Comparative analysis of NK- or NK-CTL-mediated lysis of immature or mature autologous dendritic cells
Abstract
Natural killer (NK) cells have been shown to kill efficiently autologous immature dendritic cells (iDC), while sparing those undergone maturation. In this study we investigated the effect of the interaction between autologous DC and NK-cytolytic T lymphocytes (NK-CTL), a subset of HLA-E-restricted CD8+ T cells that express HLA class I-specific inhibitory NK receptors. Although these cells share with NK cells various phenotypic and functional features (such as the capacity to lyse most allogeneic, NK-susceptible tumor cell lines), different from NK cells, NK-CTL failed to lyse autologous DC. However, after pulsing DC with a cytomegalovirus-derived, HLA-E-binding peptide recognized by NK-CTL, both iDC and mature DC became highly susceptible to lysis. On the other hand,the addition of the peptide resulted in the down-regulation of the NK-mediated lysis of the same autologous iDC. The capability of killing autologous DC, presenting a non-self, HLA-E-binding peptide, may represent a feedback mechanism by which NK-CTL down-regulate HLA-E-restricted responses to certain pathogens.
Abbreviations:
-
- iNKR:
-
Inhibitory NK receptors
-
- iDC:
-
Immature DC
-
- mDC:
-
Mature DC
1 Introduction
Recent data have provided evidence that two major players of the innate immunity, i.e. dendritic cells (DC) 1, 2 and natural killer (NK) cells 3, may interact each other, leading to remarkable functional consequences. Thus, upon encountering DC, NK cells undergo extensive proliferation and acquire the ability to kill autologous DC, particularly those at an immature stage 4, 5; in turn, immature DC (iDC) undergo maturation 4, 6–9, thus becoming competent for optimal antigen presentation and migration to lymph nodes 4, 10. Interestingly, the NK-mediated lysis of iDC is induced by one of the several receptors involved in NK cell triggering during the process of tumor cell lysis 11. Indeed, the antibody-mediated blocking of the activating NKp30 receptor 12 has been shown to abrogate the NK-mediated lysis of both iDC and mature DC (mDC), while mAb to other NK triggering receptors had no effect 4. Notably, NKp30-mediated lysis of mDC can be better appreciated upon mAb-mediated disruption of the interaction between inhibitory NK receptors (iNKR) and HLA class I molecules on mDC 4, 13.
Another lymphoid cell type shares with NK cells various phenotypic and functional characteristics. These cells, termed NK-CTL, represent a subset of CD3+ TCR α/β+ CD8+ T lymphocytes that express NK cell markers including CD56 and different HLA class I-specific iNKR 14–17. In addition, as assessed by cytolytic assays, NK-CTL lyse a large panel of NK-susceptible tumor cell lines 14. Despite these functional similarities, recent studies indicated a rather different molecular mechanism by which NK-CTL recognize target cells. Thus, we showed that NK-CTL recognize the poorly polymorphic HLA-E molecule 18, 19 via their TCR α/β 20, and that this recognition is dependent on the HLA-E-bound peptide 21, 22. Notably, HLA-E was previously known only as the ligand for the CD94/NKG2 NK receptor 23, 24. In particular, NK-CTL isolated from different individuals have been shown to recognize with high avidity two nonameric peptides derived from the UL40 protein 25 isolated from different human cytomegalovirus (HCMV) strains and presented at the cell surface in the context of the HLA-E molecule 26, 27.
In the present study, we analyze the effect of the interaction between NK-CTL and autologous DC. We show that, different from NK cells, NK-CTL fail to lyse both autologous iDC and mDC. However, both iDC and mDC become highly susceptible to NK-CTL-mediated lysis when pulsed with the relevant UL40-derived peptide.
2 Results
2.1 Different capacity of NK cells and NK-CTL to lyse autologous DC
In the first set of experiments we analyzed the ability of NK-CTL to lyse autologous DC in comparison to NK cells derived from the same individual. iDC were obtained from plastic adherent peripheral blood mononuclear cells (PBMC) cultured for 5 days in the presence of GM-CSF and IL-4 28. mDC were obtained by culturing cells for additional 2 days in GM-CSF, IL-4 and LPS 28. Both iDC (characterized by the CD14–, CD1a+, CD83– and HLA class Ilow surface phenotype) and mDC (characterized by the CD14–, CD1a+, CD83+, CD86+, CD25+ and HLA class Ihigh surface phenotype) were analyzed for their susceptibility to lysis by NK or NK-CTL populations derived from donors GG and KK. Fig. 1A, B shows a representative experiment in which effector cells were derived from donor GG. In agreement with previous data 4, NK cells efficiently lysed iDC, while they were poorly cytolytic against mDC. In contrast, NK-CTL failed to lyse both types of DC although they efficiently killed two representative NK-susceptible tumor cell lines (the adenocarcinoma cell line HeLa and the melanoma cell line M14 are shown). These data indicate a substantial difference between NK cells and NK-CTL in their ability to lyse autologous DC, although they display a similar cytolytic activity against the two tumor cell lines analyzed.
On the basis of recent data showing that NK-CTL kill target cells upon the TCR-mediated recognition of HLA-E/peptide complexes 20–22, 25, it is conceivable that the failure to lyse autologous DC may reflect the nature of the HLA-E-bound peptides. Thus, HLA-E molecules expressed on autologous DC are likely to bind available peptides, i.e. those derived from the leader sequences of self HLA class I molecules 19.
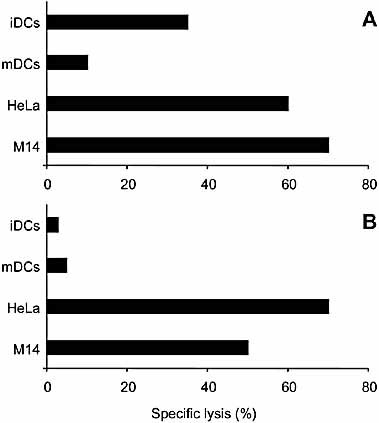
Different ability of NK cells and NK-CTL to lyse autologous DC. NK cell (A) and NK-CTL populations (B) derived from donor GG were analyzed for their cytolytic activity against both autologous iDC and mDC (in a 4-h 51Cr-release assay). As control, the same effector cells were analyzed for their ability to lyse two representative NK-susceptible tumor cell lines (HeLa and M14). The results are representative of seven independent experiments; the SD of the mean of the triplicates was <10%.
2.2 NK-CTL lyse both autologous iDC and mDC pulsed with a non-self CMV-derived peptide
In these experiments we investigated whether autologous DC pulsed with an HLA-E-binding peptide, known to be recognized by NK-CTL of donors GG and KK, would become susceptible to lysis. Thus, we used the VMAPRTLIL peptide present in the leader sequence of the UL40 protein that was recognized with high avidity by NK-CTL of both donors 25. As shown in Fig. 2A, B, both iDC and mDC became highly susceptible to lysis when pulsed with the non-self CMV-derived VMAPRTLIL peptide. Notably, no substantial differences could be detected in the susceptibility to NK-CTL-mediated lysis of pulsed iDC versus pulsed mDC. On the other hand, pulsing DC with either a self HLA class I-derived peptide (VMAPRALLL) or a different HLA-E-binding CMV-derived peptide (VMAPRTLVL) 26 did not result in any significant cytolytic activity (Fig. 2A, B). Hence, only the non-self viral VMAPRTLIL peptide could render both autologous iDC and mDC susceptible to NK-CTL-mediated lysis.
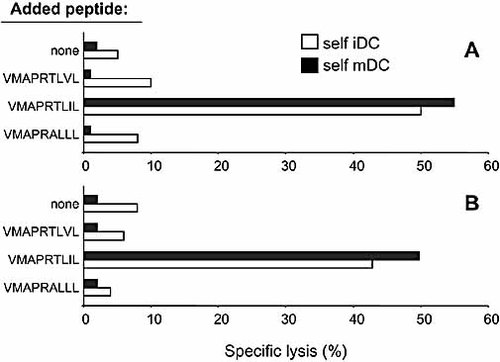
NK-CTL-mediated lysis of autologous iDC or mDC following pulsing with different HLA-E-binding peptides. NK-CTL populations derived from donor GG (A) and from donor KK (B) were analyzed for their ability to lyse autologous iDC (white bars) or mDC (black bars) (in a 4-h 51Cr-release assay). Target cells were either unpulsed or pulsed overnight at 37°C with the indicated HLA-E-binding peptides. The results are representative of four independent experiments; the SD of the mean of the triplicates was <10%.
2.3 Effect of DC pulsing with the VMAPRTLIL peptide on the NK- and NK-CTL-mediated lysis of autologous iDC
We next investigated whether the exogenously added HLA-E-binding VMAPRTLIL peptide could affect the NK-mediated killing of autologous iDC. NK and NK-CTL populations derived from donor GG were analyzed for their cytolytic activity against autologous iDC either unpulsed or pulsed with the VMAPRTLIL peptide.
Different from NK-CTL, NK cells were able to kill autologous MHC class I+, unpulsed iDC. In agreement with previous data, this recognition was mediated almost entirely by the NKp30 activating receptor, and it was blocked by anti-NKp30 mAb (Fig. 3A) 4. It is of note that peptide-pulsed iDC were less susceptible to NK cell-mediated lysis (Fig. 3A). This inhibition was likely to reflect the peptide-induced up-regulation of HLA-E molecule expression on iDC cell surface. In this context, the NK cell populations analyzed expressed the HLA-E-specific inhibitory CD94/NKG2A receptor (not shown). Accordingly, the NK-mediated lysis of pulsed iDC was comparable to that of unpulsed iDC only upon antibody blocking of CD94/NKG2A receptor (Fig. 3A). Again, as in the case of unpulsed iDC, lysis of peptide-pulsed iDC was NKp30 dependent (Fig. 3A). On the other hand, in agreement with the above results, unpulsed autologous iDC were resistant to NK-CTL-mediated lysis, whereas pulsing of DC with the VMAPRTLIL peptide resulted in high susceptibility to lysis that could be inhibited by an anti-TCR pan α/β mAb (Fig. 3B). Remarkably, NK-CTL-mediated lysis of DC was not affected by masking with anti-CD94 mAb.
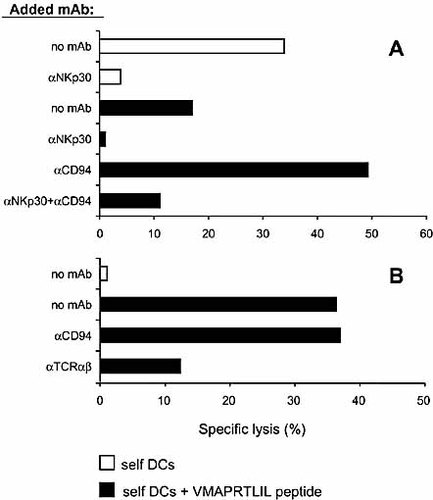
Differences between NK cell- and NK-CTL-mediated lysis of autologous immature DC either unpulsed or pulsed with HLA-E-binding VMAPRTLIL peptide. NK cell (A) or NK-CTL populations (B) derived from donor GG were analyzed (in a 4-h 51Cr-release assay) for their cytolytic activity against autologous iDC either unpulsed or pulsed with the CMV UL40-derived VMAPRTLIL peptide, in the absence or in the presence of mAb to the indicated molecules. Black bars represent lysis of peptide-pulsed iDC; white bars refer to lysis of unpulsed iDC. The results are representative of three independent experiments; the SD of the mean of the triplicates was <10%.
3 Discussion
In the present study we show that, different from NK cells, NK-CTL fail to lyse autologous DC both at immature and mature stage. However, both iDC and mDC become highly susceptible to lysis when pulsed with a viral peptide (VMAPRTLIL) that was known to be recognized with high avidity by NK-CTL of the donors analyzed 25. This implies that also iDC express sufficient amounts of HLA-E/peptide complexes to allow efficient triggering of NK-CTL. These data are further supported by the finding that NK-CTL lyse with high efficiency a large panel of allogeneic iDC expressing appropriate HLA alleles (i.e. containing in their signal sequences the peptides recognized with high avidity by NK-CTL). This functional behavior of NK-CTL was also shown previously using other types of allogeneic target cells including B-EBV cell lines and PHA-blasts 21.
Another interesting finding is the different effect exerted by the HLA-E-binding peptide (VMAPRTLIL) on NK and NK-CTL cytolytic activity against DC. Thus, while NK-CTL acquired ex novo the ability to lyse peptide-pulsed DC, these cells became less susceptible to lysis mediated by NK cells. Our data strongly suggest that the inhibitory effect of the added VMAPRTLIL peptide may bedue to the up-regulation of HLA-E surface expression on peptide-loaded DC. Indeed, mAb-mediated blocking of the HLA-E-specific CD94/NKG2A inhibitory receptor expressed by NK cells completely restored the NK-mediated lysis of pulsed DC.
Perhaps, it may appear surprising that major differences occurred in the ability of NK cells and NK-CTL to lyse peptide-pulsed mDC. Thus, while NK cells were poorly cytolytic, NK-CTL lysed pulsed mDC with similar efficiency as pulsed iDC. As previously shown, the inability of NK cells to efficiently kill mDC is related to the inhibitory interaction occurring between iNKR and HLA class I molecules expressed in high amounts on mDC. Indeed, mAb-mediated disruption of this inhibitory interaction completely restored cell lysis. Since also NK-CTL are characterized by the expression of iNKR, one may ask why their cytolytic activity is not inhibited by the iNKR/HLA class I engagement upon interaction with peptide-pulsed autologous mDC. This finding can be explained by different mechanisms. First, the NK-CTL populations analyzed were largely composed of NK-CTL expressing CD94/NKG2A receptors. As shown in a previous report, the surface density of these receptors on NK-CTL is relatively low and insufficient to deliver an inhibitory signal 21. A second explanation is that, in some donors, the NK-CTL populations expressed the CD94 molecule that is not associated with either NKG2A (to form an inhibitory receptor) or other known members of the NKG2 family 21. A third explanation is that the interaction between TCR α/β and HLA-E molecule could, at least in part, prevent the CD94/NKG2A binding to HLA-E, thus resulting in the lack of inhibition of cytotoxicity mediated by the CD94/NKG2A receptor. However, it should be noted that the cytolytic activity can actually be down-regulated in those NK-CTL expressing appropriate inhibitory receptors. This effect can be easily detected by the analysis of NK-CTL clones. Indeed, those expressing KIR (such as p58.1/KIR2DL1 and p58.2/KIR2DL2) can be sharply inhibited upon interaction with specific HLA class I ligands expressed on mDC (not shown) or other target cells 21.
In conclusion, our study provides experimental evidence that two cell types (NK cells and NK-CTL) that largely overlap for their surface phenotype and function (e.g. lysis of allogeneic tumor cell lines) greatly differ in their ability to lyse autologous DC, reflecting the different molecular mechanisms involved in their effector functions.
T cell-mediated killing of APC has been proposed as a feedback mechanism resulting in regulation of immune response. In the early 1990s, Del Prete et al. 29 suggested that addition of high numbers of Th1 cells could inhibit the B cell antibody production by killing APC. Recent data have shown that conventional TCR α/β+ CD8+ memory T cells can kill DC 30, 31. Our present data suggest that also HLA-E restricted NK-CTL may exert a similar immune regulatory role by efficiently killing DC. Remarkably, they are memory CD8+ T cells and may express CCR7, a receptor that would allow these cells to migrate, in response to specific chemokines, to lymph nodes and thus encountering DC. This regulatory function of NK-CTL would be restricted to those infections (such as CMV infection) during which HLA-E-binding peptides are generated 26, 27.
4 Materials and methods
4.1 mAb and flow cytofluorimetric analysis
The following mAb were used in this study: GL183 (IgG1, anti-KIR2DL2/S2/L3), Y249 (IgM, anti-KIR2DL2/S2/L3), EB6 (IgG1, anti-KIR2DL1/S1), XA141 (IgM, anti-KIR2DL1/S1), Y9 (IgM, anti-CD94), Z270 (IgG1, anti-NKG2A), KD1 (IgG2a, anti-CD16), A13 (IgG1, anti-Vδ1), BB3 (IgG1, anti-Vδ2), BAB-281 (IgG1, anti-NKp46,), AZ20 and F252 (IgG1 and IgM, respectively, anti-NKp30) and A6.136 (IgM,anti-HLA class I); all produced in our laboratory. LeuTM-4 (IgG1, anti-CD3), LeuTM-2a (IgG1, anti-CD8), LeuTM-3a (IgG1, anti-CD4) and LeuTM-12 (IgG1, anti-CD19) mAb were purchased from Becton Dickinson. mAb anti-TCR α/β (IgG2b), anti-TCRVβ16 (IgG1) and anti-TCRVβ9 (IgG2a) were purchased from Immunotech (Marseille, France). FITC- and PE-conjugated anti-isotype goat anti-mouse (GAM) mAb were purchased from Southern Biotechnology (Birmingham, AL). Analysis of DC surface markers was performed using the following mAb: PE-conjugated anti-CD1a (T6-RD1; Beckman Coulter, Hialeah, FL), PE-conjugated anti-CD80 (Becton Dickinson), PE-conjugated anti-CD83, FITC-conjugated anti-CD86 (Ancell, Bayport, MN), FITC-conjugated Leu M3 (anti-CD14, Becton Dickinson) and FITC-conjugated anti-CD25 (Becton Dickinson). Negative controls consisted of FITC-conjugated IgG1 and PE-conjugated IgG1 mAb specific for irrelevant antigens (Becton Dickinson). Thereactivity of mAb with NK cell and NK-CTL populations was assessed by indirect immunofluorescence and cytofluorimetric analysis as described 32. All samples were analyzed on a FACScan flow cytometer (Becton Dickinson).
4.2 Isolation and culture of NK-CTL and NK cell populations
PBMC were isolated from blood of normal donors by a Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient, as previously described 32. PBMC were depleted of CD4+, CD16+, NKp46+, Vδ1+, Vδ2+ cells by negative selection using appropriate mAb and magnetic beads coated with anti-mouse IgG (Dynal, Oslo, Norway). The culture medium used was RPMI 1640 (Seromed, Berlin, Germany) supplemented with 10% FCS (Sigma-Aldrich), 1% antibiotic mixture (5 mg/ml penicillin, 5 mg/ml streptomycin stock solution) and human rIL-2 (Proleukin, Chiron Corp., Emeryville, CA). NK-CTL populations were obtained in mixed lymphocyte culture (MLC). MLC were set up by culturing CD8+ T cells in round-bottom microwells (105 cells/well) in the presence of irradiated (5,000 rad) allogeneic PBMC (2×105 cells). On day 3, rIL-2 (25 U/ml) was added to cultures. On day 8, the specificity of the NK-CTL expanded after MLC was evaluated in a cytolytic assay and immunofluorescence analysis was performed.
Enriched NK cells were isolated by depleting PBMC of CD3+, CD4+ and CD19+ cells using appropriate mAb and goat anti-mouse coated Dynabeads (Dynal). The percentage ofNK cells in that population was evaluated using FITC-conjugated anti-CD3 and PE-conjugated anti-CD56 mAb (Becton Dickinson) and flow cytometry. CD3–CD4–CD19– cells were cultured on irradiated feeder cells in the presence of rIL-2 (100 U/ml) and PHA (1.5 ng/ml, Gibco, Paisley, GB) to obtain polyclonal NK cell populations.
4.3 Generation of DC from PBMC
PBMC from healthy donors were isolated by using centrifugation of blood samples on Ficoll-Hypaque density gradient, resuspended in RPMI 1640–10% FCS, and allowed to adhere for 2 h at 37°C in 150-cm2 culture flasks (Corning, NY). The adherent fraction (>90% CD14+) was used to generate myeloid DC using culture with rHuGM-CSF (50 ng/ml, Mielogen; Schering-Plough, Milan, Italy) and IL-4 (20 ng/ml; PeproTech, Rocky Hill, NJ), as described 28. The cultures were fed every 2 days by removing one half of the culture volume and adding an equal volumeof fresh media containing sufficient GM-CSF and IL-4 for the entire culture volume. After 5 days of culture cells were characterized by the CD14– CD1a+ and CD83– phenotype corresponding to iDC. To generate CD14–, CD1a+, CD83+, CD86+, CD25+ and HLA class Ihigh mDC, iDC were treated for 48 h with LPS from Escherichia coli (40 ng/ml; Sigma-Aldrich).
4.4 Cytolytic activity
NK cell and NK-CTL populations derived from two different donors were tested for cytolytic activity in a 4 h 51Cr-release assay as previously described 33. The following target cells were used: the cervical adenocarcinoma cell line HeLa, the melanoma cell line M14, and, finally, autologous DC, in the presence or in the absence of mAb. The concentrations of thevarious mAb added for masking experiments were 10 μg/ml. In some experiments autologous DC, either immature or mature, were previously incubated overnight at 37°C either alone or in the presence of saturating amounts (105 nM) of synthetic peptides. The peptides used in these experiments were the following: VMAPRTLIL, VMAPRTLVL and VMAPRALLL. The E/T ratio used in all experiments was 10:1.
Acknowledgements
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.); Istituto Superiore di Sanità (I.S.S.); Ministero della Salute; Ministero dell'Istruzione dell'Università e della Ricerca (M.I.U.R.); Ministero dell'Università e della Ricerca Scientifica e Tecnologica (M.U.R.S.T.), MURST 5%-CNR Biotechnology program 95/95; Consiglio Nazionale delle Ricerche, and Compagnia di San Paolo. We thank Stefano Canu for secretarial assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




