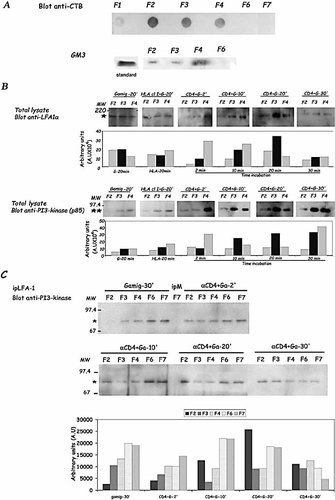
Anti-CD4 Ab induced association between LFA-1 and PI3-kinase in raft microdomains. (A) As described in Sect. 4, 3 μg of each fraction were tested in dot blot (A). GM1 was detected with a CT-HRP-conjugate and GM3 by HPTLC as described. This experiment is derived from one representative experiment out of ten. (B) Jurkat T cells (25×106) were incubated for 20 min at 37°C with F(ab′)2 GAMIg (Gamig) or anti-HLA class I Ab used as control, or with an anti-CD4 Ab (15 min at 4°C), washed and cross-linked during the time indicated at 37°C with F(ab′)2 GAMIg. After washing, cells were lysed, ultracentrifugated, and fractions containing detergent-resistant membranes (F2-F4) were collected and quantified as described in Sect. 4. Five micrograms of each raft fractions were analyzed and Western blotted with an anti-LFA-1α (upper panel, one asterisk indicates LFA-1) and with an anti-p85-PI3-kinase (lower panel, two asterisks indicate PI3-kinase). Molecular weight markers (MW) are indicated. Data depict one representative experiment out of six. Histograms represent the quantification of the different fractions in arbitrary units measured with a Fluorimager (FUJI-FLA 3000) and analyzed with Image Gauge. (C) Jurkat T cells (25×106) were incubated with the different Ab as described in (B), lysed, and ultracentrifugated as previously described. Proteins isolated from these different fractions were immunoprecipitated with an anti-LFA-1 Ab, or an anti-mouse Ig (ipM), analyzed by SDS-PAGE and Western blotting with an anti-PI3-kinase Ab (blot anti-PI3K). The asterisk indicates the p85 subunit of PI3-kinase. Molecular weight markers (MW) are indicated. Histograms represent the quantification of the different fractions in arbitrary units measured with a Fluorimager (FUJI-FLA 3000) and analyzed with Image Gauge. Data depict one representative experiment out of eight.
The different localizations of LFA-1 and PI3-kinase in raft compartments following CD4 cross-linking, were confirmed by using confocal microscopy analysis. In Fig. 3, following an incubation with GAMIg only or with a control antibody, GM1 (A1, A7; B1, B7), p85-PI3-kinase (A2, A8, A11, A17) and LFA-1 (B2, B8, B11, B17) were found uniformly distributed. Patches formation of GM1 (A4; B4), p85-PI3-kinase (A5, A14) and LFA-1 (B5, B14) were detected, following CD4 cross-linking. It was shown that p85-PI3-kinase (A6) and LFA-1 (B6) colocalized in patches with GM1 in the membrane following anti-CD4 cross-linking. Of note, only a fraction of LFA-1 colocalized with GM1. Therefore, it was investigated, whether or not LFA-1 and PI3-kinase could also localize within GM3-containing rafts. Following CD4 cross-linking, it was observed that PI3-kinase (A15) and LFA-1 (B15) indeed also colocalized with GM3. All these patches and colocalizations were not detected following incubation with a control antibody cross-linked with GAMIg (A9, A18; B9, B18). Same results were obtained with A201-CD4 (Fig. S3, Supporting Information). It has been determined that the GM1+ rafts and the GM3+ rafts partially colocalized following incubation with GAMIg (C-Gamig), control antibody (C-control) or anti-CD4 Ab (C-aCD4).
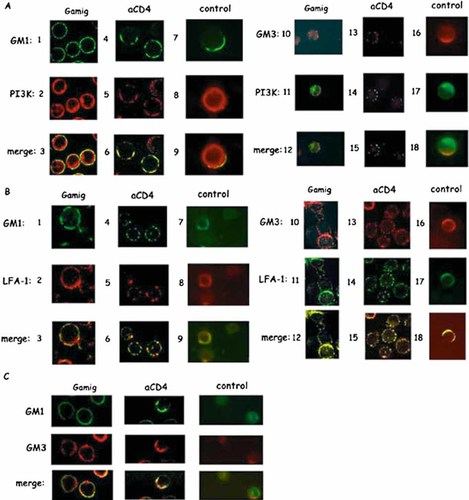
Anti-CD4 Ab induced a colocalization of PI3-kinase and LFA-1 with GM1 and GM3 ganglioside containing rafts. Jurkat T cells were treated with GAMIg for 2 min at 37°C: [(A): 1–3, 10–12; (B): 1–3, 10–12; (C): GAMIg], or incubated with an anti-CD4 Ab (15 min at 4°C), washed and cross-linked 2 min at 37°C with GAMIg [(A): 4–6, 13–15; (B): 4–6, 13–15; (C): aCD4). In some experiments, cells were incubated with an anti-transferrin receptor used as control Ab, (15 min at 4°C), washed and cross-linked 2 min at 37°C with GAMIg [(A):7–9, 16–18; (B):7–9, 16–18; (C): control]. After washing, cells were fixed/permeabilized/stained as described in Sect. 4. Rafts-GM1+ were detected in following panels: A1, A4, A7; B1, B4, B7; C-GM1 and rafts-GM3+ were detected in panels: A10, A13, A16; B10, B13, B16; C-GM3). Following activation with cross-linked anti-CD4 Ab, the colocalization of PI3-kinase with rafts-GM1+ and with rafts-GM3+ is shown, respectively in panel A6, A9 and A15, A18. The colocalization of LFA-1 with rafts-GM1+ and with rafts-GM3+ is shown, respectively in panel B6, B9 and B15, B18. The colocalization between rafts-GM1+ and GM3+ is shown in panel C-merge.
It was also observed that LFA-1, PI3 K, GM1 and GM3 partially colocalized with CD4, following cross-linking of CD4 (Fig. 4). These four molecules were not detected in patches following a GAMIg or a control Ab cross-linking.
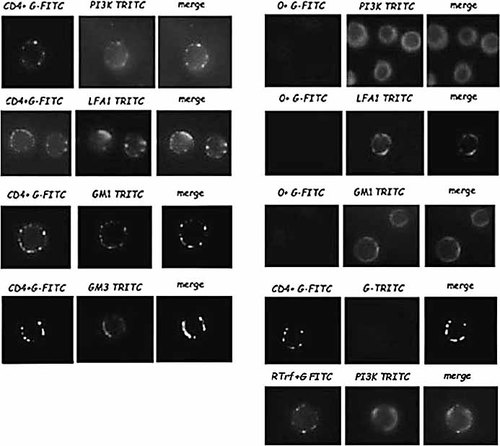
Colocalization of LFA-1, PI3-kinase and CD4 in GM1+ or GM3+ rafts, following CD4 ligation. As described in Sect. 4, Jurkat T cells were incubated or not (GAMIg), with an anti-CD4 Ab or an anti-transferrin receptor Ab used as control (RTrf), for 15 min at 4°C, then cross-linked during 2 min at 37°C with a FITC-coupled GAMIg. PI3-kinase, LFA-1, GM1, and GM3 were detected with appropriate Ab. All the colocalizations are shown in merge panels.
2.3 Role of the raft microdomains in the down-regulation of LFA-1-dependent adhesion induced by CD4 ligands
Previous results have suggested that the formation of rafts and the localization of LFA-1 and PI3-kinase in GM1+ and/or GM3+ microdomains could be necessary for CD4-mediated down-regulation of LFA-1-dependent adhesion. In order to investigate the role of raft microdomains in CD4-dependent down-regulation of LFA-1-mediated adhesion, we have examined in Fig. 5, the effects of the cholesterol-binding agent: methyl-β-cyclodextrin (MBCD) on Jurkat T cells adhesion to B cells. MBCD is a selective, surface-acting, cholesterol-depleting agent that does not incorporate into cell membranes 21 for solubilizing most of the raft-associated proteins. Its effect on LFA-1-mediated T cell adhesion to B cells was assessed. Down-regulation of adhesion induced by CD4/HLA class II interaction after a 20-min incubation with B cells was reduced following treatment of Jurkat T cells with MBCD (1 mM) (Fig. 5A). We have also observed that preincubation with MBCD (1 mM) neutralized the inhibitory effect of an anti-CD4 Ab on T cell adhesion, while the inhibitory effect of an anti-LFA-1 Ab was preserved (Fig. 5B). This inhibitory effect was specific since it was reversed by adding cholesterol during 30 min, after MBCD incubation (Fig. 5B), showing a dependence on intact lipid rafts for adhesion regulation. Same results were obtained with an other T cell line, A201-CD4 (Fig. S4, Supporting Information). Since LFA-1 and PI3-kinase were also found in GM3+ rafts, the role of GM3 in this down-regulation of adhesion was also investigated by using a GM3-CD4 interaction inhibitor, 3′sialyl lactose 22. This inhibitor also neutralized the CD4 ligand-induced down-regulation of adhesion between T cells and B cells (Fig. 5A), and the inhibitory effect of an anti-CD4 Ab on T cell adhesion (Fig. 5B), while the inhibitory effect of an anti-LFA-1 Ab was preserved (Fig. 5B).
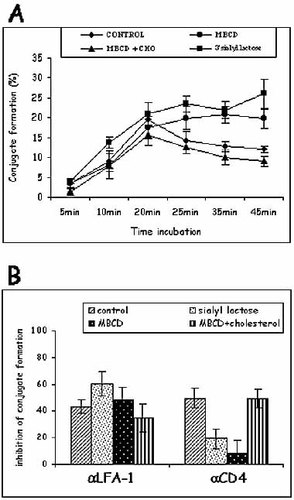
Down-regulation of LFA-1-dependent adhesion of Jurkat T cells to B cells requires rafts integrity and the association of CD4 to GM3. Prior to adhesion, Jurkat T cells were untreated (♦, □) or preincubated for 20 min at 37°C with 1 mM MBCD (•, □), or with 3′sialyl lactose (1.85 mg/ml) for 30 min at room temperature (▪, □). In some experiments, cells were washed and incubated with cholesterol 60 μg/ml for 30 min at 37°C, after incubation with MBCD (▴, □). Cells were then washed, incubated with B cells and the different mAb for conjugate formation. Results are given as the mean of ten independent experiments ± SD.
3 Discussion
To understand the mechanism by which CD4 ligands inhibit LFA-1-dependent adhesion of CD4+ Jurkat T cells to B cells, we have investigated whether protein interactions, putatively involved in the process, are modified after incubation with CD4 ligands. In co-immunoprecipitation experiments, we found that this incubation induced a transient increase in the association between PI3-kinase and LFA-1, which decreased after a 20-min incubation. Similar findings were obtained by using either Jurkat or A201-CD4 T cell lines. This result corroborates previous findings reported by Kolanus and our group. Indeed, Kolanus has shown that D-3 phospholipids synthesized by PI3-kinase up-regulate adhesion by enhancing the association between LFA-1 and a cytoplasmic protein, cytohesin-1 16 while we have found that LFA-1 and PI3-kinase dissociate from cytohesin following a CD4-mediated signal 6. These dissociations were dependent upon PI3-kinase activity that is increased by CD4 ligand binding 6.This suggests that PI3-kinase could interact with LFA-1. The present results confirm this hypothesis. In parallel, we investigated whether the association detected between LFA-1 and PI3-kinase was related to a modification of their localization. Immunofluorescence experiments showed that in the absence of CD4 ligand binding, PI3-kinase was cytoplasmic and translocated to the membrane following CD4 activation. It was also observed that incubation with the anti-CD4 Ab induced a colocalization in small patches to the membrane of LFA-1 with PI3-kinase, suggesting a specific compartmentalization. We have therefore investigated whether PI3-kinase and LFA-1 could localize to lipid raft domains potentially involved in CD4 signaling. Lipid rafts are heterogeneous in cholesterol and sphingolipid content and GM1, a well-known raft marker, is actually not the main ganglioside component of rafts. In peripheral T lymphocytes, the monoganglioside GM3 represents the main ganglioside component of the plasma membrane (72% of total gangliosides) 23. In addition, Iwabuchi et al. 24 have proposed that GM3 within rafts is involved in cell adhesion as well as in initiation of cell signal transduction. Thus, cell adhesion coupled with signal transduction operates through raft, and GM3-enriched microdomains are involved in TCR signaling 25 and CD4 signaling 22, 26. We found that the anti-CD4 antibody increases the recruitment of both proteins in some raft fractions, partially different in their ganglioside composition. Following short-time activation, PI3-kinase and LFA-1 were more immunoprecipitated from fraction containing more GM3 ganglioside than GM1. Following a longer activation, LFA-1 was also detected in fraction containing more GM1 than GM3 and PI3-kinase was identically detected in fractions containing GM1 or GM3. This recruitment is transient for LFA-1 and more stable for PI3-kinase. In contrast, the LFA-1/PI3-kinase association was mainly increased in the raft fraction containing mainly GM1 ganglioside. These results suggest that the increased association between LFA-1 and PI3-kinase observed in one of the GM1-containing fraction was not apparently related to a new recruitment of the two partners. Leitinger et al. 11 previously reported that activated LFA-1, but not inactived LFA-1, is preferentially found in lipid rafts. How integrins are held within the lipid raft compartment is an unresolved issue, since neither subunit of the integrin αβ heterodimer is modified by palmitoylation, a characteristic of many raft containing transmembrane proteins 27. Leitinger et al. 11 and Brown 27] have proposed that raft localization was dependent on complex formation between LFA-1 and other proteins. Following CD4 ligand binding, the association between LFA-1 and PI3-kinase could form one of the complexes necessary for localization of other partners, such as cytohesin, or others proteins containing Pleckstrin homology domains to bind phosphatidylinositol-3-4-5-triphosphate synthesized by PI3-kinase. In addition, CD4 activation also induced in different raft compartments, the recruitment of LFA-1 and of PI3-kinase without increasing their association, suggesting the participation of other partners. Indeed, other proteins such as SHP-2, p56lck or Gab2 which are important for the regulation of LFA-1 dependent adhesion 6 are also localized in these raft domains and their association with PI3-kinase were modified following CD4 signaling (manuscript in preparation). Because of the localization of LFA-1 and PI3-kinase in fractions partially different in their ganglioside composition, we investigated whether LFA-1 and PI3-kinase colocalized with GM1 and/or GM3. We observed that cross-linking of CD4 induced a more frequent aggregation of GM3-enriched lipid rafts than of GM1-enriched rafts. The higher frequency of GM3 rafts and the known GM3-CD4 association 26 could explain this. In addition, we found that PI3-kinase colocalized both with GM1 and GM3 as previously observed in the immunoprecipitation experiments. LFA-1 that partially colocalizes with GM1 was more strongly associated with GM3 following CD4 ligation. All of these proteins colocalized with CD4 following its cross-linking, showing that this colocalization was induced by CD4 signaling. It is not clear whether GM1 and GM3 are components of the same raft or segregate into distinct structure. The partial colocalization of GM1 and GM3 as observed in the absence, or following CD4 ligand binding, suggests that both gangliosides could in part be localized in different membrane compartments with potentially distinct functions 28, 29.
The functional role, in CD4-mediated down-regulation of adhesion, of lipid rafts in which LFA-1 and PI3-kinase translocate upon CD4 signaling, and more specifically of the CD4/GM3 interaction, was assessed by using a cholesterol-binding agent and an inhibitor of CD4/GM3 interaction. It was found that the integrity of lipid rafts was effectively required for the down-regulation of LFA-1-dependent adhesion induced by CD4, as this down-regulation was no longer observed when rafts were disrupted by MBCD, a molecule depleting cholesterol from lipid rafts. In addition, the association between GM3 and CD4 was also essential since the inhibition of CD4/GM3 interaction neutralized down regulation of adhesion. These results also establish the role of microdomain structure in antigen-independent CD4 signaling. Leitinger et al. 11 have previously shown that lipid rafts play a key role in positively regulating integrin activity. Our results show that lipid rafts are also involved in the negative regulation of LFA-1 adhesion. We have previously observed that the down-regulation of LFA-1-dependent adhesion induced by CD4-binding also requires the CD4-p56lck association 30. Recently Fragoso et al. 31 have demonstrated that the CD4 association with p56lck is important for maintaining CD4 highly concentrated in lipid rafts. The maintained localization of CD4 in rafts could thus be necessary to the recruitment of other proteins such as LFA-1 and PI3-kinase, or other kinases and phosphatases important for this down-regulation event. Further work is thus needed to assess mechanism and significance, in other T cells and in particular in peripheral T cells. Indeed, these associations and colocalization of LFA-1 and PI3-kinase in raft domains was not specific to the Jurkat cell line since similar results were obtained with an other T cell line, A201-CD4 (see Supporting Information).
One can therefore suggest that, following CD4 ligand binding, a fraction of PI3-kinase and LFA-1 translocates from non-raft to different raft compartments. Following CD4 signaling, activated-PI3-kinase could promote recruitment of proteins potentially involved in the regulation of adhesion. PI3-kinase could play distinct roles inside and outside of the raft compartments. Harriague et al. 32 and Costello et al. 33 have shown that PI3-kinase is detected both inside and outside the T cell-APC contact zone, suggesting that a sustained signal transduction is not restricted to the contact area and that different sites of the cell membrane are important. Constantin et al. 17 have also shown that PI3-kinase could also control lateral mobility of LFA-1 induced by chemokines, a cytoskeleton-dependent process. We have also previously described that CD4 binding induced dissociation between PI3-kinase, LFA-1 and cytohesin, an important cytoplasmic protein for the regulation of LFA-1 avidity for its ligand 6. Studying the localization of cytohesin in the different raft fraction, following CD4 signaling should therefore help to understand the mechanism of adhesion regulation. It is possible that a displacement of cytohesin localization in membrane domains, associated or not with LFA-1, could regulate adhesion. A study of parallel compartmentalization of cytohesin, LFA-1, PI3-kinase, and probably other interacting molecules is thus now warranted.
4 Materials and methods
4.1 Antibodies and reagents
The following antibodies (Ab) were used: 13B8.2, 25.3 or CD18 (IgG1, anti-CD4, anti-LFA-1α and anti-LFA-1β mAb, respectively), anti-HLA class I Ab from Immunotech, Marseille, France, anti-LFA1α mAb from Transduction Laboratories was used for Western blot experiments, anti-p85-PI3-kinase polyclonal Ab (Upstate Biotechnology, Inc, Lake Placid, NY). F(ab′)2 GAMIgG, was used for cross-linking experiments. For confocal immunofluorescence experiments, the following Ab were used: TRITC-conjugated F(ab′)2 GAMIgG and FITC-conjugated sheep anti-rabbit IgG (Jackson Immunoresearch Laboratories, Inc). FITC-conjugated cholera toxin (CT) (Sigma) and anti-GM3 Ab (Seikagaku Corp. Japan). The control antibody, OKT9, an anti-RTrf Ab was a gift of A. Alcover (Pasteur, France).
4.2 Cells culture
The human leukemia T cell line Jurkat, clone J77cl 20, has been previously described 34. The A201 T cell line was a CEM-derived T cell line, transfected with wild-type CD4 (A201-CD4). Both T cell lines and the EBV-B cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine.
4.3 Immunoprecipitation experiments and Western blot analysis
Jurkat T cells (20×106) were washed in RPMI 1640 (Life Technologies, UK), incubated for different time intervals with CD4 ligands, then pelleted, washed and lysed for 20 min on ice in lysis buffer A (50 mM Tris, pH 7.5; 110 mM NaCl; 10 mM EGTA; 1 mM orthovanadate (Na3VO4); 1% Brij 58; 5 mM iodoacetamide; 2 mM MgCl2, antipain, pepstatin, leupeptin (each 2 μg/ml); 2 μg/ml aprotinin and 1 mM PMSF). Lysates were clarified by centrifugation at 12,000×g for 15 min. The protein concentration was determined in postnuclear supernatants (PNS) by using the Bio-Rad kit (Bio-Rad, Richmond, CA) with bovine serum albumin as standard. The same amount of each PNS was incubated for 1 h at 4°C with 3 μg of rabbit, rat or mouse immunoglobulins, recovered by incubation with 40 μl of protein-G-Sepharose (PGS) beads for 45 min at 4°C. Then, the supernatant was incubated for 2 h or overnight at 4°C with the specific antibodies: anti-p85-PI3kinase (1 μg/ml), anti-LFA1α or β (5 μg/ml). Immunoprecipitates were recovered by incubation with 40 μl of PGS beads for 45 min at 4°C and washed in lysis buffer A prior to dissociation in reduced Laemmli sample buffer before resolution on SDS-PAGE using 8% gels. Then, they were electrophoretically transferred to a polyvinylidine difluoride membrane (Immobilon P, Millipore, Bedford, MA). Nonspecific binding was blocked with 5% bovine serum albumin in PBS and blots were hybridized with the different antibodies. Proteins were visualized using a chemiluminescence detection system (ECL-Amersham, Arlington Heights, IL) with an anti-rabbit, or an anti-mouse Ig coupled to horseradish peroxidase (HRP) as secondary Ab (Amersham). Experiments have been quantified by densitometric scanning.
4.4 Flotation experiments
For analysis of detergent-insoluble complexes in flotation gradients, Jurkat T cells were depleted in serum-free medium overnight before incubation with anti-CD4 antibody. Cells (25×106) were incubated 20 min at 4°C with anti-CD4 antibody, or anti-HLA-class I Ab used as control, then washed and incubated during time indicated at 37°C with a GAMIg. Cells were then lysed on ice for 15 min in 900 μl of lysate buffer A without detergent. Cells were sonicated gently (5-s bursts, 5 W; Branson sonifier 250) in 900 μl of ice–cold lysate buffer. After centrifugation at 800×g at 4°C for 5 min, the PNS was incubated with Brij 58 at a 1% final concentration for 1 h at 4°C. Detergent-resistant membranes were isolated by ultracentrifugation (28,000 rpm for 4 h at 4°C) in a SW41 rotor (Beckman Instruments Inc), in a 40-30-5% optiprep gradient (Sigma). Seven fractions were collected from the top to the bottom of the gradient [500 μl for each fraction except F2 (1 ml) and F7 (700 μl)]. Detergent insoluble fractions were recovered mainly from the low-density fractions 2-3-4 and soluble fractions were recovered from the high-density fractions 6-7, then immunoprecipitated and analyzed by SDS-PAGE and Western blotting.
To detect the GM1 glycosphingolipid in optiprep gradient fraction, we used a 1 μg quantity of individual fractions. Fractions were applied on PVDF membrane, then the membrane was blocked with 5% BSA in PBS/0.05% Tween 20 and probed for 45 min at room temperature with 0.5 μg/ml of CT-HRP conjugate. The membrane was washed with PBS/0.05% Tween 20, and the HRP activity was detected by chemiluminescence, as described.
The detection of GM3 was performed by High Performance Thin Layer Chromatography (HPTLC) as previously described 25.
4.5 Confocal microscopy
After activation, cell suspensions containing 8×104 cells/slide were layered onto poly-L-lysine-coated coverslips, for 45 min at room temperature, fixed for 15 min at 4°C in 4% paraformaldehyde then incubated in 50 mM NH4Cl 15 min at 4°C, to stop the fixation. Immunofluorescence staining of cell surface molecules was performed with the appropriate mAb in the absence of permeabilizing agent. Intracellular proteins were stained in 2 min 0.05% saponin-permeabilized cells. The first incubation with the appropriate Ab was followed by TRITC- or FITC-coupled appropriate secondary Ab. In some experiments, in order to follow the localization of LFA1 and PI3 K as a function of CD4 ligation, cells were incubated with an anti-CD4 Ab during15 min at 4°C and cross-linked with a FITC-coupled GAMIg during 2 min at 37°C. Then, 40 μg of GAMIg F(ab′)2 were added during 15 min at 4°C, to saturate the free sites. Immunofluorescence staining of the different molecules was performed as previously described. Images were acquired in a Zeiss LSM-510 confocal microscope with settings allowing in each case the maximum signal detection below the saturation limits of the detectors.
4.6 Conjugate formation
Conjugate formation was performed as described 35. Briefly, Jurkat T cells were incubated with hydroethidine (40 μg/ml; Polysciences, Warrington, PA) for 30 min at room temperature. EBV-B cell lines were incubated with bis-carboxyethyl-carboxyfluorescein acetoxymethyl ester (100 μg/ml, Calbiochem, San Diego, CA) for 30 min at 37°C. After washing, cells were incubated with methyl-β-cyclodextrin (Sigma) 10 min at 37°C or with 3′ sialyl lactose (Dextra Lab LTD, GB) 30 min at room temperature. In some experiments, cells were washed after MBCD incubation, then incubated with cholesterol (60 μg/ml) 30 min at 37°C. Cells were further washed, incubated with EBV-B cell lines during 20 min or time indicated and cooled at 4°C to block adhesion. Two-to-three hundred fluorescent cells were counted blindly in each experiment under a fluorescence microscope. Results are expressed as the percentage of T-B conjugates among all T cells. Statistical analysis was performed by using the Student's t-test.
Acknowledgements
We thank Y. Goureau and J. Lipecka for valuable help on image acquisition with confocal microscope. M. Trucy is supported by a doctoral fellowship from La Ligue National contre le cancer. This work was supported by INSERM.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




