Structural and kinetic basis for low affinity cross-reactivity in T cell allorecognition
Abstract
The alloreactive BM3.3TCR interacts with high affinity with H-2Kb loaded with the endogenous peptide pBM1 (INFDFNTI), and shows low affinity cross-reactivity for H-2Kb loadedwith a viral peptide VSV8 (RGYVYQGL), CTL activity requiring 103-fold higher peptide concentration and being highly sensitive to inhibition by anti-CD8 monoclonal antibody. VSV8 peptides substituted with pBM1/TCR contact residues (N6 and T7) retained low affinity characteristics and among pBM1 peptides substituted with residues Q6 and/or G7 present in VSV8, only pBM1(G7) was recognized, albeit with characteristics akin to those of VSV8. Despite the difference in KD values and the faster dissociation rate of multimeric VSV8/H-2Kb as compared to pBM1/H-2Kb complexes, similar TCR occupancy could be achieved with both multimers either at 4 or 37°C. Only TCR engagement with pBM1/H-2Kb, however, resulted in early (Ca2+ flux) and late(CD69 expression) activation events in naive BM3.3TCR CD8 T cells. CD8 coreceptor, essential for binding of the weak agonists, was dispensable for binding of pBM1/H-2Kb multimers and their induction of signaling in naive T cells. Hence, high number of TCR and coreceptor engagement by weak agonists fail to substitute for strong agonist TCR engagement that can be coreceptor-independent and involve a limited number of TCR.
1 Introduction
Polymorphic MHC products are the targets of the rapid rejection of allogeneic grafts by effector T cells. Acute rejection involves direct killing of parenchymal graft cells, whereas sensitization of T cells to alloantigen-derived peptides presented by host APC is known to contribute to chronic rejection 1. The TCR repertoire resulting from positive selection for low avidity fit between TCR and selfMHC/peptide 2, is armed for interaction with selfMHC presenting foreign antigens. It also contains a high frequency of receptors capable of interaction with alloMHC. These include T cells recognizing specific peptides presented by alloMHC 3, the peptide-free alloMHC molecule itself 4, or a peptide-dependent conformation of the alloMHC molecule 5. In view of the potential of using defined TCR of an allogeneic source for cellular therapies 6, 7, it is important to better understand the specificity of the T cell repertoire with respect to recognition of polymorphic MHC features together with the repertoire of natural self peptides and those generated upon cell transformation or infection. Analyses of the extent of cross-reactivity of allorestricted CTL, its structural basis and consequences upon T cell activation are relevant in that context.
The in vivo selected BM3.3TCR shows high affinity binding characteristics for H-2Kb-bound pBM1. It also showed high selectivity as pBM1 was the only peptide among the whole repertoire of H-2Kb-bound endogenous peptides that was recognized by the BM3.3TCR 8. Oddly, we had previously found that an exogenous peptide, VSV8, the main H-2Kb-binding VSV nucleoprotein epitope [9, behaved as a cross-reactive epitope for this TCR 10. The increased EC50 and high susceptibility to inhibition byanti-CD8 mAb, however, suggested distinct characteristics of TCR/peptide/MHC binding as compared to pBM1 8. Use of dimer and multimer H-2Kb constructs loaded with pBM1, VSV8, or variant pBM1 peptide allowed us to determine directly the binding characteristics and their consequences on TCR signaling. Two main characteristics distinguished the strong agonist pBM1 from the weak agonists VSV8 and pBM1(G7): (i) in the presence of CD8, quantitatively comparable TCR occupancy could be obtained with multimers containing strong or weak agonist, but only the former led to TCR-mediated signaling in naive T cells; (ii) in the absence of CD8, no binding of the weak agonists could be observed, whereas pBM1 multimers could bind to and induce coreceptor-independent signaling in naive T cells.
2 Results
2.1 Structural basis for recognition of pBM1 and VSV8/H-2Kb complexes by CTL BM3.3
Resolution of the crystal structure of the BM3.3TCR/pBM1/H-2Kb complex identified amino acids N6 and T7 of pBM1 as the essential TCR contact residues with the CDR3 region of the TCRβ chain 11. In VSV8, the corresponding positions are Q6 and G7: N/Q is a conserved change and substitution of T for G would potentially restore TCR contact sites (G is small and polar, T is larger with a polar OH group). We wondered if reciprocal exchange of N6T7 with Q6G7 in pBM1 and VSV8 would imprint the characteristics of the original peptides. We produced peptides with single and double substitutions between p6 and p7 residues present at those positions in pBM1 and VSV8. All these peptides had similar H-2Kb binding properties (results not shown), but varied in their capacity to be recognized by the BM3.3 CTL (Fig. 1A). Double-substituted pBM1(Q6G7) and single-substituted pBM1(Q6) failed to be recognized by the CTL, whereas single-substituted pBM1(G7) was recognized when 100 nM of peptide was used (Fig. 1A). The EC50 for pBM1(G7) was, however, increased 4,000-fold as compared to pBM1 ("wt", Fig. 1B). Of the VSV8 peptides in which pBM1 residues were substituted at p6 and/or p7, none was found to improve significantly the EC50 for recognition by CTL BM3.3. For both VSV8 and VSV8(N6T7), the EC50 was around 1,000-fold higher than for pBM1, whereas for VSV8(T7) and VSV8(N6), it was, respectively 2,000- and 3,000-fold higher (Fig. 1B).
Thus in the context of VSV8, residues N6T7 did not provide more favorable interactions with the BM3.3TCR than residues Q6G7 originally present in VSV8, suggesting a distinct mode of interaction of the TCR with the two peptide/MHC complexes, consistent with the recent crystallographic analysis 12. In the context of pBM1, the G7 substituted peptide maintained a low interaction with the BM3.3TCR, and could thus provide an altered peptide ligand, as an alternative to VSV8 which sequence totally differs from pBM1.
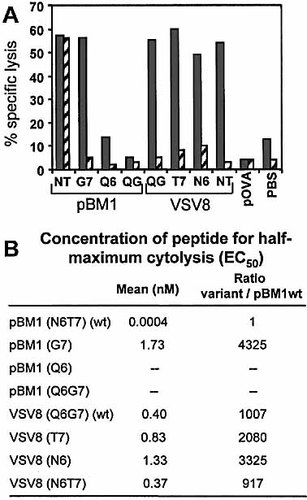
Characterization of CTL activity induced on RMA-S cells loaded with pBM1, VSV8 and variant peptides. (A) Lysis of RMA-S cells loaded with 10–7 M of various peptides in the presence (dashed bars) or absence (gray bars) of 20 μg/ml anti-CD8 mAb. Sequences of wild-type (wt) peptides are INFDFNTI (pBM1) and RGYVYQGL (VSV8). Amino acids at position 6 and 7 are noted, without numbers for double substitution (NT for N6T7 and QG for Q6G7). (B) Concentration of peptide inducing 50% of maximum lysis (EC50). (A, B) Effector/target ratio =5/1. One representative experiment out of three is shown.
2.2 Functional CD8 independence is unique for TCR BM3.3 interacting with pBM1/H-2Kb
Because of its structure the CD8 coreceptor can play a dual function, as (i) its intrinsic affinity for MHC class I molecules allows it to influence binding characteristics of TCR/peptide/MHC interactions, while (ii) its association with lck through the cytoplasmic domain of CD8α, and with lipid rafts through the palmitoylated CD8β chain 13 allow for a co-signaling function. Naive T cells expressing the BM3.3TCR are relatively independent of CD8 for both these functions, as even naive CD4 T cells could develop into pBM1/H-2Kb specific CTL effectors 14. This characteristic was correlated with the absence of inhibition by anti-CD8 mAb of both induction of CTL from naive BM3.3 CD8 T cells and target cell killing by the CTL 15. In contrast, anti-CD8 mAb totally inhibited lysis of VSV8-expressing target cells by the same CTL 8. Results in Fig. 1A confirmed the respective resistance and sensitivity to anti-CD8 mAb of the pBM1- and VSV8-dependent cytolysis by CTL BM3.3. Interestingly, cytolysis of target cells presenting the substituted pBM1(G7) peptide was totally inhibited by anti-CD8 mAb. Similarly, for all of the substituted VSV8 peptides cytolysis remained sensitive to inhibition by anti-CD8 mAb (Fig. 1A). As all the peptides built on pBM1 or VSV8 H–2Kb anchor residues have similar H-2Kb binding properties 8, differences observed are to be attributed solely to interactions between TCR and peptide/MHC complexes. Hence, we find a correlation between the EC50 of the peptides, and the susceptibility to inhibition of cytolysis by anti-CD8 mAb.
2.3 Comparison of affinity and kinetics of BM3.3 TCR interaction with pBM1/H-2Kb and with VSV8/H-2Kb
T cell activation was generally correlated with the level of TCR affinity for its ligand. Measurements of apparent KD values for the interaction between the isolated BM3.3TCR and immobilized peptide/Kb complexes confirmed the higher affinity (around 40-fold) of the TCR for the pBM1 (KD =2.6 μM) as compared to the VSV8 (KD =114 μM)/Kb ligand 11, 12. Using dimeric peptide/MHC complexes as previously characterized 16 as well as peptide/MHC multimers (see Sect. 4) allowed us to evaluate the kinetic parameters of the TCR/peptide/MHC interaction directly on CD8 T cells, therefore including the contribution of the coreceptor CD8.
The apparent KD values for the binding of pBM1/Kb dimers or multimers to TgBM3.3 CD8 T cells were, respectively, 1.5 nM and 1.3 nM (as monomeric peptide/Kb equivalents; see Table I). For VSV8/Kb complexes, the corresponding KD values were 60 times higher for dimers (78.3 nM) and 8 times higher for multimers (10.4 nM). Of note, for pBM1/Kb complexes apparent KD values were similar whether interaction was measured with dimeric or higher valency complexes, whereas for VSV8/Kb complexes dimers were 8 times less efficient than multimers.
The duration of receptor engagement also appears to be a key parameter for T cell activation. Analysis of the rate of dissociation of pBM1/Kb multimers from TgBM3.3 CD8 T cells (at 4°C, in the presence of anti-Kb mAb to avoid re-fixation of peptide/MHC complexes) indicated that the complex half-life was 2.9 min. In the same conditions, VSV8/Kb multimers dissociated from the BM3.3 T cells more rapidly with a half-life of 0.84 min. Thus, BM3.3 TCR/VSV8/Kb complexes dissociated 3.5 times faster than BM3.3 TCR/pBM1/Kb (Table 1).
|
Parameters analyzed |
|
pBM1 |
VSV8 |
pBM1(G7) |
|---|---|---|---|---|
|
Half-time (min) |
Pep/Kb multimer |
2.86±0.6 |
0.84±0.15 |
ntb) |
|
KDc) |
Pep/Kb dimer |
1.51±0.24 |
78.3±8.2d) |
nt |
|
|
Pep/Kb multimer |
1.27±0.53 |
10.36±3.03 |
8.25±0.05 |
|
EC5O CD69c) |
Pep/Kb multimer |
0.56±0.2 |
200 |
200 |
|
EC5O Cac) |
Pep/Kb dimer |
8.1±2.5e) |
undetectable |
nt |
|
|
Pep/Kb multimer |
0.85±0.35 |
200 |
200 |
- a) Results are expressed as mean ± s.d. calculated from three or four independent experiments.
- b) nt: not tested.
- c) KD and EC50 are expressed as nM of monomeric pep/Kb equivalents.
- d) Extrapolated to the plateau observed with pBM1/Kb dimers that was not reached with VSV8/Kb dimers.
- e) After anti-IgG1 cross-linking.
2.4 TCR-mediated calcium signaling depends upon affinity and oligomerization of ligand peptide/MHC complexes
To measure directly ligand binding to T cells and the resulting signaling, CD8 T cells were loaded with Indo-1 and peptide/MHC binding as well as Ca2+ signaling were recorded at 37°C.
Data show the increase in cytoplasmic Ca2+ level in naive TgBM3.3 CD8 T cells in response to pBM1/Kb (Fig. 2A) or VSV8/Kb (Fig. 2B) dimers before and after addition of anti-mouse IgG1 antibody as cross-linker. At a concentration of 16 nM, dimeric pBM1/Kb was able to induce a weak Ca2+ flux (increment 35), that was strongly enhanced upon cross-linking of the ligand (Fig. 2A). For lower concentrations of dimeric pBM1/Kb (4 nM in Fig. 2A), a Ca2+ transient (increment 65) could be detected only after addition of the cross-linker. The extent of pBM1/Kb binding (revealed by the PE-cross-linker, right panels of Fig. 2A) was observed to increase with the ligand concentration. When similar experiments were performed with VSV8/Kb dimers, no Ca2+ transient could be detected in the CD8 T cells, even for dimer concentrations up to 320 nM, and after addition of the cross-linker (Fig. 2B). For the latter concentration of VSV8/Kb dimers (320 nM), the extent of ligand binding to the T cells was slightly higher than that obtained with 4 nM pBM1/Kb dimers (right panel of Fig. 2A), in agreement with their respective KD values (see Table 1). Thus, for similar levels of ligand binding to the TCR, signaling was induced when TCR engagement was with pBM1/Kb, but not with VSV8/Kb.
To evaluate whether further ligand oligomerization could correct the signaling defect of VSV8/Kb for TgBM3.3 CD8 T cells, similar experiments were performed using preparations of multimeric peptide/Kb complexes. Data (Fig. 2C) show a rapid Ca2+ flux (increment 195) in response to 2 nM pBM1/Kb multimers. At a concentration as low as 0.4 nM, no ligand binding could be detected, yet pBM1/Kb induced a Ca2+ increase, which, however, lacked the rapid peak response observed for the higher concentrations of ligand. For VSV8/Kb multimers in contrast, at 100 nM a significant binding was observed (Fig. 2D, right panel), that did not generate any Ca2+ signal (Fig. 2D, left panel). Even for concentrations as high as 200 nM VSV8/Kb, only a very weak Ca2+ transient (increment 25) could be observed. Thus, even for peptide/Kb multimers that could generate a high degree of ligand binding, VSV8/Kb, as well as pBM1(G7)/Kb complexes (results not shown), remained deficient to induce early TCR signaling.
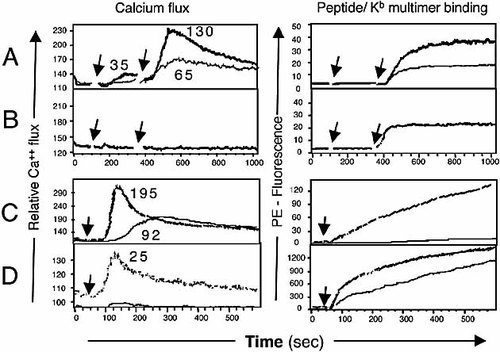
Ca2+ flux induced by dimeric and multimeric peptide/Kb complexes. (A–D) Indo-1AM-loaded TgBM3.3 CD8 T cells were analyzed by flow cytometry before and after addition of dimeric (A, B) or multimeric (C, D) peptide/Kb complexes. Ca2+ flux (left panels) and peptide/Kb binding (right panels) were recorded at 37°C on the same cells as a function of time. (A, B) IgG-Kb/peptide, first arrow, followed by PE-anti-IgG1 (second arrow). In (A), heavy line for IgG-Kb/pBM1 16 nM, light line for 4 nM. In (B), IgG-Kb/VSV8, 320 nM. (C, D) PE-peptide/Kb multimers (single arrow). In (C), heavy line for pBM1/Kb 2 nM, light line for 0.4 nM, and in (D), heavy line for VSV8/ Kb 200 nM and light line for 100 nM. Numbers in left panels indicate the increment of relative Ca2+ flux. (A, D) Irrelevant pKB1/Kb dimer or multimer complexes used as controls did not elicit significant Ca2+ flux (not shown). One representative experiment out of four is shown.
2.5 Multimers pBM1/H-2Kb, but not pBM1(G7)/H-2Kb or VSV8/H-2Kb, bind to CD4 T cells expressing the BM3.3 TCR and induce CD8-independent signaling
The difference between the strong agonist pBM1 and the weak agonists VSV8 and pBM1(G7) was much more pronounced when EC50 of peptides for CTL activity (4,000-fold, Fig. 1C) rather than multimer binding was considered (8-fold, Table 1). Hence, we wondered whether the relative coreceptor-independence of the stimulation of BM3.3 CD8 T cells by pBM1 as compared to VSV8 and pBM1(G7), that had so far only been shown indirectly through functional assays, would also be apparent in binding studies. For this we labeled CD4 and CD8 T cells from TgBM3.3 mice and analyzed BM3.3TCR expression and peptide/Kb multimer binding on gated CD4 or CD8 T cells. As shown by the binding of the anti-TCR mAb Ti98, these CD4 T cells are very heterogeneous with respect to expression of the BM3.3TCR (Fig. 3A) and only 39% of CD4 T cells express high level of BM3.3TCR, as compared to 91% of CD8 T cells. Their expression of CD3 was high and homogeneous, however, (results not shown), consistent with the fact that these CD4 T cells are selected on the basis of their expression of a second TCRα chain 14. Interestingly, the pattern of binding of pBM1/Kb multimers was also heterogeneous and similar to that of the anti-clonotypic mAb (Fig. 3B), with 38% of CD4 T cells binding a high level of pBM1/Kb multimers. In contrast to the binding pattern of pBM1/Kb, no fixation could be detected on the CD4 T cells with pBM1(G7)/Kb or with VSV8/Kb (Fig. 3B). The curves of peptide/Kb binding for increasing concentrations of multimers (Fig. 3C), clearly show that in spite of their efficient binding on CD8 T cells (upper graph), no binding was observed on CD4 T cells, except for pBM1/Kb (lower graph). An irrelevant clonotypic mAb (Désiré, Fig. 3A) and Kb multimers loaded with an irrelevant peptide (pKB1/Kb) are shown as controls (Fig. 3B and C).
To further analyze whether the exclusive engagement of the BM3.3TCR by pBM1/Kb multimers was able to activate T cells, the induction of CD69 expression was measured in response to stimulation of the BM3.3TCR on CD4 and CD8 T cells. Data (Fig. 4A, right panel) show that all of the BM3.3 CD8 T cells (97%) bound pBM1/Kb and the majority (74%) were induced to express CD69. In contrast, a similar level of binding of pBM1(G7)/Kb or VSV8/Kb multimers (87%) induced a very low level of CD69 expression on, respectively, 7 and 10% of the BM3.3 CD8 T cells (Fig. 4B and C, right panels). These two multimers failed to bind and did not induce CD69 on the BM3.3 CD4 T cells (Fig. 4B and C, left panels). Stimulation of BM3.3 CD4 T cells with pBM1/Kb multimers induced a significant level of CD69 expression on a fraction of cells binding a high level of pBM1/Kb (Fig. 4A, left panel). The proportion of CD69-positive cells (18%) among pBM1/Kb multimer binding CD4 cells (45%) was, however, far from that observed with CD8 T cells (74% of CD69 expressing cells among 97% of pBM1/Kb-binding cells). The curves of peptide/Kb-induced CD69 expression for increasing concentrations of multimers (Fig. 4E) clearly show that only pBM1/Kb complexes induced significant expression of CD69 on CD8 T cells (upper graph), as well as a moderate expression on CD4 T cells (lower graph). As a positive control for stimulation of the BM3.3TCR without engagement of coreceptor, we used the anti-clonotypic mAb Ti98 (Fig. 4F). This reagent was less efficient than pBM1/Kb multimers for the induction of CD69 on CD8 T cells, whereas on CD4 T cells it had a similar degree of efficiency, being slightly superior at high concentration.
The data showed that CD8 contributed to peptide/Kb multimer binding for poor agonist peptides, but was dispensable for binding of a strong agonist peptide. Additionally, engagement of the BM3.3TCR by the strong agonist pBM1/Kb could lead to signal transduction (measured by late induction of CD69 expression) in the absence of the signaling function of CD8α β, whereas the engagement of the same receptor by the weak agonists pBM1(G7)/Kb or VSV8/Kb was inefficient at eliciting similar signaling on naive T cells even in the presence of CD8.
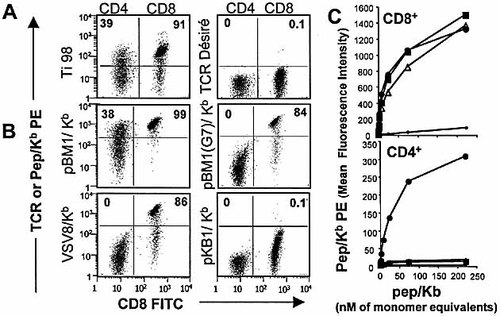
Binding of peptide/Kb multimers or anti-clonotypic mAb to naive CD8 and CD4 T cells expressing the TCR BM3.3. Dot plots indicate the binding of PE-anti-TCR (A) or -peptide/Kb multimer (B) on CD4 (left part of the dot plot) and CD8 (right part) T cells. Numbers in upper quadrants indicate the percentage of PE positive cells among CD4 or CD8 cells. Cells were incubated for 90 min with 2 μg/ml mAb (A) or 222 nM peptide/Kb multimers (B). In (C), binding of peptide/Kb multimers as a function of concentration is shown for the CD8 (upper graph) and for the CD4 (lower graph) T cells as measured in (B). Symbols in (C) are: closed circles for pBM1; closed squares for pBM1(G7); open triangles for VSV8; dots for pKB1. One representative experiment out of four4 is shown.
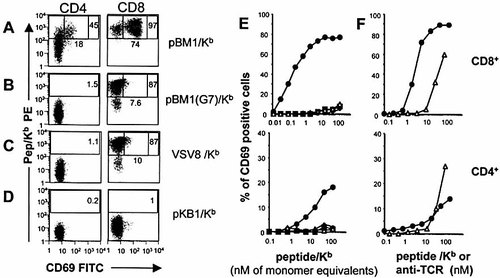
Induction of CD69 on CD8 and CD4 T cells expressing TCR BM3.3 in response to peptide/Kb multimers or anti-clonotypic mAb. T cells from TgBM3.3 mice were incubated 90 min at 4°C with various concentrations of peptide/Kb multimers or anti-TCR mAb Ti98 and then transferred to 37°C for a 24-h incubation. Dot plots (A–D) show expression of CD69 and peptide/Kb multimer binding on CD4 (left column) and CD8 (right column) T cells after incubation with 222 nM peptide/Kb multimers. Rectangular boxes delineate the cells with high level peptide/Kb binding and the square boxes indicate the fraction of those cells that are activated as measured by CD69 expression. (E) reports the percentage of CD69 expressing cells as a function of multimer concentration on the total CD8 (upper graph) and on the high peptide/Kb binding CD4 (lower graph) T cells as measured in (A–D). Symbols in (E) are as in Fig. 3C. One representative experiment out of three is shown. Data in (F) report the comparison of pBM1/Kb multimers (closed circles) and anti-clonotypic mAb Ti98 (open triangles) for their capacity to induce expression of CD69 on CD8 (upper graph) and on CD4 (lower graph) T cells.
3 Discussion
3.1 Optimal TCR/ligand binding characteristics for efficient signaling
This question is still a matter of debate. Some stress the importance of ligand affinity for the TCR, while others consider the main parameter the rate of dissociation of the ligand from the TCR, with an optimal at which TCR engagement is of sufficient duration to allow assembly of intracellular signaling complexes, but is sufficiently short to permit reengagement of new TCR entities with the limiting amount of peptide/MHC complexes available (serial engagement model) 17–19. Another major issue is whether the TCR is a "rigid" structure, endowed with an on/off switch relaying information through cross-linking associated CD3 components or by inducing their association with rafts upon ligand binding. The alternative view is that depending on the nature of the ligand, conformational changes may be induced within the TCR or within the TCR/CD3 complex, that may qualitatively affect early signaling events 20.
In the present study we compared the ligand-binding parameters and their functional consequences for naive CD8 T cells expressing an alloreactive TCR (BM3.3) that recognizes an endogenous peptide pBM1 (INFDFNTI) complexed to Kb and that cross-reacts with a viral peptide of unrelated sequence, VSV8 (RGYVYQGL), also in complex with Kb.
3.2 Distinct position of TCR contact residues with two peptide/Kb complexes
The characteristics of BM3.3TCR/pBM1/H-2Kb interactions were critically controlled by two residues (N6T7) as any substitution of N6 (except for D where partial recognition is maintained, result not shown) led to total loss of recognition by the BM3.3TCR, whereas substitution of T7 with G7 (pBM1(G7)) generated a peptide/MHC complex of low affinity for the BM3.3TCR, that was coreceptor dependent for killing (Fig. 1).
Our attempts at shuffling the N6 and/or T7 residues in the VSV8 sequence led to peptides that were not improved in their affinity for the BM3.3TCR, and that remained dependent on coreceptor for induction of killing. Additionally, Ala-substitution of residue D4 in pBM1 did not lead to any loss of activity, whereas the more conserved substitution of V4 to Ala in VSV8 was not permissive (not shown). These data are consistent with the recent crystallographic resolutions of the BM3.3TCR/pBM1/H-2Kb 11 and BM3.3TCR/VSV8/H-2Kb 12 complexes that elegantly revealed N6 and T7 as the unique pBM1 contact residues with the BM3.3TCRβ chain, in the absence of peptide interaction with the TCRα chain, that bends away from the peptide binding groove. In contrast, in the case of VSV8, the highest number of TCR/peptide bounds were between the BM3.3TCRα chain and residue V4, whereas bounds between the TCRβ chain and Q6 and particularly G7 were reduced, as compared to the corresponding residues in pBM1. While this structural analysis showed the flexibility of the BM3.3TCRα CDR3 loop that allows for the interaction of the TCR with distinct residues in pBM1 and VSV8, it also confirmed the stability of the general mode of docking of the TCR on peptide/Kb complexes, unlikely to explain differences in signaling or coreceptor interactions (see hereafter).
3.3 High TCR occupancy by low affinity ligand fails to substitute for high affinity agonist engagement of a limited number of TCR in naive CD8 T cell activation
KD values for binding of pBM1/H-2Kb complexes to TgBM3.3 CD8 T cells were 1.2 nM and 1.5 nM for multimers or dimers, respectively, as compared to 2.6 μM for their bindingto soluble BM3.3TCR 11. For VSV8/H-2Kb binding the corresponding values were 8-, 60- and 31-fold higher (Table 1, 12), respectively. Clearly the relative KD values did not reflect the relative "functional" avidities of the two TCR ligands (EC50 in Table 1 and Fig. 1). Indeed, high concentrations of VSV8/Kb multimers led to high TCR occupancy on naive CD8 T cells (Fig. 2–4), but failed to activate these cells.
Consistent with "kinetic proof reading" models 17, 21, 22, the rate of dissociation of peptide/H-2Kb multimers was 3.5 times faster for VSV8 as compared to pBM1 loaded multimers (Table 1). It is thus possible that in spite of similar TCR occupancy, the more rapid dissociation of VSV8/H-2Kb complexes from individual TCR molecules is incompatible with efficient signaling on naive TgBM3.3 CD8 T cells, whether early (Ca2+ flux) or late (CD69 expression) signaling was measured. Interestingly, for the OT-I TCR on naive CD8 T cells, dissociation kinetics from agonist OVA-peptide/H-2Kb multimers were also about 3.5 times slower than from G4-variant peptide/H-2Kb multimers (7 as compared to 2 min). Yet, G4-variant peptide/H-2Kb multimers could induce a delayed, but efficient signaling as judged by induction of CD69, although no Ca2+ transient was detected 22. The KD values for binding to isolated TCR were, respectively of 5 and 10 μM for strong and weak agonist in the OVA-peptide model, as compared to 2.6 and 114 μM with the ligands considered in our study. The low intrinsic affinity between TCR and peptide/MHC 23 combined with an increased dissociation rate may thus set a threshold where naive CD8 T cells fail to be activated. The fact that these weak agonists could nevertheless trigger effector CTL for lytic activity is consistent with the enhanced signaling potential of preactivated CD8 T cells (24, 25 and data not shown).
We further showed that in the absence of CD8, no binding of the weak agonists could be observed, whereas pBM1 multimers could bind to and induce coreceptor-independent signaling in naive T cells (Fig. 4). Thus in contrast to previous reports 26, 27, we show that provided a multimerized peptide/MHC complex is endowed with appropriate TCR binding characteristics, it can lead to CD8-independent signaling on naive T cells. Presence of CD8 on naive T cells, however, greatly improved both binding and extent of signaling even in the response to the strong agonist (Fig. 4). For the weak agonists CD8 was essential to permit binding of antigenic multimers to the T cells.
3.4 Alloreactivity is no exception: CD8 dependence is correlated with TCR affinity for peptide/MHC complexes and peptide contributes an essential energy to the TCR/peptide/MHC interaction
In another extensively studied alloreactive T cell model, the 2C TCR has been shown to interact with the alloMHC molecule Ld with peptide p2Ca(QL9) in a manner that is largely CD8 independent, while its interaction with the selfMHC Kb with peptide dEV8 is CD8 dependent 28. This correlated with a shift in TCR binding towards the N terminus of the QL9 peptide associated with Ld as compared to its binding in the context of the selfMHC molecule 29, leading to the suggestion that this TCR shift could hinder CD8 interactionwith Ld and therefore lead to CD8 independence 28. It has also been proposed that a higher intrinsic affinity may exist between TCR and alloMHC compared to selfMHC because only the latter is constrained by the low affinity requirement to allow positive selection to occur on selfMHC in the thymus. Therefore, it was reasoned, the peptide needs only contribute a little energy above that existing for the intrinsic TCR/alloMHC interaction 30. As indicated above, the crystal structure of the TCR BM3.3 indeed revealed a limited interaction between the TCRβ chain and residues p6 and p7 of peptide pBM1 11. The interaction of the same TCR with VSV8 in the same alloMHC molecule, however, involves a repositioning of the TCRα chain to contact the N terminus residue p4, while losing many of the TCRβ chain interactions with residues p6 and p7 in VSV8 12. In this case, this repositioning of the TCR is associated with a drastic increase in the dependency on CD8 (8, Fig. 1A and 3). Another CD8-dependent alloreactive TCR with low affinity for peptide/Kb [8 also presented limited interactions involving mainly the TCRß chain and peptide residues p4 and p6 31. Therefore, no correlation appears to exist between TCRαß contact sites on the peptide/MHC complex, CD8 dependency and peptide/alloMHC -versus self-recognition.
The single substitution of residue T7 to G7 in pBM1 likewise generated a pBM1(G7)/Kb complex with properties akin to those of VSV8/Kb in terms of CD8 dependency in CTL activity (Fig. 1) and in T cell binding of pBM1(G7)/Kb multimers (Fig. 3). Furthermore pBM1/Kb, but not VSV8/Kb or pBM1(G7)/Kb multimers were capable of activating naive CD8 T cells (Fig. 4). Therefore the contribution of the peptide to the "energy" of the TCR/peptide/alloMHC interaction is essential in this model to reach the threshold for activation of naive CD8 T cells, as it is for T cell responses to peptides seen in the context of selfMHC.
Altogether these results show that an alloreactive TCR can recognize endogenous peptide/alloMHC complexes with exquisite specificity. T cell cross reactivity for a viral peptide associated with the same alloMHC molecule is based on TCR interaction with non homologous peptide residues generating a weak agonist, whose binding to the TCR is dependent on the CD8 coreceptor. Modification of asingle residue within the endogenous peptide likewise generates a coreceptor-dependent weak agonist, indicating that in spite of the high degeneracy of TCR/peptide/MHC interactions, optimal fit mayseldom occurs.
4 Materials and methods
4.1 Media, buffers, antibodies
Incubation at 31°C in RPMI 1640 (25 mM HEPES, 5% PBS-dialyzed FCS), cultures at 37°C in supplemented RPMI 1640 and buffers for FACS analysis were as described 8. Directly-coupled mAb were from PharMingen (Becton Dickinson, CA).
4.2 Mice and cells
LN CD8 T cells from mice expressing the BM3.3TCR (TgBM3.3) 15 (on either normal or RAG-1-deficient H 2k background) were used as naive T cells or as CTL effectors after 3 days culture as described 8. RMA-S (TAP-2 deficient), RMA (H-2b) and RDM4 (H-2k) were used as target cells as described 8.
4.3 Synthetic peptides
Peptides were synthesized by solid-phase F-moc chemistry, purified and analyzed as described 8.
4.4 Dimeric and multimeric peptide/H-2Kb preparations
Dimeric peptide/H-2Kb complexes (IgG1-Kb), kindly given by J. Schneck (Baltimore, MD), were prepared as described 16 or purchased (Dimers X1, Becton Dickinson). The peptide/Kb multimers were prepared by Immunomics Operations (Beckman-Coulter, Marseille, France) using H-2Kb monomers produced as described 32 and mixed with a polymeric PE-streptavidin conjugate (Immunotech, Beckman-Coulter Marseille France). The peptide/Kb multimers were tested by size exclusion chromatography to be free of soluble monomers.
4.5 CTL assay
Tumor cell labeling with 51Cr (Na2CrO4) and CTL assay were as described 8.
4.6 Peptide/Kb dimer and multimer binding
LN T cells were incubated for 90 min at 4°C with various concentrations of either (1) H-2Kb dimers, washed and re-incubated with PE-goat anti-mouse IgG1 (Southern Biotech Ass., AL) for 30 min at 4°C, washed again and formaldehyde-fixed, or (2) PE-peptide/Kb multimers, washed and formaldehyde-fixed until FACScan analysis. To measure binding to CD4 and CD8 T cells, LN T cells were incubated with anti-CD4(PerCPCy5) or anti-CD8(FITC) mAb after incubation with peptide/Kb multimers. PE-fluorescence was analyzed after gating on CD4 or CD8 T cells using FlowJo software (Treestar Inc, CA). For comparison between dimeric and multimeric complexes all concentrations were expressed as monomeric peptide/Kb equivalents.
4.7 Decay
CD8 T cells were incubated with 15 nM pBM1/Kb or VSV8/Kb multimers for 90 min at 4°C, washed thrice with cold PBS(1% FCS) and incubated for various times at 4°C in the same buffer with 50 μg/ml anti-Kb mAb 5F1 to avoid re-fixation of peptide/Kb to the TCR. At each time point, cells were washed, formaldehyde-fixed and analyzed (FACScan, Becton Dickinson).
4.8 KD and half-life determination
Mean fluorescence intensities observed as a function of dimer or multimer concentration, or as a function of time, were analyzed using Prism software (Prism3, GrahPad Software, Inc).
4.9 Calcium signaling
LN T cells from RAG-1°/° TgBM3.3 mice (107 cells/ml) were incubated with 1 μM Indo-1AM (Calbiochem) in RPMI(3% FCS) for 45 min. After washing at room temperature, T cells were distributed in aliquots of 106 cells/ml, and kept at 37°C. For each test sample, a background line was recorded for 1 min and calcium levels were recorded during 10–15 min at 37°C after addition of PE-peptide/Kb multimers. Peptide/Kb dimers were present for 4 min before addition of PE-goat anti-mouse IgG1 for cross-linking. PE fixation and calcium flux were quantified on a FACStar+ cytofluorimeter (Becton Dickinson), and kinetic analyses were performed using FlowJo software (TreeStar, CA).
4.10 Peptide/Kb multimer-induced CD69 expression
LN T cells were incubated with peptide/Kb multimers or anti-TCR mAb Ti98 for 90 min at 4°C. After addition of 100 μl culture medium, incubation was pursued at 37°C for 24 h. Cellswere washed and labeled with anti-CD4(PerCPCy5), anti-CD8(APC) and anti-CD69(FITC) mAb. Fluorescence was analyzed on a FACScalibur (Becton Dickinson).
Acknowledgements
We thank Jonathan Schneck for gift of IgG1-Kb dimers. N. Auphan-Anezin, C. Boyer, S. Guerder, L. Leserman, P. Machy and B. Malissen for helpful discussions, P. Fourquet for peptide synthesis, N. Brun and M. Barad for help with calcium analysis and C. Beziers-Guigue for artwork. This work was supported by institutional grants from "Institut National de la Santé et de la Recherche Médicale" and "Centre National de la Recherche Scientifique" and by a grant from "Association pour la Recherche sur le Cancer".
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




