The activation of Csk by CD4 interferes with TCR-mediated activatory signaling
Abstract
CD4-Lck recruitment to TCR/CD3, as well as Lck activation is essential for T cell activation. Indeed, the blockage of CD4-Lck recruitment to TCR during antigen recognition exerts a drastic inhibitory effect on T cell activation by interfering with both early and late phases of T cell signaling. In the present work, we report a novel inhibitory mechanism by which CD4 can shut down proximal T cell-activating signals. Indeed, we show that upon ligation of CD4 by antibodies the inhibitory kinase, p50csk, is strongly induced and prolonged during the time. In contrast, p50csk was not activated when TCR and CD4 were properly engaged by their ligands. We also demonstrate that anti-CD4 treatment stimulated Csk kinase associated to the membrane adapter, PAG/Cbp, without affecting the total amount of Csk bound to PAG/Cbp. As a consequence, early tyrosine phosphorylation events as well as downstream signaling pathways leading to IL-2 gene expression induced by TCRwere inhibited in anti-CD4 pretreated cells. We suggest a new model to explain the activation of negative signals by CD4 molecule.
Abbreviations:
-
- PAG:
-
Glycosphingolipid-enriched microdomains
-
- Cbp:
-
Csk binding protein
-
- SEE:
-
Staphylococcal enterotoxin E
1 Introduction
The coreceptor molecule CD4 plays a crucial role in the early events of T cell activation by contributing to both MHC recognition and intracellular signal transduction. CD4 binds to both engaged TCR and non-polymorphic regions of MHC class II molecules 1, 2, and its cytoplasmic tail interacts with the Src family tyrosine kinase p56lck 3 that is necessary to initiate the immunoreceptor signaling. The earliest event induced by TCR recognition of peptide-MHC molecules is the phosphorylation of tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITAM) of the CD3 subunits and TCR-associated ζ chain, which leads to the recruitment and activation of ZAP-70 kinase 4. CD4-associated p56lck contributes to the initial tyrosine phosphorylation of ITAM and interacts with the ζ/ZAP-70 complex, mechanisms proposed to favor CD4 recruitment to the activated TCR 5. Moreover, p56lck is also involved in the phosphorylation and activation of ζ-associated ZAP-70, which in turn phosphorylates several adapter proteins (i.e. LAT and SLP-76) resulting in the activation of downstream signaling events that lead to functional T cell responses [6.
In addition to initiate immunoreceptor signaling, CD4-associated p56lck can also have inhibitory roles. Indeed, CD4 ligation by mAb or HIV gp120 transmits negative signals able to both inhibit TCR signaling 7, 8 and activate apoptotic programs 9, 10. Several mechanisms have been proposed for the CD4-mediatedinhibitory effects, including sequestration of p56lck to the actin-cytoskeleton 11 or sequestration of key components of the TCR/CD3 by the CD4-Lck complexes 8. A still open question is whether the modulation of CD4-associated Lck kinase activity modifies TCR-dependent kinase signaling cascades by transforming activatory signals into suppressive ones.
Lck kinase activity is regulated by an inhibitory C-terminal tyrosine residue (Tyr505) that when phosphorylated associates with the SH2 domain of p56lck itself, thus leading to the down-regulation of its catalytic activity 12, 13. A balance between phosphorylated and dephosphorylated p56lck at this C-terminal residue must be maintained to avoid hypo– as well as hyper-reactivity in response to TCR engagement. The tyrosine kinase Csk phosphorylates the inhibitory Tyr505 residue, whereas the CD45 tyrosine phosphatase antagonizes Csk by dephosphorylating this inhibitory residue 14. p50csk is a cytoplasmic tyrosine kinase expressed ubiquitously, albeit in greater amounts in hemopoietic cells 15. p50csk contains an SH3 domain, an SH2 domain and a C-terminal kinase domain. Elimination of p50csk in thymocytes abrogates the requirement for preTCR-mediated signals for the development of mature α β T cells 16. p50csk overexpression strongly inhibits TCR signaling, an effect correlated with its ability to phosphorylate and inactivate Lck and Fyn 17. More recently, a cooperation between p50csk and the associated proline-enriched protein tyrosine phosphatase PEP, in inhibiting Src kinase mediated signals in T cells, has been described 18. To negatively regulate Src kinase activities, p50csk must translocate from the cytoplasm to the membrane, specifically to the glycosphingolipid-enriched domains or lipid rafts, which play an important role for the initiation of receptor-mediated signaling, and where the majority of Src kinases are localized 19–21. One mechanism that has been proposed relies on the interaction of p50csk with a recently cloned phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) or Csk binding protein (Cbp) 22, 23. Indeed, in resting normal T cells p50csk is present in lipid rafts through interaction with tyrosine phosphorylated PAG/Cbp, thus inducing a tonic inhibition of T cell activation. The dephosphorylation of PAG/Cbp induced by TCR engagement leads to the dissociation of Csk from lipid rafts and to Lck activation 24. Therefore, the signals that activate Csk and at the same time inhibit its dissociation from membrane PAG/Cbp may interfere with Lck activation by blocking TCR activating signaling.
Here we report a novel inhibitory mechanism by which CD4 can shut down proximal T cell activating signals. Indeed, we show that upon ligation of CD4 p50csk kinase activity was strongly induced and prolonged during the time. On the contrary, p50csk was not activated when TCR was properly stimulated. We also demonstrate that anti-CD4 treatment induced PAG/Cbp-associated Csk kinase activity without affecting the total amount of Csk bound to PAG/Cbp. As a consequence, TCR-mediated early tyrosine phosphorylation events as well as downstream signaling pathways, which lead to IL-2 gene expression, were inhibited in anti-CD4 pretreated cells. These results support data on the negative effects mediated by Csk, and provide a new model to explain the activation of negative signals by CD4 molecule.
2 Results
2.1 CD4 dissociation from TCR inhibits both earliest and late TCR-mediated signaling events in staphylococcal enterotoxin E-stimulated Jurkat cells
To elucidate the molecular mechanisms regulating anti-CD4 antibodies-mediated inhibition of T cell responses, we used CD3+CD4+CD28+ Jurkat cells that, when stimulated with staphylococcal enterotoxin E (SEE) presented by B7+ HLA-DR1-expressing cells (5-3.1/B7) or EBV-B cells, provide a good physiological model of T cell activation 10. CD4 ligation by Leu3a mAb separately from TCR inhibited both NF-AT-and IL-2-luciferase activities induced by SEE (Fig. 1A, B). The tyrosine phosphorylation of TCR-associated CD3 subunits and ζ chains is one of the earliest events that precede the activation of the downstream signaling pathways regulating T cell activation. Tyrosine phosphorylated ζ chains in turn recruit ZAP-70 that is itself tyrosine phosphorylated by the receptor-associated src kinases, and activated 4. To determine whether the inhibition of NF-AT and IL-2 promoter activity reflected a blockage of early TCR signaling, Jurkat cells were both untreated or treated with Leu3a and then stimulated with EBV-B cells pre-pulsed with an optimal concentration of SEE. ZAP-70 was then immunoprecipitated and tyrosine phosphorylation was analyzed by Western blotting. As shown in Fig. 2, Leu3a treatment inhibited the tyrosine phosphorylation of both ZAP-70 (upper panel) and coprecipitating ζ chain (middle panel, lane 4 vs. 2). Similar to that observed in primary T cells 25, CD4 engagement before TCR triggering inhibits in Jurkat cells the earliest TCR-induced tyrosine phosphorylation events necessary for IL-2 gene expression.
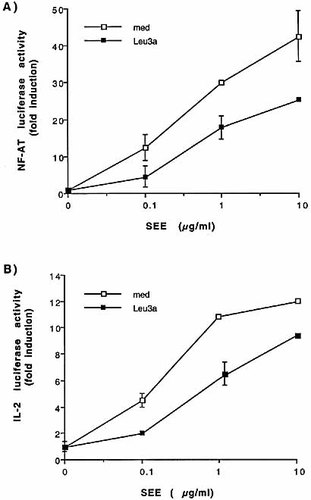
CD4 engagement by Leu3a inhibits TCR-induced NF-AT and IL-2 promoter activity. (A, B) Jurkat cells were transfected with 10 μg NF-AT (A) or IL-2 (B) luciferase reporter constructs. Cells were then pre-cultured for 15 min in the presence or absence of Leu3a (1:100 dilution) and then stimulated for 8 h with 5-3.1/B7 cells pulsed with different concentrations of SEE. The results are expressed as fold induction over the basal luciferase activity after normalization to β-galactosidase values. The results express the mean ± SD of three different experiments.
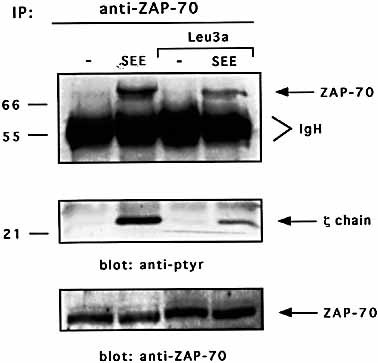
CD4 engagement by Leu3a inhibits TCR-induced ZAP-70 tyrosine phosphorylation. Jurkat cells were pre-cultured for 15 min in the presence or absence of Leu3a (1:100 dilution) and then stimulated with EBV-B cells pulsed with 1 μg/ml SEE. ZAP-70 was immunoprecipitated and anti-phosphotyrosine Western blotting was performed (upper and middle panels). Each sample was analyzed by Western blotting with anti ZAP-70 antibodies (lower panel). Sizes are indicated in kilodaltons. The results are representative of three independent experiments.
2.2 CD4 engagement by antibodies induces Csk kinase activity
In most T cells, immunoreceptor signaling is dependent on full activation of Src family tyrosine kinases 4. In contrast, the activation of Csk inhibits TCR signaling 17. Thus, we verified whether the inhibitory signals induced by dissociating CD4 from TCR following Leu3a treatment were associated to the activation of p50csk. To test this hypothesis, Jurkat cells were treated with Leu3a for different times and both p56lck and p50csk kinase activities were measured in vitro. Fig. 3A shows that cross-linking of CD4 by Leu3a results in a twofold increase of Lck kinase activity that rapidly decreased to nearly the basal level after 15 min of stimulation (upper panel, lanes 1–4). In contrast, CD4 engagement induced a high and sustained activation of Csk (Fig. 3A upper panel, lanes 5–8). CD4-induced activation of Csk was independent of Lck as demonstrated by the absence of any detectable Lck in anti-Csk immunoprecipitates (Fig. 3B, lower panel), and was also observed using a second anti-Csk antiserum (Fig. 3C, upper panel, lanes 1–4). Similar results were obtained in primary CD4+ T cells (Fig. 3D), indicating that CD4-mediated activation of Csk was not peculiar to Jurkat cells but was also a feature of normal T lymphocytes.
Although Leu3a mAb was an IgG1, with a low binding affinity for protein A, and lysates were subjected to two preclearing steps with protein A-Sepharose beads before Csk immunoprecipitation, we ensured that the kinase activity observed was exclusively due to immunoprecipitated Csk. Thus, we performed in vitro competition assays using the Csk peptide used for generating the anti-Csk antibodies. The anti-Csk antibodies were incubated overnight at 4°C with a saturating concentration of blocking peptide, and anti-Csk immunoprecipitations were performed in unstimulated or Leu3a-activated Jurkat cells. As shown in Fig. 4A, the addition of blocking peptide strongly reduced the total amount of immunoprecipitated Csk (lower panel, lanes 3 and 4 vs. 1 and 2) as well as the kinase activity observed in Leu3a stimulated Jurkat cells (upper panel, lanes 3 and 4 vs.1 and 2). In contrast, the same peptide did not inhibit either CD4-associated Lck activity (Fig. 4A, upper panel, lane 6 vs. 5) or the amount of CD4-bound Lck (lower panel). The ability of Csk to undergo strong autophosphorylation in vitro has been previously shown 26, but no obvious sites of tyrosine autophosphorylation were found in Csk. Thus, we verified whether the robust autophosphorylation of Csk induced by anti-CD4 antibodies was also accompanied by an increase of its ability to phosphorylate a specific exogenous substrate. CD4 stimulation increased Csk kinase activity on the exogenous substrate pEY (Fig. 4B, upper panel). This peptide has been previously described to be highly specific for Csk 26. Indeed, pEY was not phosphorylated by CD4-associated Lck (Fig. 4C, upper panel lanes 3 and 4), despite the strong Lck kinase activity observed in anti-CD4 immunoprecipitates (Fig. 4C, upper panel, lanes 1 and 2). Altogether these data clearly demonstrate that CD4 stimulation of both Jurkat cells and primary CD4+ T cells induced the activation of Csk.
We next examined the effect of CD4 cross-linking on Csk kinase activity in TCR stimulated Jurkat cells. Jurkat cells were pretreated for 15 min with Leu3a and then stimulated with 5-3.1/B7 cells pulsed with an optimal dose of SEE. Consistent with several lines of evidence that for an efficient TCR signaling Csk must not be active 17, 18, SEE stimulation did not induce a significant up-regulation of Csk kinase activity (Fig. 5, upper panel, lanes 1–4). No Csk activity was observed when Jurkat cells were stimulated with an optimal concentration of SEE (10 μg/ml, data not shown). In contrast, when Leu3a engaged CD4 before SEE stimulation, Csk kinase activity increases of at least 23-fold (Fig. 5, upper panel, lanes 5–7). Similar results were obtained in normal CD4+ T cells stimulated with anti-CD3 Ab (data not shown). These data demonstrate that the inhibitory signals elicited by CD4 engagement, separately from TCR, are associated at a very early step with the induction of Csk kinase activity, which through the negative regulation of Src kinases abolishes TCR activation.
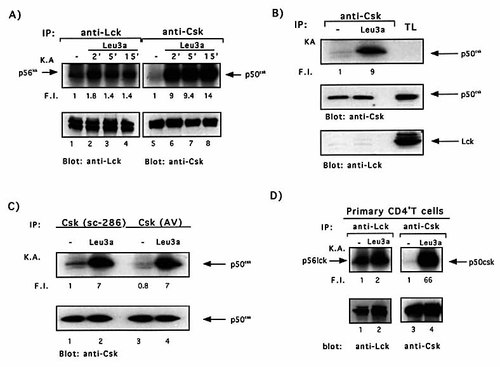
Csk kinase activity is induced following CD4 engagement in T cells. (A) Jurkat cells were stimulated for different times with Leu3a and in vitro kinase assays were performed in anti-p56lck (lanes 1–4) or anti-p50csk (lanes 5–8) immunoprecipitates. (B) Jurkat cells were stimulated for 5 min with Leu3a and Csk was immunoprecipitated by using sc-286 antibody (lanes 1–2). The kinase activities of immunoprecipitated Csk was evaluated in vitro (upper panel). (C) Jurkat cells were stimulated for 5 min with Leu3a and Csk was immunoprecipitated by using sc-286 antibody (lanes 1–2) or anti-Csk antiserum from A. Veillette's Laboratory (AV, lanes 3–4). The kinase activities of immunoprecipitated Csk were evaluated in vitro (upper panel). (D) Primary CD4+ T cells were stimulated for 5 min with Leu3a and the kinase activities of Lck and Csk were measured as in (A). Fold induction over the basal kinase activity (F.I.) was quantitated by phosphorimager. Each IP was analyzed for Lck or Csk content using specific antibodies followed by peroxidase-conjugated protein A. The results are representative of three independent experiments.
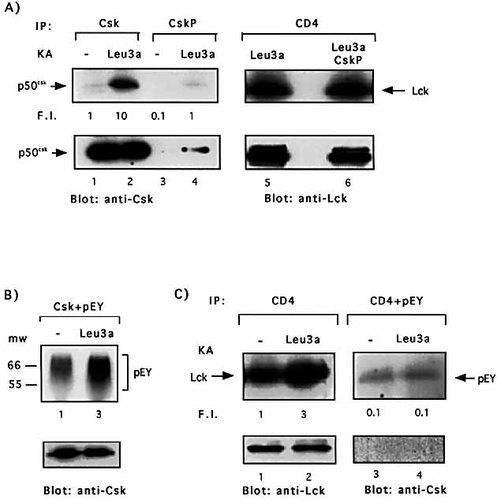
CD4 engagement increases Csk kinase activity on exogenous substrates. (A) Jurkat cells were stimulated for 5 min with Leu3a, Csk and CD4 were immunoprecipitated with specific antibodies (upper panel, lanes 1, 2 and 5) or specific antibodies incubated overnight with a tenfold excess of Csk blocking peptide (CskP) (upper panel, lanes 3, 4 and 6) and in vitro kinase assays were performed. (B) Csk was immunoprecipitated from both unstimulated or anti-CD4 activated Jurkat cells and the kinase activity was measured on the exogenous substrate poly(Glu, Tyr) (pEY). Position of molecular weight markers (mw) are shown in kDa. (C) CD4 was immunoprecipitated from both unstimulated or anti-CD4 activated Jurkat cells and in vitro kinase assays for Lck activity (upper panel, lanes 1, 2) or on the exogenous substrate pEY (upper panel, lanes 3, 4) were performed. F.I. and IP were determined as in legend for Fig. 3. The results are representative of three independent experiments.
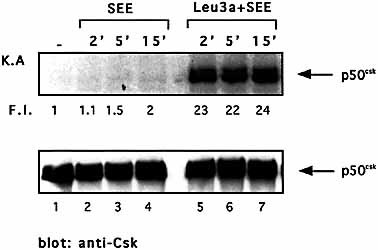
CD4 ligation before TCR engagement induces Csk kinase activity. Jurkat cells were pre-cultured for 15 min in the presence or absence of Leu3a and then stimulated with adherent 5-3.1/B7 cells prepulsed with 1 μg/ml SEE for the indicated times. Csk was immunoprecipitated and the kinase activity was measured by in vitro kinase assays. F.I. and IP were determined as in legend for Fig. 3. The results are representative of three independent experiments.
2.3 Leu3a treatment induces Csk kinase activity associated to PAG/Cbp without affecting the ratio of PAG/Cbp-Csk complexes
The relocation of Csk to the lipid rafts, where Src kinases are active, is essential for its role as negative regulator of TCR signaling. Indeed, the identification of lipid rafts-associated protein PAG/Cbp, which when tyrosinephosphorylated binds Csk 22, 23 and up-regulates its kinase activity 27, has suggested a mechanism by which Csk may turn off the signaling events initiated by Src kinases. PAG/Cbp was immunoprecipitated from both Jurkat cells and primary CD4+ T cells treated with Leu3a, and Csk kinase activity was measured. CD4 engagement by Leu3a increased PAG/Cbp-associated Csk autophosphorylation (Fig. 6A, upper panel). We were sure that the autophosphorylated band was PAG/Cbp-associated Csk and not CD4-bound Lck on the basis of their different migration mobility on 7% SDS-PAGE (data not shown). The increase of in vitro PAG/Cbp-associated Csk autophosphorylation was also accompanied by an increase of Csk kinase activity on the exogenous substrate pEY (Fig. 6B, upper panel). Similar results were obtained in primary CD4+ T cells (Fig. 6C). The induction of Csk kinase activity induced by Leu3a was not associated to an increase of Csk binding to PAG/Cbp as demonstrated by the equal amounts of Csk coprecipitated with PAG/Cbp in both unstimulated and Leu3a-treated cells (Fig. 6, middle panels). We next examined if CD4 treatment before TCR engagement was also able to activate PAG/Cbp-associated Csk activity. Jurkat cells were pretreated for 15 min with Leu3a and then stimulated for different times with 5-3.1/B7 cells prepulsed with an optimal concentration of SEE. As shown in Fig. 7, SEE stimulation did not affect PAG/Cbp-associated Csk kinase activity (upper panel, lanes 1–3). It is interesting to note that in contrast to the results obtained using anti-CD3 and anti-CD28 mAb as stimulators 22, the amount of PAG/Cbp-associated Csk did not change in Jurkat cells physiologically activated with SEE presented by B7+ APC (Fig. 7, middle panel). Interestingly, PAG/Cbp-associated Csk kinase activity was up-regulated in SEE-stimulated Jurkat cells pretreated with Leu3a (upper panel, lanes 5–7 vs. 1–4). Thus, CD4 engagement was able to up-regulate PAG/Cbp-associated Csk kinase activity also in SEE-stimulated cells without affecting the amount of Csk bound to PAG/Cbp.
To assess the significance of Csk activation by CD4 in inhibiting TCR-mediated signaling, Jurkat cells were transfected with a NF-AT-luciferase reporter construct together with a Csk mutant lacking its kinase activity (Csk KR). Overexpression of Csk (KR) increased NF-AT basal activity (at least tenfold) and completely reverted Leu3a-mediated inhibition of NF-AT activity in SEE-stimulated Jurkat cells (Fig. 8, panel A).
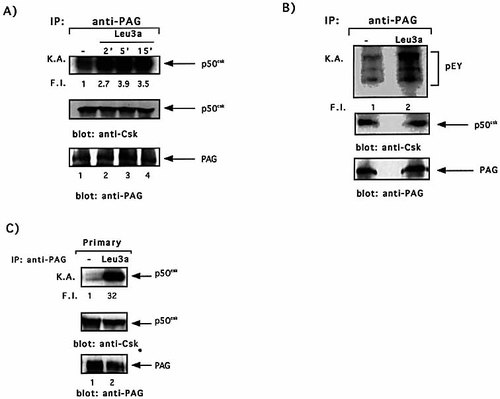
CD4 ligation induces the activation of PAG/Cbp-associated Csk without affecting the amount of Csk bound to PAG/Cbp. (A) Jurkat cells were activated with Leu3a for the indicated times. Cells were lysed in buffer containing n-octyl β-glucopiranoside, PAG/Cbp was immunoprecipitated using specific antibodies and in vitro kinase assays were performed. (B) Jurkat cells were activated with Leu3a for 5 min and Csk activity was measured in anti-PAG/Cbp immunoprecipitates using pEY as substrate. (C) Primary CD4+ T cells were activated with Leu3a for 5 min and PAG/Cbp-associated Csk kinase activity was evaluated as in (A). Each IP was analyzed for Csk and PAG content using specific antibodies followed by peroxidase-conjugated protein A. The results are representative of three independent experiments.
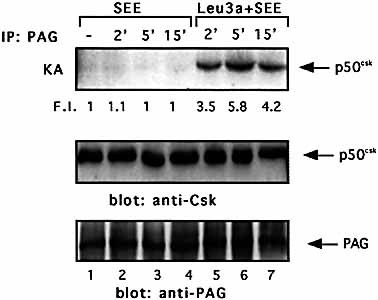
CD4 engagement separately from TCR increases PAG/Cbp-associated Csk kinase activity. Jurkat cells were pre-cultured in the presence or absence of Leu3a and then stimulated with adherent 5-3.1/B7 cells prepulsed with 1 μg/ml SEE. Csk kinase activity in anti-PAG/Cbp immunoprecipitates was measured. Each IP was analyzed for Csk or PAG/Cbp content using specific antibodies. The results are representative of three independent experiments.
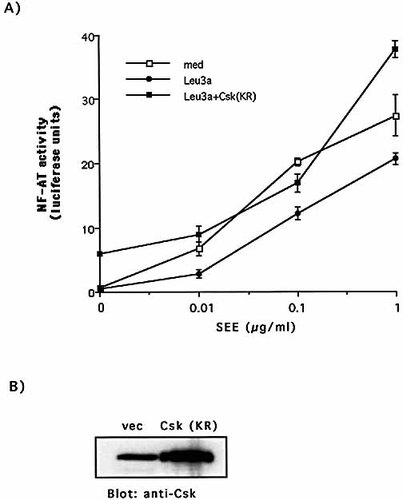
Expression of dominant negative Csk (KR) restores TCR-induced NF-AT activation in Leu3a-treated cells. (A) Jurkat cells were transfected with NF-AT luciferase reporter construct together with vector control or dominant negative Csk (KR) mutant and after 24 h cells were stimulated for additional 8 h with SEE-pulsed 5-3.1/B7 cells. The results are expressed as the mean of arbitrary units of the luciferase activity ± SD. The data represent at least four independent experiments. (B) The overexpression of Csk (KR) mutant was analyzed by western blotting using anti-Csk antibodies.
3 Discussion
The reorganization of surface receptors and signaling molecules at the T cell/APC interface plays a key role in T cell activation. This process, known as immunological synapse (IS) formation, co-ordinates engagement of the TCR by peptide/MHC complex, thus facilitating signaling through the TCR. Recent models suggest that the main role of CD4 is to "boost" TCR recognition 28, facilitating the rapid recruitment of Lck to TCR/CD3, an event that is essential to initiate signaling and trigger the early phase of T cell activation 29. Indeed, Lck is autophosphorylated and activated during IS formation, an event that require both CD4 and CD28 29. The blockage of CD4-Lck recruitment to TCR by anti-CD4 antibodies or HIV gp120 during antigen recognition exerts a drastic inhibitory effect on T cell activation by interfering with both early and late phases of T cell signaling 7, 30. Several mechanisms have been proposed to explain the inhibitory effects mediated by CD4 on TCR signaling, such as decreased cellular levels of p56lck 31, sequestration of CD4-associated p56lck to the cytoskeleton 11, or sequestration of crucial components of the TCR/CD3 signaling pathway to the CD4-p56lck complexes 8. Less is known about the regulation of p56lck kinase activity following CD4 engagement separately from TCR. Here we present evidence that a mechanism regulating p56lck activity relies on the induction of Csk kinase activity by CD4.
p56lck kinase activity is regulated by a conserved tyrosine residue, Tyr505, within the C-terminal tail that, when phosphorylated, inhibits kinase activity. CD45, a trans-membrane tyrosine phosphatase expressed by all hemopoietic cells, appears to be required for activation of p56lck by dephosphorylating its inhibitory site 32. In contrast, the tyrosine kinase p50csk phosphorylates the Tyr505, thus inhibiting p56lck kinase activity 15. CD45 and Csk are both constitutively active enzymes. However, data from CD45-deficient mice indicate that the balance between CD45 and Csk is a net dephosphorylation of the inhibitory site at the steady state 33. A current model proposes that, under resting conditions, CD45 maintains a basal level of CD4-Lck activity that is responsible for generating the p21 ζ phosphoisoform associating inactive ZAP-70 34. Engagement of TCR by peptide-MHC molecules brings CD4-Lck into closer proximity of CD3/TCR-ζ, thus leading to the generation of p23 ζ phosphoisomer, and phosphorylation and activation of ζ-bound ZAP-70 by p56lck 35. The subsequent interaction between ZAP-70 and p56lck will result in the activation of both proteins 36, 37. In this model, if the balance between CD45 and Csk will shift in favor of Csk, the dominance of Tyr505-phosphorylated p56lck species in a "closed" conformation and with a reduced ability of their SH2/SH3 domains to dock with the TCR/ZAP-70 complex will be induced. Indeed, we show that CD4 engagement by anti-CD4 mAb induced a reduction of both ZAP-70 and ZAP-70-associated TCR-ζ tyrosine phosphorylations (Fig. 2). These events were associated to a transient increase of Lck kinase activity. In contrast, CD4 stimulation induced a stronger and persistent increase of Csk kinase activity that was not observed when cells were correctly stimulated through TCR (Fig. 5). Although, a direct correlation between CD4-induced Csk activation and the impairment of TCR-mediated signaling cannot be made, our data on the loss of CD4-mediated inhibition of TCR signaling in kinase-deficient Csk-overexpressing cells, strongly support this hypothesis. Moreover, it is intriguing that the reduction in TCR-ζ phosphorylation and ZAP-70 recruitment in anti-CD4-treated cells is similar to that observed in Csk-overexpressing cells 18.
Similarly to the Src kinases, Csk consists of an SH3 domain, an SH2 domain and a kinase domain, but lacks the C-terminal regulatory tyrosine residue as well as the N-terminal myristoylation signal for targeting to lipid rafts. Recently, a transmembrane protein known as PAG or Cbp has been identified that, when phosphorylated, can recruit Csk via its SH2 domain to the membrane lipid rafts where the Src kinases are located 22, 23. PAG/Cbp is involved not only in the membrane recruitment of Csk but also in Csk-mediated inhibition of Src, by directly activating Csk 27. In unstimulated T cells, PAG/Cbp is constitutively tyrosine phosphorylated and associated to Csk. TCR engagement by antibodies has been described to induce the rapid dephosphorylation of PAG/Cbp, thus leading to a transient displacement of Csk from lipid rafts. This event has been reported to allow a stronger and prolonged activation of Src kinases and sustained TCR signaling 22, 24. Physiological stimulation of Jurkat cells by SEE presented on B7+ APC did not affect either the amount of PAG/Cbp-associated Csk or PAG/Cbp-associated Csk kinase activity. The failure to see PAG dephosphoryation in Jurkat cells following TCR stimulation may be due to the fact that Jurkat express not very much PAG/Cbp but a lot of phosphatases, which results in a constitutive dephosphorylation of PAG/Cbp. Indeed, when the expression levels of PAG/Cbp are raised, PAG becomes dephosphorylated after TCR stimulation also in Jurkat cells 38. In contrast, CD4 engagement alone or before TCR stimulation was able to activate PAG/Cbp-associated Csk kinase activity, again without modifying the total amount of Csk bound to PAG/Cbp (Fig. 6, 7). It may be possible that Lckactivated by Leu3a phosphorylates PAG, thus inducing an increase of Csk recruitment and activation. However, our results showing that Leu3a did not change the amount of Csk associated with PAG suggest that Csk kinase activity may be regulated by another mechanism. Csk has also been described to down-regulate Src activity by redistributing to sites of Src activation through a direct interaction with tyrosine phosphorylated GTPase-activating protein (GAP)-associated p62dok 39, 40. In T cells, p62dok is tyrosine phosphorylated following CD2 41 or CD28 stimulation 42. Recent data from Nemorin et al. have shown that p62dok tyrosine phosphorylation is mediated by Lck 41 and that p62dok overexpression impairs both CD3-and CD2-induced NF-AT activation and IL-2 secretion 43. Thus, the transient increase of Lck kinase activity induced by Leu3a may lead to the tyrosine phosphorylation of p62dok, which in turn may recruit and activate Csk thus leading to the termination of Lck activity. CD4 engagement by Leu3a induced in Jurkat cells the tyrosine phosphorylation of a ∼62 kDa protein that may be p62dok (data not shown). Experiments are in progress to verify this hypothesis.
A disregulation between CD3- and CD4-mediated signals has been observed following exposure of T lymphocytes to IL-16, a natural ligand of CD4 44, or in lymphocytes derived from HIV-1-infected patients. Moreover, binding of HIV gp120 with CD4 separately from TCR results in the activation of programmed cell death 9, 45. An increase of Csk kinase activity has also been observed in T cells committed to apoptosis 46. Our data represent the first demonstration that CD4 may regulate the activity of an inhibitory kinase that, altering the proximal signaling events generated by TCR, gives rise to a dominant negative signals that, in some circumstances, may contribute to the death of T cells 47. This characterizes a novel biological role for CD4 that may contribute to a greater understanding of TCR signaling both in normal and pathological conditions.
4 Materials and methods
4.1 Cell lines, antibodies and reagents
CD4+ Jurkat T cell line was maintained in RPMI 1640 supplemented with 10% FCS, L-glutamine, penicillin and streptomycin (Gibco). The L cell transfectants expressing HLA-DRB1*0101 (5-3.1) and co-transfected with human B7.1 (5-3.1/B7) were previously described 45. Human peripheral blood CD4+ T cells were purified by negative selection using an indirect magnetic cell sorting kit (Miltenyi Biotec, Auburn, CA). Mouse anti-CD4 mAb (Leu3a) was from Becton Dickinson (Mountain View, CA). Mouse anti-p56lck (3A5), rabbit anti-p56lck, rabbit anti-p50csk antibodies and the Csk blocking peptide were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-Csk antiserum was kindly provided by A. Veillette (IRCM, Montreal, Canada). Anti-human ZAP-70 and ζ chain mAb were from Upstate Biotechnology (Lake Placid, NY). Anti-PAG antibodies were generated as previously described 22. SEE was purchased from Toxin Technology (Sarasota, FL).
4.2 Plasmids, cell transfection, activation and luciferase assay
The NF-AT luciferase reporter construct containing the luciferase gene under the control of the human IL-2 promoter NF-AT binding site was kindly provided by C. Baldari (University of Siena, Siena, Italy). The IL-2 luciferase construct 48 was kindly provided by J.F. Peyron (Faculté de Medicine Pasteur, Nice, France). Kinase-dead Csk, Csk (K222R), was kindly provided by A. Veillette (IRCM). The pSVβ-gal vector (Promega Corp., Madison, WI) contains the β-galactosidase gene driven by the SV40 promoter/enhancer.
Transient transfections were performed by electroporating (at 260 V, 960 μF) 107 Jurkat cells in 0.5 ml RPMI 1640 supplemented with 20% FCS with 10 μg of the indicated reporter constructs together with 20 μg pSV-βgal plasmid, keeping the total amount of DNA constant (50 μg) with empty vector. At 24 h after transfection 105 cells were pretreated for 15 min with medium or Leu3a (1:100 dilution) at 37°C and then stimulated for 8 h with confluent 5-3.1/B7 cells cultured overnight with the indicated concentration of SEE in round-bottom 96-well plates. β-Galactosidase and luciferase assays were performed according to the manufacturer's instruction (Promega). Luciferase activity, determined in triplicates samples using an automated luminometer (Lumat LB 9501, EG&G Berthold, Wildbad, Germany) was expressed as fold induction over the basal activity of cells cultured with medium alone or in arbritary units (AU) after normalization to the β-galactosidase values.
4.3 Cell stimulation, immunoprecipitation and in vitro kinase assay
Jurkat cells were washed twice, resuspended in medium (108/ml) and incubated at 37°C in the presence or absence of Leu3a mAb for different times. When indicated, Leu3a pretreated cells were then stimulated with adherent 5-3.1/B7 cells pulsed with different concentration of SEE. At the end of incubation, cells were harvested and lysed for 30 min on ice in 1% NP40 lysis buffer containing 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EGTA in the presence of inhibitors of proteases and phosphatases: 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM Pefabloc-sc, 50 mM NaF, 10 mM Na4P2O7 and 1 mM NaVO4. Cells were lysed in buffer containing 60 mM N-octyl β-glucopiranoside (Sigma) instead of 1% NP-40 for co-immunoprecipitation experiments of PAG/Csk complexes. Post-nuclear lysates were subjected to two rounds of preclearing with protein A-Sepharose beads before immunoprecipitations with anti-Csk, or anti-Lck or anti-PAG antibodies preadsorbed on protein A-Sepharose beads. In the competition assays with the Csk blocking peptide, the anti-Csk antibodies were incubated overnight at 4°C with a tenfold excess of peptide before immunoprecipitation. The kinase activity of immunoprecipitated p56lck or p50csk was assayed at 30°C for 15 min in 25 μl p56lck kinase buffer (1 mM Tris-HCl pH 7.5, 7.5 mMNaCl, 25 mM Hepes pH 7.3, 10 mM MnCl2, 0.05% NP-40) or p50csk kinase buffer (50 mM Hepes, pH 7.4, 5 mM MgCl2, 0.05% NP-40) in the presence of 10 μCi [γ–32P]ATP (10 Ci/mmol). Similar assays with the same buffer were performed with the synthetic polyamino acid poly(Glu, Tyr) 4:1 (Sigma), abbreviated pEY (0.5 μg), as substrate of Csk. The reactionswere terminated with 2× Laemmli sample buffers. Samples were analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were fixed, treated with 1 M KOH for 1 h at 55°C to remove the alkali-labile phosphate groups from serine and threonine-phosphorylated proteins, dried and autoradiographed. Radioactivity in the phosphorylated proteins was quantitated by a phosphorimager. Immunoblotting and detections of proteins by enhanced chemiluminescence (Amersham Pharmacia Biotech) were performed as previously described 5. For anti-Csk and anti-Lck Western blotting, peroxidase-conjugated protein A was used instead of secondary Ab to avoid Ig heavy chain nonspecific binding.
Acknowledgements
We thank C. Baldari, J. F. Peyron and A. Veillette for providing reagents. This work was supported by grants from the National Health Ministry research project on AIDS (2001), the Ministry for the University and Scientific and Technological Research (MIUR-COFIN 2001, and "Progetto Giovani Ricercatori 2000"), and the National Council of Research (CNR, "Progetto Giovani Ricercatori 2000").
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




