Somatic mutations can lead to a loss of superantigenic and polyreactive binding
Abstract
Although antibodies have been assumed to bind a specific antigen, evidence exists showing that a single antibody can bind to multiple unrelated antigens. We previously studied a human monoclonal antibody expressing a mutated form of the VH3–73 gene and displaying anti-tubulin activity in a patient suffering from an immunocytic lymphoma. Despite its expression of a VH3 family member, this immunoglobulin failed to react with protein A (SpA), suggesting that somatic mutations could account for its change in specificity. To examine this possibility, we produced recombinant Ig expressing germ-line (IgMκ-Germ) or the mutated form (IgMκ-PER) of the VH3–73 fragment. Comparison of the respective affinities of the two Ig demonstrated that IgMκ-Germ restores its SpA-binding capacity, and shows a moderate decrease in its affinity for tubulin. Interestingly, IgMκ-Germ displayed polyreactive specificity for different autoantigens, which contrasted to the monospecific binding of IgMκ-PER to tubulin. These results suggest that the monoreactive IgMκ-PER antibody may be derived from a natural polyreactive antibody through somatic mutation. In addition, both temperature modification and mild denaturation succeeded in recovering the polyreactivity of IgMκ-PER, which favors the view that conformational modifications of the tertiary structure of antibodies may play a key role in the genesis of polyreactivity.
Abbreviation:
-
- CDR:
-
Complementarity-determining region
-
- FR:
-
Framework region
-
- SpA:
-
protein A
-
- SAg:
-
Superantigen
1 Introduction
Immunoglobulins in their monomeric form are four-chain macromolecules composed of two heavy (H) and two light (L) chains. Each chain contains variable domains, which define the antigen (Ag)-combining site of the antibody, and several constant domains aimed to define effector function. Both H and L chain variable domains contain three regions of extensive sequence variability, termed the complementarity-determining regions (CDR), and four regions of relative sequence stability, termed framework regions (FR). The three L chain CDR and the three H chain CDR are juxtaposed to form the antigen-combining site of the antibody, as classically defined. In turn, the FR creates a scaffold that surrounds, supports, and influences the conformation and structure of the CDR 1–4. Although the historical and canonical basis of immunology was the antibody monospecificity dogma, the binding of a single antibody to different unrelated antigens has been extensively documented 5–7. In addition, data derived from crystallographic studies elegantly visualized the simultaneous binding of two different antigens with a single paratope.
Together with their ability to bind antigens via CDR, lg can react with unconventional antigens via the FR 1. These unconventional Ag are the B cell superantigens (SAg), andmost of them include microbial proteins, such as protein A (SpA) from Staphylococcus aureus 4, gp120 from HIV-1 8, and the staphylococcal enterotoxin A 9. SpA contains five highly homologous extracellular Ig-binding domains, which can bind Fcγ and Fab fragments 4. The Fab specificity involves a site on the variable region of the Ig H chain 8 and is restricted to products of the human VH3 family that represent as many as 43% of inherited human VH genes 3, 9–11. It has been proposed that in vitro stimulation with SpA can contribute to selection of these B cells and promote the production of antibodies that may include rheumatoid factor autoantibodies 12, whereas in vivo exposure to recombinant SpA can result in supraclonal suppression and deletion of B lymphocytes expressing a given VH family 13, 14. The structural basis for these Fab-binding interactions was recently revealed in crystallographic analysis of the complex between a VH3-expressing Fab and domain D of SpA 15. A VH3-binding human SAg has also been reported 9, 16.
In a previous study, we analyzed four different human monoclonal antibodies (mAb) displaying different isotypes (IgA1κ, IgG1κ, IgG2κ, and IgG4κ) and anti-tubulin activity that were isolated from the serum of a patient (patient PER) suffering from an immunocytic lymphoma 17. The complete sequence of these mAb showed that they shared the same VH and VL domains associated with mutations compatible with an antigen-driven process. However, despite the identity in their variable domains, IgA1κ, IgG1κ, and their Fab fragments bound to a common motif of β-tubulin with striking differences in affinity 18, 19. Interestingly, despite their expression of a VH3 gene family, the different Ig from patient PER were unable to bind SpA, suggesting that the mutational process undergone by this Ig gene resulted in the loss of this activity.
To better assess the effect of somatic mutations in the VH3–73 gene, we constructed two recombinant antibodies, one containing the complete mutated κ chain from IgA1κ-PER and the corresponding VH3–73 region in its germ-line configuration (IgMκ-Germ) and the other containing the same mutated κ chain and the mutated form of the VH3–73 gene expressed by patient PER (IgMκ-PER). We compared the antigen and SAg binding affinities of the recombinant Ig, and our findings demonstrate that the germ-line transformation of VH3–73 results in recovery of SpA-binding activity, does not introduce significant modifications in the affinity for tubulin, and induces the appearance of a polyreactive-binding pattern to the different autoantigens. This polyreactive binding was also observed when IgMκ-PER was submitted to a dissociating process, providing new evidence that conformational changes could play an important role in the genesis of polyreactivity.
2 Results
2.1 Comparison of recombinant mutated (IgMκ-PER) and germ-line (IgMκ-Germ) VH3-encoded antibodies
To evaluate the role of the somatic mutation process in the generation of IgMκ-PER antibody specificity and to avoid misinterpretation related to V gene polymorphisms, we isolated and sequenced the germ-line VH3–73 from autologous genomic DNA of patient's T cells 18. The differences between the germ-line 3–73 VH domain and the mutated form expressed by patient PER are depicted (Fig. 1a, b). There were eight and four codons which underwent replacement (R) and silent (S) mutations, respectively, in the FR (R:S ratio of 2), as compared to nine replacement and three silent mutations in CDR 1 and 2 (R:S ratio of 3). Mutations and replacements predominant in CDR induced a significant hydrophobicity shift in these domains (Fig. 1a, b). In addition, the structural model of an Ab with mutated VH3–73 is shown, with the mutated residues highlighted (Fig. 1c). Somatic mutations resulting in multiple replacements in the CDR with preservation of the peptide sequence in the FR are typical of Ab that have undergone selection by Ag. In addition, mutations in FR predominated in the FR3 domain of both H and L chains, a region that may itself contribute to Ag recognition 18.
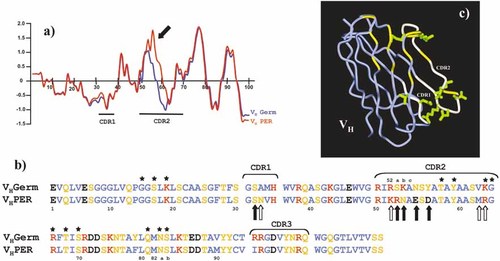
Comparison of the germ-line and mutated sequences expressed by patient PER at the protein level. (a) Replacement mutations predominate in the VH CDR2 region, which induce important changes in the Hopp and Woods hydropathicity plot. (b) Amino acid changes are shown. Filled arrows depict major replacements, and open arrows point out amino acid changes without variation in the lateral chain. Stars indicate the essential amino acids involved in the binding of VH3 Ig to SpA. (c) Structural model of the VH3–73 domain. Somatic mutations are indicated in green, and CDR1/CDR2 regions are highlighted in white. Binding sites of SpA protein interaction are shown in yellow.
2.2 Expression and purification of IgMκ-PER and IgMκ-Germ antibodies
Expression vectors containing the mutated human μ H chain (pRTM1μ-PER, Fig. 2a) and the germ-line-encoded human μ H chain (pRTM1μ-Germ, Fig. 2b) were constructed and co-transfected into the murine myeloma X-63 cell line together with a vector for the expression of the mutated human κ L chain (pSVκ-PER, Fig. 2c). Transfected clones were selected on the basis of their resistance to neomycin, and supernatants were tested by ELISA and Western blot for secretion of complete IgMκ. "Transfectomas" secreting complete pentameric human IgMκ-PER or IgMκ-Germ were expanded, and culture supernatants were purified by affinity chromatography (Fig. 2d). The presence of pentameric IgMκ recombinant Ig was substantiated by gel filtration chromatography for both recombinant Ig (data not shown).
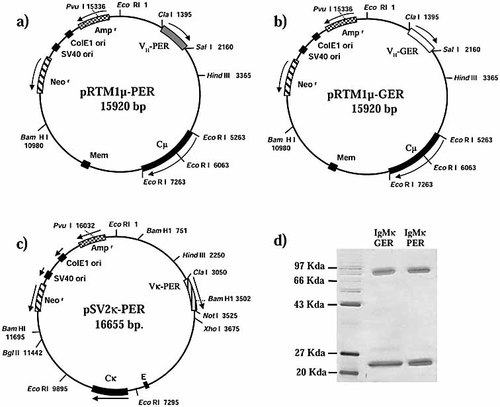
Schematic representation of vectors pRTM1μ-PER, pRTM1μ-Germ and pSV2κ-PER. pRTM1μ contains the functionally rearranged VH PER (a) and VH Germ (b) genes, respectively (Cμ: exons encoding the human Cμ constant region; Mem: Cμ membrane exon; Neor: neomycin resistance gene; SV40 and ColE1: origins of replication; Ampr: ampicillin resistance gene). (c) pSVκ-PER contains the functionally rearranged Vκ-PER gene and all the necessary genetic information for expression of a complete human κ chain. (Cκ: the κ L chain constant region exon; arrows: direction of gene transcription; open labeled boxes: important regions of vectors expression). (d) SDS-PAGE analysis of recombinant antibodies after affinity chromatography purification. One hundred nanograms of each antibody was run on a 10% polyacrylamide gel under reduction conditions and then stained with silver nitrate.
2.3 Germ-line recovery of IgMκ-PER results in the rescue of binding capacity for SpA
To better define the reactivity differences between the two engineered Ig, we analyzed the binding affinity of mutated IgMκ-PER and germ-line-encoded IgMκ-Germ to SpA by surface plasmon resonance (Biacore technology). The affinity constant for IgMκ-PER was KD =1.47 × 10–7 M (kASS =2.48×103 M–1s–1 and kDISS =3.66×10–4 s–1), whereas for IgMκ-Germ it was KD =6.86×10–9 M (kASS =8.32×104 M–1s–1 and kDISS =5.71×10–4 s–1) (Fig. 3 and Table 1).
2.4 Germ-line recovery of IgMκ-PER results in a moderate decrease in the affinity for tubulin
The affinity constants of both antibodies with the tubulin antigenic determinant (QQYQDATAEEEEDFGEEAE peptide motif) were also analyzed: KD =9.8×10–9 M (kASS=5.57×104 M–1s–1 and kDISS =5.46×10–4 s–1) for recombinant IgMκ-PER and KD =1.7×10–8 M (kASS = 5.04 × 104 M–1s–1 and kDISS =8.41×10–4 s–1) for recombinant IgMκ-Germ (Fig. 3 and Table 1).
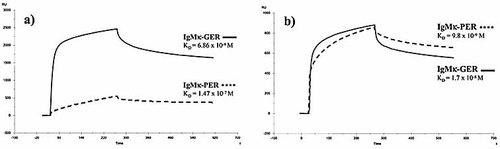
Affinity measurement of recombinant IgMκ-Germ and IgMκ-PER antibodies. (a) Binding of both Ig with immobilized SpA. (b) Binding of both Ig against immobilized tubulin antigenic determinant (RU: Resonance units). Kinetic association and dissociation constants for all antibodies are reported in Table 1.
|
|
Protein A |
Tubulin peptide |
||
|---|---|---|---|---|
|
|
IgMκ-PER |
IgMκ-Germ |
IgMκ-PER |
IgMκ-Germ |
|
kASS (M–1s–1) |
2.48 × 103 |
8.32 × 104 |
5.57 × 104 |
5.04 × 104 |
|
kDISS (s–1) |
3.66 × 10–4 |
5.71 × 10–4 |
5.46 × 10–4 |
8.41 × 10–4 |
|
KD (M) |
1.47 × 10–7 |
6.86 × 10–9 |
9.8 × 10–9 |
1.7 × 10–8 |
2.5 Germ-line recovery of IgMκ-PER results in the appearance of polyreactive binding activity
To analyze the effects of somatic mutations on polyreactivity, we carried out ELISA with a panel of known autoantigens (actin, myosin, tubulin, and DNA). The utilization of the germ-line VH3–73 segments in the IgMκ-Germ construction resulted in the appearance of significant binding to actin, myosin, and DNA, in addition to the binding to tubulin (Fig. 4). Because the two recombinant antibodies exhibited a similar reactivity to anti-κ Ig, differences in the antibody concentrations were disregarded (Fig. 4).
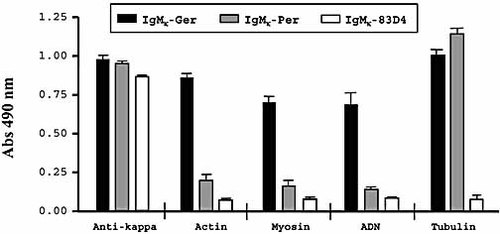
Polyreactivity of IgMκ-Germ and IgMκ-PER recombinant antibodies. Binding of purified recombinant antibodies was tested in ELISA plates coated with actin, myosin, ADN or tubulin. Plates coated with anti-κ antibodies were used as a positive control. Irrelevant IgMκ-83D4 mAb was used as negative control. Values represent the mean of triplicates (± SD).
2.6 Dissociating treatment of IgMκ-PER and IgMκ-Germ results in the appearance of polyreactive binding activity
To determine whether the observed differences in the binding of the recombinant Ig could be related to conformational modifications, we used a method of mild denaturation of the antigen-combining site 20. Both recombinant Ig were treated with 6 M urea as a dissociating agent and renaturated by dialysis in PBS. The binding activities of IgMκ-PER and IgMκ-Germ against the autoantigen panel were measured by ELISA before and after the dissociating treatment. While no significant binding changes occurred with IgMκ-Germ (Fig. 5A), the dissociating process induced the appearance of significant polyreactive binding activity by IgMκ-PER, without introduction of a significant change in the binding to tubulin (Fig. 5B).
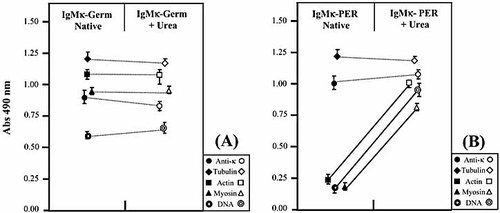
Effect of denaturation-renaturation treatment on the polyreactivity of IgMκ-Germ and IgMκ-PER recombinant antibodies. Capture ELISA was used to evaluate the interaction of purified IgMκ-Germ (A) and IgMκ-PER (B) with the same panel of antigens. "Native" and "+ Urea" correspond to purified antibodies before and after the denaturation-renaturation process (treatment with 6 M urea), respectively. The bold lines show the increased reactivity of IgMκ-PER against auto-antigens following denaturation-renaturation.
2.7 Temperature variations are able to reconstitute the polyreactive binding specificity of IgMκ-PER
Previous work indicated that treatments such as acid pH 21 or denaturing agents 20 are able to increase polyreactive binding. To further evaluate the role of conformation modifications, both recombinant IgMκ-PER and IgMκ-Germ were tested in ELISA at two different temperatures (37°C and 4°C). Incubation of ELISA at 4°C did not modify the binding specificity of IgMκ-Germ (Fig. 6A), whereas diminution of temperature induced a significant increase in the binding of IgMκ-PER to actin, myosin, and DNA (Fig. 6B).
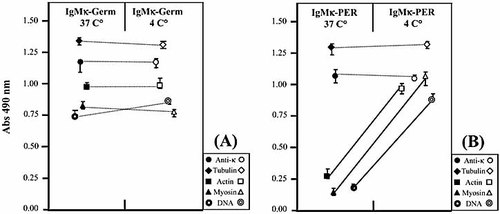
Effect of temperature on the polyreactivity of IgMκ-Germ and IgMκ-PER recombinant antibodies. The reactivity of purified IgMκ-Germ (A) and IgMκ-PER (B) was assessed by ELISA at 4°C and 37°C. The bold lines depict the increased reactivity of IgMκ-PER against auto-antigens at 4°C. Values represent the mean of triplicates (± SD).
3 Discussion
Antigen binding by the B cell receptor is an important event in B cell activation and selection. It has long been assumed that only the hypervariable regions of the Ab are involved in ligand binding. However, it has been demonstrated that together with their ability to bind antigens via CDR, lg can react with SAg via the FR 1. The Fab specificity of the human VH3 family against SpA involves a site on the variable region of the Ig H chain 8. One current opinion is that microbial SAg are involved in pathogenicity by stimulation of a high percentage of B cells, leading either to expansion or cellular apoptosis of these B cells 22–24.
To analyze the role of somatic mutations in the binding to SpA, we constructed two recombinant IgM antibodies (IgMκ-PER and IgMκ-Germ). The only difference between these two IgM constructions was the replacement of the mutated VH3 segment in IgMκ-PER with its non-mutated germ-line counterpart in IgMκ-Germ. Recovery of the VH3–73 germ-line sequence resulted in a striking recovery of SpA binding, suggesting that somatic mutations in the VH domain are responsible for this phenomenon. Graille et al. determined the crystal structure of the SpA domain D complexed with the Fab fragment of a human IgM antibody and described the key contact residues from both partners in this interaction 15. In the Fab, the contact residues are located in the FR and interact with helix II and helix III of the SpA domain D. The site of SpA-Fab interaction is remote from the antigen-combining site and the H and L chain constant domains. The following variable H chain residues mediate this interaction: Gly-H15, Ser-H17, Arg-H19, Lys-H57, Tyr-H59, Lys-H64, Gly-H65, Arg-H66; Thr-H68, Ser-H70; Gln-H81; Asn-H82a and Ser-H82b. Among the 13 VH residues involved as SpA contacts, VH3 genes include frequent germ-line sequence variations only at position H57. It is not a core residue interface, whereas Lys, Ile, and Thr are permissive at this position 25, 26. The other 12 VH residues in contact with domain D are highly conserved in members of this family. Indeed, amongthe 22 potentially functional human VH3 gene segments, there are only two germ-line variations: the conservative change to Lys at H19 in VH3–73 and the nonconservative change to Gly at H82a in VH3–64. However, each of these genes is the source of less than 2% of the VH3 Ig repertoire expressed in adults 27. From a structural point of view, a core of seven critical residues can be defined: Arg/Lys-H19, Gly-H65, Arg-H66, Thr-H68, Ser-H70, Gln-H81, and Asn-H82a. Apart from the requisite salt bridge at position H19, all other residues are virtually unexposed to solvents, making their replacement by a larger residue highly disruptive. Hence, this core of seven VH residues forms the structural motif for SpA binding, and this conveys the restricted specificity for VH3-encoded Ig and their homologues.
Despite important variations in binding affinities to SpA, the discrepancy between the germ-line and mutated domains in terms of SpA reactivity depends on a single mutation from Lys to Arg. These two residues are alkaline, and Arg is the residue observed at this position in the VH3 sequences. It is presently unclear whether this single change could, by itself, account for the different binding affinities.
We simultaneously compared the anti-tubulin activity of IgMκ-PER and IgMκ-Germ. Although, the mutation pattern observed for the PER clone is in agreement with a selective process driven by the antigen, we could not substantiate significant affinity differences between these two constructions. In the case of IgMκ-PER, important changes occurred in the CDR2 segment: Ser to Arg, Lys to Asn, Asn to Glu, Tyr to Asp, and Val to Arg. This suggests that a significant proportion of the CDR is not involved in the binding to tubulin and remains free for other Ag binding. Thepossibility that hydrophobic charges could hide the contact with the antigen is ruled out by the hydrophilic nature of the new amino acids (Fig. 1A). Thus, the somatic mutations present in the VH segment did not appear to play a major role in the binding to tubulin. In agreement with this possibility, Carayannopoulos et al. 28 showed that the affinities of recombinant antibodies using somatically mutated parental rheumatoid factor (RF) VH segments displayed a decreased affinity for IgG-Fcγ compared to their closest germ-linecounterparts (germ-line revertants). This indicates that the antibodies posses the highest affinity in their germ-line configuration. However, if we assume that tubulin is the true antigen that selected this clone, it is likely that the Vκ domain and/or the VH-CDR3 could play a central role in the anti-tubulin reactivity.
Interestingly, replacement of the mutated VH3–73 segment with its germ-line counterpart resulted in the acquisition of a polyreactive binding specificity absent in the mutated form. To date it is unclear whether somatic mutations induce conformational changes sufficient to preclude polyreactive binding specificities. In agreement with this possibility, Wademeyer et al. compared the crystal structures of two antigen-antibody complexes; the first included the antibody in its germ-line configuration and the bound hapten, whereas the second comprised the mutated affinity-matured antibody and the same bound hapten 29. As a result of nine replacements by somatic mutations, affinity maturation induced a 30,000 times greater affinity for the hapten than the germ-line-encoded antibody. Significant changes in the configuration of the combining site occurred upon binding of the hapten to the germ-line-encoded antibody, whereas the hapten bound to the mature antibody by a key-and-lock mechanism. The reorganization of the combining site nucleated by hapten binding is further optimized by somatic mutations that occur as far as 15 Å from the bound hapten. These results suggest that the binding potential of the primary antibody repertoire may be largely expanded by the ability of germ-line antibodies to adopt several configurations of the combiningsite, with both antigen binding and somatic mutation stabilizing the configuration with optimal hapten fit. When the germ-line antibody binds the hapten, its structure is substantially altered. In contrast, there is only a slight alteration of the structure of the mature antibody upon hapten binding. Interestingly, the structure of the mature antibody, in either the bound or unbound state, closely resembles that of the germ-line antibody in the bound state. The same mechanism could be evoked to explain the binding variation of Ig with SpA.
It appears that the germ-line-encoded antibody is pluripotent with respect to the conformations it can assume upon contacting various antigens. The mutations may consolidate a specific conformation that is especially well suited for binding the antigen or SAg that drives the immune response. The set of proteins that constitutes the germ-line antibody repertoire must recognize an almost limitless array of potential antigens. If each of these proteins can adopt a variety of conformations, then the effective diversity of the germ-line repertoire is greatly expanded. Mutations that preserve antigen binding while modifying some of the alternative conformations are expected to occur more frequently at positions that lie distant from the site of hapten binding.
There is consistent evidence demonstrating that humoral immune diversity is mainly obtained through combinatorial and somatic mutation processes. However, crystallographic evidence of antibodystructures obtained with or without the ligands, showing the presence of conformation dimorphisms, favors the existence of an additional source of diversity at the level of antibody tertiary structure 30, 31. The presence of conformational isomers of the same antibody binding to the hapten with different affinities has also been suggested by the kinetic studies of Foote and Milstein 32 and has recently been substantiated by x-ray crystallographic studies by James et al. 33. In addition, Labrousse et al. 34 suggested that polyreactive antibodies possess a more "plastic" structure than monoreactive species and can thus more easily undergo alternative conformations when they are subjected to unusual microenvironments. Overall, these data indicate that the binding site of a single antibody is able to adopt different conformations and thereby bind unrelated antigens. The fact that temperature variation and dissociating treatment, which are both able to provoke conformational changes, induce the appearance of polyreactive binding is in agreement with this hypothesis.
In conclusion, our results suggest that the monoclonal IgMκ-PER was derived by somatic mutation from a natural polyreactive antibody, which lost its SAg and polyreactive binding capacities following this process. This finding lends support to the hypothesis that polyreactive antibodies are a product of the germ-line repertoire and constitute a template upon which Ag-driven selection and somatic mutation operate to derive highly specific immune antibodies 35. The study of Naparstek et al. 36 provided evidence supporting this view. In the present case, the key mutation may have randomly occurred during the development of a malignant clone. Alternatively, the mutation may have preceded the malignant transformation in a clone already engaged in a specific immune response. A fascinating hypothesis would be that a staphylococcal infection was involved in this process and that this clone escaped SAg-induced apoptosis through a mutational process. If true, this case could constitute a new type of immune selection of B cells induced by SAg.
4 Materials and methods
4.1 Construction pRTM1μ-PER
A recombinant IgM containing the VH domain from IgA1κ-PER associated with the μ constant domain and containing the complete κ chain from Ig PER was constructed as previously described 19. Genomic DNA isolated from patients IgA1κ-expressing lymphocytes was amplified using a 5′ primer annealing to the leader region (5′ LH3-ClaI: ATG CAT CGA TAA TCA CCA TGG AGT TTG GGC TGA GCT GG) and 3′ consensus primer annealing to the JH region (JH-SalI: TGT GTC GAC TCA CCT GAG GAG ACG GTG ACC AGG G). Thecomplete sequences of VH and VL domains of this recombinant Ig were sequenced to discard Taq infidelities.
4.2 Construction of pRTM1μ-Germ
We previously isolated the germ-line form of VH3–73 from autologous genomic DNA patient's T cells 18. Using PCR, we constructed a fragment containing the FR1-CDR1-FR2-CDR2-FR3 from the germ-line-encoded VH3–73 domain associated with the CDR3 and FR4 from Ig PER and the constant μ domain. The germ-line FR1-CDR1-FR2-CDR2-FR3 fragment was obtained byamplification of the plasmid containing the germ-line VH3–73 segment using the following primers: 5′ LH3-ClaI-forward, ATG CAT CGA TAA TCA CCA TGG AGT TTG GGC TGA GCT GG;and 3′ γ FR3-μ CDR3-reverse, GGT TGT AAA CAT CTC CCC GTC TAG TAC AGT AAT ACA CGG. The CDR3-FR4 fragment from Ig PER was amplified from a plasmid containing the mutated form using the following primers: 5′ γ FR3-μ CDR3-foward, CCG TGT ATT ACT GTA CTA GAC GGG GAG ATG TTT ACA ACC and JH-SalI: TGT GTC GAC TCA CCT GAG GAG ACG GTG ACC AGG G. Of note, γ FR3-μ CDR3-reverse and -forward share complementary sequences allowing their assembly. In a subsequent step, both fragments were purified in an agarose gel and assembled using a mixture of the fragments at an equimolecular ratio and 5′ LH3-ClaI and JH-SalI primers. The amplified fragment obtained was subsequently cloned into a PKS plasmid and sequenced.
4.3 Construction of pSV2κ-PER
An Lκ-Jκ PER fragment was obtained by genomic DNA amplification with 5′ leader-specific LκII-ClaI (CAT ATC GAT TTC TCA CAA TGA GGC TCC CTG CTC AGC) and 3′ Jκ5-NotI (AGA GCG GCC GCA AAT GCT TAC GTT TAA TCT CCA GTC GTG TCC C) primers. A fragment containing a confirmed Vκ sequence was ligated into the pSVκ vector 19.
4.4 Construction of IgMκ-PER and IgMκ-Germ-secreting transfectomas
Both H and L chain expression vectors were transfected into the non-secreting murine myeloma P3X63Ag8.653 cell line by electroporation. Briefly, 5×106 X-63 cells in RPMI medium without FCS were co-electroporated with 10 μg linearized DNA from pRTM1μ-PER and pSVκ-PER using a 250 V-1500 μF pulse in an EasyJect+ device (Eurogentec, Belgium). Transfected cells were cultured in RPMI medium containing 20% FCS, selected with neomycin (G418, Gibco, France) at a final concentration of 1 mg/ml, and cloned by limiting dilution. Expression of complete engineered IgMκ-PER and IgMκ-Germ molecules was analyzed by Western blot and ELISA, as previously reported 19.
4.5 Expression and purification of secreted IgMκ antibodies
Selected clones secreting IgMκ-PER or IgMκ-Germ were subcloned, stabilized, and grown in large scale for purification. Purification from the supernatant was performed by affinity chromatography on a CNBr-activated Sepharose CL-4B column (Pharmacia Biotech, USA) coupled with rabbit anti-human κ chain antibody (DAKO, France). Bound antibodies were eluted with 0.1 M glycine-HCl (pH 3.0) and neutralized with 1 M Tris-HCl (pH 8.0). The final antibody concentration was determined by absorbance measurement at 280 nm using an extinction coefficient of 1.18 for a 1 mg/ml IgM solution, and purity was evaluated by SDS-PAGE followed by silver staining. To assess the relative molecular weight of native recombinant IgMκ antibodies, FPLC on a Superose 6 HR 10/30 column (Pharmacia Biotech, USA) was carried out. The column was equilibrated and eluted with 0.1 M ammonium acetate (pH 7.0). Each antibody (200 μl, 100 μg/ml) was injected at a flow rate of 0.35 ml/min 37.
4.6 Surface plasmon resonance
Equilibrium and kinetic constants for the interactions were determined using the BIAcoreTM system (Pharmacia Biosensor AB, Uppsala, Sweden). Immobilization of SpA (Sigma-Aldrich, France) via primary amines to the Sensor Chip CM5 was performed using an amine-coupling kit. Activation of the carboxymethylated dextran matrix was obtained by injecting a mixture of N-ethyl-N′-(dimethyl-aminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). A 20 μg/ml solution of SpA in 0.1 M sodium acetate was used for coupling, and it rendered a signal of 250 resonance units. A biotinylated peptide, Biot-Q-19-E (biotinyl-QQYQDAT-AEEEEDFGEEAE), was synthesized (Neosystem Laboratoire, Strasbourg, France) for affinity measurement. Immobilization of biotinylated peptide was achieved using a Sensor Chip SA containing streptavidin covalently coupled to a carboxymethylated dextran surface. Kinetic rate constants (kASS and kDISS) as well as apparent equilibrium affinity constants (KD = kDISS/kASS) were determined using BIAcoreTM Kinetics Evaluation software 19.
4.7 ELISA
ELISA was used to evaluate the binding of recombinant antibodies IgMκ-PER and IgMκ-Germ to different antigens. Plates were coated with 10 μg/ml actin from human muscle, 5 μg/ml myosin, 10 μg/ml DNA or 5 μg/ml tubulin (Sigma). Nunc 96-well microtiter plates (Roskilde, Denmark) were coated overnight at room temperature with 100 μl/well Ag diluted in carbonate buffer (pH 9.6). After five washes with PBS containing 0.1% v/v Tween 20, the plates were blocked for 1 h at 37°C with PBS containing 1% gelatin. After five washes, serial dilutions of IgMκ-PER and IgMκ-Germ were incubated for 3 h at 37°C, and after five additional washes, the plates were incubated for 1 h at 37°C with peroxidase-conjugated rabbit anti-human μ chain Ab (DAKO, France). All washes were carried out at 37°C. For assays at low temperature, a similar protocol was utilized, but all incubation steps and washes were performed at 4°C. Theplates were developed with o-phenylenediamine and read at 490 nm.
4.8 Molecular modeling of IgMκ-Germ and IgMκ-PER
Models of the L and H chains for both engineered Ab were generated using the WAM algorithm (web Ab modeling, http://antibody.bath.ac.uk), which combines energy- and knowledge-based methods 38. Since the H2 CDR found in both Ig does not belong to any canonical class, we proceeded by homology modeling, using the known crystal structures from Ab having sequence identitiesgreater than 70% in this region as templates. Visualization and analysis of the predicted structures were performed using SPDB-viewer, and the figures were realized using MOLSCRIPT software 39.
Acknowledgements
We thank our colleagues G. Bentley and P. Alzari from the Pasteur Institute for helpful discussions on structural antibody conformation and Mrs. Reine Bouyssié for secretarial assistance. P. Oppezzo is a recipient of a Grant from the Académie Nationale de Médecine.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




