Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein and retinal S-antigen
Abstract
Antigenic mimicry of infectious agents and autoantigens is a proposed pathomechanism for autoimmune diseases. Here, we describe antigenic mimicry of a peptide from rotavirus, a nutritional protein from bovine milk (αs2-casein) and a peptide thereof as well as a highly pathogenic peptide from retinal S-antigen (PDSAg), a major autoantigen in experimental autoimmune uveitis in Lewis rats. Immunization of rats with the peptides and the casein protein induced uveitis, an intraocular inflammation leading to decreased vision and even blindness. The peptides elicited cross-reactive T cell responses and uveitis in rats and were also recognized by lymphocytes and sera from uveitis patients. Oral tolerization with PDSAg, but not with rotavirus- and casein-derived peptides or caseinprotein, prevented PDSAg-induced uveitis in rats. Cofeeding of casein with cholera toxin induced uveitis in rats, suggesting that breaking oral tolerance to casein during gastrointestinal infections might also be able to initiate uveitis in humans.
Abbreviations:
-
- CTX:
-
Cholera toxin
-
- S-Ag:
-
(Retinal) S-antigen
-
- SI:
-
Stimulation index
1 Introduction
Uveitis is a sight-threatening disease caused by CD4+ Th1 cells. The intraocular inflammation can destroy the delicate tissues of the eye and thus result in visual impairment. Retinalautoantigens like interphotoreceptor retinoid-binding protein (IRBP) and S-antigen (S-Ag), which are well characterized as autoantigens for animal models 1, 2 and humans 3, are sequestered antigens and therefore normally invisible to the immune system. The blood-retina barrier allows only activated lymphocytes to enter the eye, therefore naive T cells have no access to ocular proteins in situ. We have described antigenic mimicry of the retinal S-Ag peptide PDSAg and a peptide from the sequence of disease-associated HLA-B antigens (B27PD) 4, which explains the peripheral activation of T cells that cross-react with S-Ag peptide in the eye, furthermore the association of several MHC class I antigens (B27, B51) with a disease mediated by CD4+ HLA class II-restricted T cells. The HLA peptide B27PD has successfully been used in a first clinical trial of oral tolerance induction for uveitis patients 5. For the initiation of the disease we proposed an event of immunological stress like an infection to activate B27PD-specific T cells as "innocent bystanders", because no specific infection or pathogen has been associated with the onset of uveitis.
Here we describe two peptides which are homologous to PDSAg, one derived from the highly immunogenic surface antigen vp4 of a rotavirus, and the other from αs2-casein of bovine milk. Casein is a frequent nutritional component in many countries. Proteins such as casein should be tolerated (oral tolerance) by the immune system, whereas rotaviruses should always be attacked. Rotavirus infections are very frequent all over the world; they are mostly accompanied by gastrointestinal problems, but can also be asymptomatic. Despite protective immunity directed to the surface protein vp4, repeated infections with other strains can occur 6. For the induction of uveitis we propose that during infection with rotavirus a defensive immune response to an epitope represented by peptide Rota might be generated, which also recognizes the ocular peptide PDSAg. Moreover, rotaviral or other gastrointestinal infections could break oral tolerance to casein, eliciting retinal autoimmunity by the mimicry of peptides Cas and PDSAg.
To prove this concept, we tested the capacity to induce cross-reactive T cell responses in vitro and in vivo in Lewis rats. We established T cell lines specific for the three peptides that were uveitogenic by adoptive transfer. Subcutaneous (s.c.) immunization with the peptides or casein protein caused uveitis in Lewis rats; furthermore, oral application of casein, but not S-Ag or the peptides, together with native cholera toxin (CTX), the latter initiating a Th1-response 7 and imitating gastrointestinal infection to break oral tolerance, induced uveitis as well. Enhanced antibody titers to the peptides and casein were detected in sera from uveitis patients, as well as increased peripheral lymphocyte responses compared to healthy donors.
2 Results
2.1 Sequence homologies of mimicry peptides
Amino acid (aa) alignment of peptides PDSAg, Rota and Cas are shown in Table 1. Peptide Cas has five, Rota seven continuous aa homologies with the C-terminal part of peptide PDSAg, which represents the major T cell epitope 4.
2.2 Induction of uveitis in rats by subcutaneous immunization and adoptive T cell transfer
Immunization of 30 rats with peptide Rota resulted in uveitis (scores of single eyes ranging from 0.5 to 3) in 30% of rats (22% of eyes affected). Twenty-four percent of 17 rats immunized with Cas (12% of eyes) developed uveitis (scores from 1 to 4), as did two of four rats immunized with αs2-casein (scores from 1 to 3). No disease was elicited when rats were immunized with ovalbumin or control peptide B7PD. Sixty-six percent of animals injected intraperitoneally with T cells specific for Rota or Cas developed uveitis, as well as one of four rats injected with T cells specific for casein (Table 2, Fig. 1). All animals receiving PDSAg-specific T cells developed uveitis. Although the incidence of uveitis was lower for Rota, Cas and casein compared to PDSAg, the severity of affected eyes was similar following immunization with PDSAg or Cas. Adoptive transfer of T cell lines specific for peptide antigens caused similar disease (Fig. 1). Peptide Rota was less pathogenic by active immunization than PDSAg and Cas, and casein protein was less uveitogenic with respect to both, active immunization and adoptive transfer.
In all affected eyes infiltration of CD4+ T cells and destruction of the retina was observed, irrespective of the antigen used for immunization (Fig. 2) or the specificity of the adoptively transferred T cell line. No signs of arthritis, paralysis, skin alterations or diarrhea were observed in rats immunized s.c. or orally. Autopsy revealed no other than ocular pathology.
|
|
Peptide/protein |
Animals tested |
Affected animals |
Affected eyes |
||
|---|---|---|---|---|---|---|
|
|
n |
% |
n |
% |
||
|
Active immun. |
PDSAg |
10 |
9 |
90 |
17 |
85 |
|
|
Rota |
30 |
9 |
30 |
13 |
22 |
|
|
Cas |
17 |
4 |
24 |
4 |
12 |
|
|
B7PD |
14 |
0 |
0 |
0 |
0 |
|
|
Casein |
4 |
2 |
50 |
4 |
50 |
|
|
OVA |
12 |
0 |
0 |
0 |
0 |
|
Adoptive transfer |
PDSAg |
4 |
4 |
100 |
6 |
75 |
|
|
Rota |
3 |
2 |
66 |
3 |
50 |
|
|
Cas |
6 |
4 |
66 |
6 |
50 |
|
|
Casein |
4 |
1 |
25 |
1 |
12,5 |
|
|
OVA |
12 |
0 |
0 |
0 |
0 |
- a) Active immunization: PDSAg 15 μg/animal; Rota, Cas, B7PD, casein, OVA 100 μg/animal in CFA. Adoptive transfer: 3×106–7×106 cells/animal. Uveitis was determined by histology.
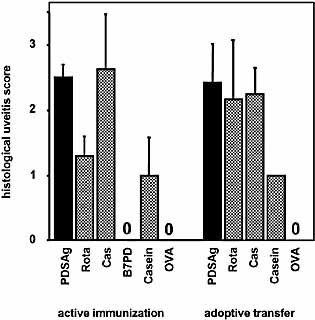
Induction of uveitis in rats. Intensity of uveitis: average uveitis score ± SE of positive eyes. Experimental groups were the same as shown in Table 2.
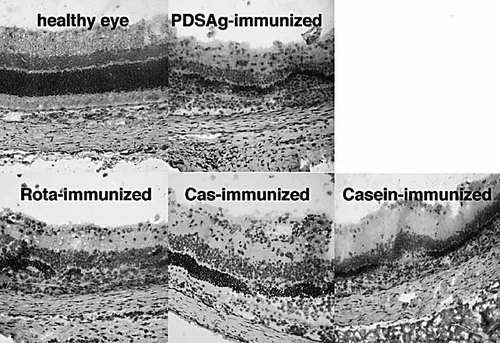
Histology of eyes from immunized rats. Destruction of photoreceptors, alteration and infiltration of cells in all retinal layers can be seen, showing uveitis after immunization as indicated.
2.3 Cross-reactive rat T cell lines
Lymph node cells from rats immunized with PDSAg, Rota, Cas or casein were propagated in vitro with their respective antigen and tested for cross-reactivity in proliferation assays (Fig. 3). Primary cultures did not reveal stimulation indices (SI) higher than 2.0; specific proliferation was not observed before the second restimulation in vitro. PDSAg-specific T cell lines did not cross-react with peptides Rota or Cas (Fig. 3A, B). Rota-specific T cell lines retained their high corecognition of PDSAg without cross-reactivity to Cas (Fig. 3C, D), whereas Cas-specific T cell lines (Fig. 3E, F) remained cross-reactive to PDSAg but lost reactivity to Rota (Fig. 3C). Cas-specific T cells also proliferated in response to the whole casein protein as in vitro antigen, suggesting that the epitope represented by peptide Cas is easily processed and presented by APC also in culture. In contrast, PDSAg-specific T cell lines never proliferated to S-Ag in culture (unpublished observations). Although the casein-specific line did not show cross-reactivity to PDSAg in vitro (Fig. 3G, H), it was uveitogenic after adoptive transfer in rats, suggesting that only a minor portion of T cells, which can not be detected in our proliferation assays, is specific for the pathogenic mimicry epitope.
All T cell lines were CD4+TCRα/β+ (data not shown). The peptide-specific lines were RT1.B-restricted, as shown by inhibition of antigen presentation with antibodies specific for RT1.B (Ox 6) and RT1.D (Ox 17) (Fig. 4). Casein-specific T cells recognized epitopes presented on both, RT1.B and RT1.D (Fig. 4). Differences in MHC class II binding affinity between the peptides were determined by competition with peptide PDSAg (Fig. 5A, B) and inhibition of proliferation of a non-cross-reactive PDSAg-specific T cell line as read-out system (Fig. 5A). At a concentration of 50 μM (tenfold excess to PDSAg) peptide Cas showed more efficient competition with PDSAg for RT1.B binding than Rota (Fig. 5B), which suggests a better presentation of Cas than of peptide Rota.
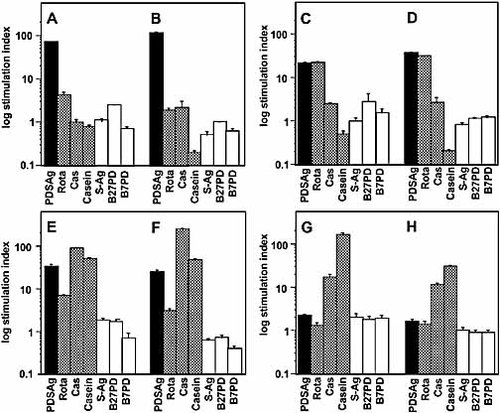
Cross-reactivity of rat T cell lines. Proliferation is shown as log of SI for each T cell line after the second (A, C, E, G) and third (B, D, F, H) restimulation in vitro. (A, B): PDSAg-specific T cell line; (C, D): Rota-specific line; (E, F): Cas-specific line; (G, H): casein-specific line.
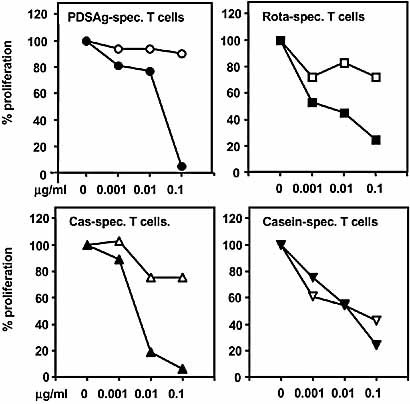
Definition of the restriction element for T cell lines. Presentation of antigens was blocked by co-culture with antibodies specific for rat MHC class II antigens. Inhibition of proliferation of rat T cells was used as read-out system. Black symbols: co-incubation with antibody Ox 6 (RT1.B-specific), open symbols: co-incubation with Ox 17 (RT1.D-specific). X-axis: the concentrations of antibodies in μg/ml. Proliferation of T cell lines without antibodies was defined as 100%.
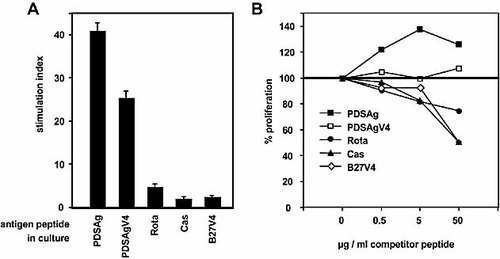
Peptide binding competition assay. Peptides competing for the presentation of PDSAg to a PDSAg-specific rat T cell line. Inhibition of proliferation of the T cell line was used as read-out system. (A) Proliferation of a PDSAg-specific T cell line to peptides PDSAg, Rota, Cas and control peptides of equivalent length (PDSAgV4 and B27V4). (B) Inhibition of proliferation of the same PDSAg-specific line by pre-incubation of APC with increasing concentrations of competitor peptides. The proliferation to PDSAg only, without competitor peptide, is represented as 100% proliferation.
2.4 Induction of oral tolerance
Only oral pretreatment of rats with peptide PDSAg resulted in significant reduction of PDSAg-induced disease, while feeding peptides Rota and Cas or casein protein had no significant ameliorating effect on uveitis compared to feeding with control peptide B7PD (Fig. 6A).
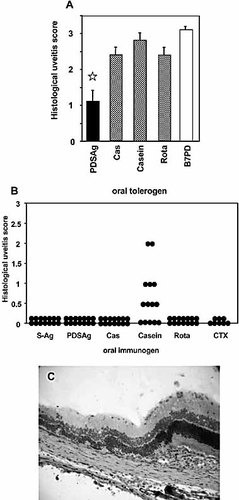
Oral tolerization and immunization with peptides and casein protein. (A) Oral tolerance induction with peptides PDSAg, Rota, Cas, control peptide B7PD and casein protein prior to induction of uveitis with PDSAg: average uveitis score/eye ± SE. Asterisks mark significant reduction of uveitis related to control feeding with B7PD (p<0.001 for PDSAg). Uveitis was not significantly reduced after oral tolerization with Rota, Cas or casein. (B) Induction of uveitis by concomitant gavage of peptides or proteins with native CTX. Each eye is represented by a black dot. Combined results from two independent experiments of feeding 1 or 2 mg of antigen with 10 or 15 μg CTX, respectively, are shown. (C) Histology of rat eye with disease induced by cofeeding casein and CTX. Destruction of the retinal architecture as shown after s.c. immunization in Fig. 2.
2.5 Oral induction of uveitis
Cofeeding rats with CTX and uveitogenic proteins/peptides was aimed at inducing eye disease due to oral immunization rather than oral tolerance induction. Only feeding casein protein (1 or 2 mg) with CTX resulted in uveitis in six of seven rats (9/14 eyes with scores between 0.5 and 2; Fig. 6B, C), whereas feeding 200 μg of casein or S-Ag or peptides at either concentration failed to induce disease. In the group fed with 1 mg casein, uveitis was clinically visible already 8 days after the first gavage. A second feeding did not increase incidence or intensity of clinical signs of disease. No difference in intensity of uveitis was observed after immunization with either 1 mg casein and 10 μg CTX applied twice or 2 mg casein and 15 μg CTX given once. Histology of rat eyes revealed no difference in uveitis induced by s.c. immunization with casein or oral immunization with casein and CTX (Fig. 6C).
2.6 ELISA with human sera
Sera from patients with anterior uveitis (iritis, iridocyclitis; n=50), posterior uveitis (n=48) and from healthy donors (n=36) were tested for binding to PDSAg, S-Ag, Cas, casein, Rota and tetanus toxoid as recall antigen control. Sera from iritis patients, but not sera from patients with posterior uveitis, were significantly (p<0.001) higher reactive to the tested antigens than sera from healthy donors. Only tetanus toxoid was significantly better recognized by sera from patients with posterior uveitis (iritis vs. healthy: p<0,016) than from iritis patients (posterior uveitis vs. healthy: not significant) or healthy controls (Fig. 7A).
We further determined the frequency of optical density (OD) for each antigen above the tolerance limit of control samples, which was set at mean OD 450 of healthy donors + 2 SD. This enabled to identify reactivities of individual sera to more than one antigen (Table 3). Only very few samples of the control sera (3%–8%) were positive for one or more of the tested antigens. Of 25 S-Ag-reactive sera from iritis patients, 3 sera exclusively recognized PDSAg or SAg and did not simultaneously react to either casein or Rota, whereas in the group of patients with posterior uveitis we found 7 of 15 sera recognizing S-Ag, but neither casein nor Rota. S-Ag and PDSAg were recognized by 50% of sera from iritis patients, followed by casein/Cas (46%) and Rota (38%). Thirty-eight percent of sera from iritis patients corecognized S-Ag and casein or S-Ag and Rota, and 32% all three antigens. More than 30% of sera from patients with posterior uveitis reacted with S-Ag, but only half of them showed also binding to casein.
Although most of our patients with posterior uveitis were under immunosuppressive therapy, reactivity to tetanus toxoid was higher than in healthy donors or patients suffering from iritis, which argues against the suppression of antibody responses in patients with posterior type of disease. Therefore, differences in treatment cannot be the reason for the reduced serological reactivity to retinal antigen, the mimicry peptides or casein. Compared to healthy donors, patients with anterior uveitis did not respond better to tetanus toxoid, whereas their reactivity to the tested retinal and mimicry antigens was significantly increased.
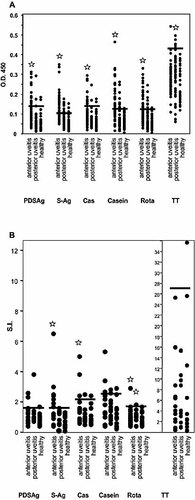
Humoral and cellular human immune response. (A) Antibody responses of sera from uveitis patients and healthy controls tested by ELISA. Mean of duplicates of OD 450 of each serum is represented by a black dot. Asterisks indicate a significantly higher mean (p<0.0001) of OD of all samples from patients with anterior uveitis compared with the mean of OD obtained from binding of sera from healthy controls to a certain antigen. Horizontal bars show the threshold for positive values calculated as mean OD 450 + 2 SD 27 for each antigen of data from healthy donors. Same or higher values were regarded as positive reaction. (B) Proliferation assay from PBL of uveitis patients and healthy donors. SI are calculated from the means of triplicates. Horizontal bars represent the threshold value for positive responses, calculated as mean SI + 2 SD of healthy donors defined for each antigen. Asterisks indicate significantly increased means of SI (p≤0.04) compared to healthy controls.
2.7 Lymphocyte proliferation of human T cells
The proliferation assay of frozen peripheral blood lymphocytes (PBL) revealed significantly (p≤0.03) higher mean SI with S-Ag, Cas, casein and Rota from iritis patients' lymphocytes compared to healthy controls. PBL from patients with posterior uveitis responded only to Rota significantly better than healthy donors (p≤0.03; Fig. 7B). Nevertheless, we found a trend to increased proliferation of cells from patients with iritis to the antigen PDSAg and with cells from patients with posterior uveitis to PDSAg, S-Ag and casein. There was no significant difference in reactivity of lymphocytes to tetanus toxoid between all groups. Determination of the frequencies of responses [exceeding the mean SI of controls + 2 SD] showed that PDSAg/S-Ag and Cas/casein was most frequently recognized by PBL from anterior uveitis patients, and Rota by lymphocytes from both, patients with anterior and posterior uveitis (Table 3). Concomitant recognition of S-Ag and casein was observed in proliferation assays from anterior uveitis patients. Healthy donors showed only rare and minor reactions to the tested autoantigen and mimicry peptides.
|
Immune response |
Type of disease |
S-Ag |
Casein |
Rota |
S-Ag + casein |
S-Ag + Rota |
Rota + casein |
S-Ag + Rota + casein |
TT |
|---|---|---|---|---|---|---|---|---|---|
|
Serolog. |
Anterior uveitis |
25 |
23 |
19 |
19 |
19 |
16 |
16 |
3 |
|
|
n=50 |
(50%) |
(46%) |
(38%) |
(38%) |
(38%) |
(32%) |
(32%) |
(6%) |
|
|
Posterior uveitis |
15 |
8 |
5 |
7 |
4 |
4 |
3 |
5 |
|
|
n=48 |
(31%) |
(17%) |
(10%) |
(15%) |
(8%) |
(8%) |
(6%) |
(10%) |
|
|
Healthy |
3 |
3 |
1 |
2 |
1 |
1 |
1 |
0 |
|
|
n=36 |
(8%) |
(8%) |
(3%) |
(5,5%) |
(3%) |
(3%) |
(3%) |
|
|
Cellular |
Anterior uveitis |
11 |
8 |
3 |
5 |
2 |
3 |
2 |
0 |
|
|
n=17 |
(65%) |
(47%) |
(18%) |
(30%) |
(12%) |
(18%) |
(12%) |
|
|
|
Posterior uveitis |
3 |
4 |
3 |
1 |
1 |
2 |
1 |
1 |
|
|
n=15 |
(20%) |
(27%) |
(20%) |
(7%) |
(7%) |
(13%) |
(7%) |
(7%) |
|
|
Healthy |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
1 |
|
|
n=14–18 |
(5,5%) |
(14%) |
|
(5,5%) |
||||
- a) Frequencies of serological and cellular immune responses are shown. The numbers represent the sera and cultures that exceeded the threshold values as calculated for each antigen in the respective assay (mean healthy donors + 2 SD). Percentages are calculated for the numbers of samples of each group. Reactivity to S-Ag or peptide PDSAg and to casein and Cas were combined, and defined as reaction to "S-Ag" or to "casein". "S-Ag + casein", e.g., represents those samples that were positively reacting to both, S-Ag and casein.
3 Discussion
Autoimmune responses to autoantigens can be induced by antigenic mimicry of autoantigen peptides and peptides from foreign antigens, e.g. from pathogens like viruses or bacteria 8 that invade the organism and induce a defensive immune response 9.Others speculate that potentially self-reactive T cells are activated by infections agents, e.g. by superantigen stimulation, bystander effects of inflammatory cytokines, or antigenic mimicry of microbial and self antigens 10. However, the immune system does not only deal with pathogens, but also with nutritional antigens that are ingested in huge amounts and in a large variety. Soluble antigens that enter the body via mucosal surfaces are usually tolerated (mucosal: oral/nasal tolerance) 11, whereas application of nutritional antigens during gastrointestinal infections can break oral tolerance and induce a Th1-driven aggressive immune response 12.
Here we describe molecular mimicry of the ocular autoantigen peptide PDSAg, a peptide from rotavirus, and in addition a peptide from bovine αs2-casein, a common food antigen from bovine milk. All peptides and even the whole casein protein were uveitogenic in Lewis rats when injected s.c. with CFA.
Rotavirus infections are common causes of gastroenteritis; more than 90% of the population have had contact with rotavirus at least once in their lives. Repetitive infections can occur at any age, since immunity is strain-specific. Vp4, a highly immunogenic surface protein of rotavirus and the source of peptide Rota, is suspected to have an enterotoxin-like activity 6. Therefore it is potentially able to break oral tolerance by eliciting a Th1-like immune response. During gastrointestinal rotavirus infection an enhanced immune response to peptide Rota might be elicited, at the same time oral tolerance to food containing bovine casein can be abrogated, and the cross-reactivity of either Rota or Cas with retinal autoantigen peptide could initiate the eye disease.
Peptide Rota is derived from an Indian rotavirus isolate and also found in a bovine strain 13. With respect to the high incidence of rotavirus infections among the population a correlation with uveitis might be as difficult to prove as for multiple sclerosis and Epstein-Barr virus infections 14. Rotavirus infections are often clinically inapparent and thus not diagnosed. Correlation of uveitis with previous rotaviral infections by screening of uveitis patients would be of limited use due to the high incidence of infections and the impossibility to define a strain expressing the respective peptide by conventional serology. Although an outbreak of uveitis in Russian children after echovirus infection was described 15, there are no data in the literature about rotaviral infections and uveitis.
Not only peptide Cas, which represents the epitope that mimics retinal peptide PDSAg, but also the whole casein protein, is uveitogenic in rats and immunogenic in humans. Casein also stimulated Cas-peptide-specific rat T cell lines in vitro, in contrast to retinal S-Ag, which is not able to induce proliferation of PDSAg-specific T cell lines in vitro. Casein lacks a well-determined three-dimensional structure 16, which probably facilitates its proteolytic processing followed by presentation on MHC class II molecules.
PDSAg-specific rat T cell lines gained monospecificity during repeated restimulations in vitro; minor cross-reactivities could only be observed in very early stages, suggesting that PDSAg is a very strong T cell epitope with respect to MHC binding and TCR activation. The Rota-specific T cell lines proliferated to PDSAg to the same extent as to peptide Rota itself, and much less to Cas (about 10%), whereas Cas-specific lines showed a much weaker cross-reactivity (tenfold higher to Cas than to Rota and three times higher than to PDSAg). This suggests that there is not a single T cell receptor cross-reacting with all three peptides, but probably more than one, either recognizing Rota and PDSAg or another one cross-reacting between PDSAg and Cas. Both, Rota- and Cas-specific lines remained highly cross-reactive with PDSAg during propagation for several restimulation cycles in vitro, whereas the casein-specific T cell line only co-recognized the peptide Cas. These data are in accordance with findings from others 14, who demonstrated multiple cross-reactivities of single human myelin basic protein-specific T cell clones.
Furthermore, we see an "unfocused cross-reactivity", as proposed by D. Mason 17, which means that not all T cells show the same pattern of cross-reactivity. At very early stages of casein-specific T cell propagation a minor cross-reactivity to PDSAg or S-Ag was observed; however, those T cell lines were pathogenic as proven by adoptive transfer. This observation suggests the presence of only a minor subpopulation of pathogenic PDSAg-reactive cells in the casein-specific line.
Only tolerance induced by peptide PDSAg resulted in a significant reduction of PDSAg-induced uveitis, whereas peptides Rota and Cas and casein protein were not efficient in inducing oral tolerance. Attempts to break oral tolerance and to revert the immune response to a Th1 type by cofeeding of antigens with native CTX 7 revealed that only casein protein induced uveitisin almost all treated rats, but not S-Ag or any of the peptides, the latter being uveitogenic only when injected s.c.
The amount of casein (1 mg) that was uveitogenic by gavage is contained in 0.5 ml of bovine milk. Casein was less pathogenic by s.c. immunization than S-Ag; the striking difference in oral disease induction observed here was surprising. The rat chow did not contain any animal proteins, e.g. milk protein, excluding presensitization of animals. We speculate that casein as a major component of bovine milk, the first nutrition of a not yet fully immunocompetent newborn calf, could have immunostimulatory properties to enhance gastrointestinal defense mechanisms, in addition to the antibacterial effect of casoicidin, a casein-derived peptide 18. It even could have immunostimulatory properties to enhance gastrointestinal defense mechanisms, mediated by its function as a "cold shock protein" 16. According to our data, casein is rather inducing an adverse immune response than tolerance.
A milk-derived protein mimicking an autoantigen has already been described for experimental autoimmune encephalitis 19, namely butyrophilin, which contains an epitope cross-reactive with myelin oligodendrocyte glycoprotein (MOG). In contrast to butyrophilin, αs2-casein is not found in human milk, and the peptide sequence of Cas, the cross-reactive epitope, is highly specific for the bovine protein. Sensitization to casein is therefore only possible by ingestion of food containing bovine milk protein under conditions that abrogate oral tolerance, e.g.gastrointestinal infections triggering Th1-responses. This could happen at any age in our lives, not only during the postnatal period where oral tolerance is not yet fully developed and feeding antigens might rather prime the immune response than induce tolerance. Breaking oral tolerance to bovine casein does more often lead to allergy than to uveitis, for casein is a frequent food allergen 20, whereas uveitis only affects about 2‰ of the population of industrialized countries 21.
The gastrointestinal induction of an uveitogenic Th1 response is obviously a rare event, and there are certainly more additional factors necessary to induce uveitis, e.g. genetic predisposition. We could observe more frequent and stronger responses to retinal S-Ag, its peptide PDSAg and to casein/Cas as well as to the peptide from rotavirus with sera and peripheral lymphocytes of patients with anterior uveitis (iritis), furthermore, concomitant recognition of more than one of the antigens, compared to sera and cells from healthy donors or patients with posterior uveitis. Patients with posterior uveitis are more frequently under immunosuppressive therapy, which could explain reduced immune reactions; but there was no decrease in response to tetanus toxoid compared to patients with iritis or healthy donors. This phenomenon suggests that anterior and posterior uveitis may have different underlying immunopathogenic mechanisms. The term "posterior uveitis" describesmany different inflammatory entities and is therefore a less homogenous group of diseases than anterior uveitis.
T cell proliferation or ELISA could not differentiate between a defensive or a suppressive immune response; however, the significantly increased responses obtained from iritis patients and thehigh frequencies of combined reactivities to retinal autoantigen, casein and Rota point to an implication of these immune reactions. Not all patients had increased responses to retinal antigens andthe mimicry peptides, for the immune responses obtained with peripheral blood cells from uveitis patients do not always reflect the disease. Most of the patients with ongoing uveitis do not show retinal antigen-specific proliferation of peripheral lymphocytes 4, 22.
Milk proteins are suspected to be responsible for autoimmune diseases 19, 23, 24, and humans are the only species that consumes milk products from other species, even as adults. We speculate that breaking oral tolerance to ingested antigens which mimic autoantigens, e.g. by gastrointestinal infections promoting a Th1 response,could induce autoimmunity. However, this is in general difficult to detect due to the huge variety of nutritional antigens, especially in processed food from industrialized countries. This might also explain the increase of allergies and autoimmune diseases within our population. The fact that we defined two external cross-reactive peptides, one derived from a pathogenic virus, the other froma usually harmless food protein, points to the possibility that there might even be more cross-reactive antigens than these that could play a role in the induction of uveitis in humans.
4 Materials and methods
4.1 Antigens
Customary peptides were purchased from Biotrend (Cologne, Germany), bovine αs2-casein, ovalbumin and CTX from Sigma (Deisenhofen, Germany). Tetanus toxoid was a generous gift from Dr. Hungerer, Chiron (Marburg, Germany), and S-Ag was purified from bovine retinas as described 25.
The peptide sequences are: PDSAg: S-Ag aa 341–354, FLGELTSSEVATEV; Rota: human rotavirus MP409, outer capsid protein vp4, aa 591–601, WTEVSEVATEV (gi:4929212, Rao, C. D., Raman, S. and Jagannath, M. R.); Cas: bovine αS2-casein, aa 73–84, SEESAEVATEEV; PDSAgV4: S-Ag aa 344–354, ELTSSEVATEV; B27PD: HLA-B aa 125–138, ALNEDLSSWTAADT; B27V4: HLA-B aa 128–138, EDLSSWTAADT; B7PD: HLA-B7 aa 125–138, ALNEDLRSWTAADT.
4.2 Induction of uveitis
All animal experiments were approved by the Review Board of the Government of Oberbayern. Treatment of the animals conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Male and female Lewis rats were obtained from RCC (Basel, Switzerland), Janvier (Le Genest St. Isle, France) or bred in our own colony, and used for experiments at the age of 6–8 weeks. Uveitis was induced by s.c. immunization into both hind legs with a total volume of 200 μl containing 100 μg peptide (except PDSAg: 15 μg) or casein protein or ovalbumin, respectively, emulsified in CFA and supplemented to a final concentration of 2.5 mg/ml with Mycobacterium tuberculosis strain H37Ra (BD, Heidelberg, Germany), or by gavage [1 mg or 200 μg peptide or protein together with 10 μg native CTX twice, every 2 weeks or as a single gavage of 2 mg antigen with 15 μg CTX (Sigma)]. Eyes were collected for histology 2–3 weeks after s.c. immunization or 1 week after the second gavage, respectively. Uveitis was graded clinically and histologically as described elsewhere 26.
Uveitis was induced by adoptive transfer of rat T cells lines that were restimulated in vitro one to three times with their respective antigen. Three days after the last antigen stimulation, 3×106–7×106 cells were intraperitoneally injected into naive animals. Eyes were collected when clinical signs of disease had subsided, and uveitis was graded as mentioned above.
4.3 Rat T cell lines
Single-cell suspensions of draining lymph node cells from immunized rats were cultivated in triplicates as described 4. Antigens were used at a concentration of 10 μg/ml, the cultures were pulsed with [3H]thymidine after 2–3 days. The results are presented as SI ± SE. To determine the presenting MHC class II molecule, increasing amounts of antibodies to RT1.B (Ox 6) or RT1.D (Ox 17) (Serotec/Biozol, Eching, Germany) were added to the cultures. For blocking of the binding groove with competitor peptides, APC (thymocytes) were pre-incubated with various concentrations of competitor peptide for 2 h before adding the PDSAg-specific T cell line and PDSAg (here: 2 μg/ml). Proliferation of cultures stimulated with 2 μg PDSAg only was defined as 100%.
4.4 Oral tolerance induction
Rats were fed with 200 μg of peptides (PDSAg, Cas, Rota or control peptide B7PD) or 1 mg casein protein in PBS three to four times every other day. Two days after the last gavage the animals were immunized with 15 μg peptide PDSAg to induce uveitis. Statistics were performed using t-test.
4.5 Proliferation assays with human blood lymphocytes
The collection and use of human peripheral blood was approved by the local ethical committee. The informed consent from uveitis patients and healthy donors was obtained. Lymphocytes from 32 uveitis patients and 14 healthy donors were separated by Ficoll density gradient centrifugation and cultivated for 6 days in triplicates in round-bottom microtiter plates (Renner, Dannstadt, Germany) at a density of 5×105/ml as described 4. Peptide and protein antigens were used at concentrations of 10 μg/ml. After 5 days, 2 μCi [3H]thymidine/well was added and cells cultured for another 18 h before harvested. Proliferation is shown as SI, which was regarded as positive when it was equal to or exceeded the mean SI of healthy controls + 2 SD, as described elsewhere 27. Statistics were performed using Mann-Whitney test.
4.6 Peptide-specific ELISA
Immunoplates (Maxisorp, Nunc, Wiesbaden, Germany) were coated with 20 μg peptide or protein/ml 0,05 M carbonate buffer (pH 9,6) and blocked with 1% bovine serum albumin (BSA) in PBS. Humansera (from 98 uveitis patients and 36 healthy donors) were diluted 1:20 in PBS/BSA and 100 μl as duplicate wells incubated overnight. Binding was detected with biotinylated rabbit anti-human IgG and peroxidase-conjugated streptavidin (both from Dianova, Hamburg, Germany); tetramethylbenzidine was used as substrate. OD was read at 450 nm and regarded as positive response when it was higheras the mean OD of healthy controls + 2 SD 20. Statistics were performed using Mann-Whitney test.
Acknowledgements
We thank I. Rädler-Angeli for excellent technical assistance, S. R. Thurau for critically reviewing the manuscript, and highly appreciate the advice of C. Guzman for oral immunization. We thank A. Kampik for his support and H.-P. Scheuber for helpful cooperation. This work was supported by the Deutsche Forschungsgemeinschaft SFB 571, the Fördergesellschaft zur Behandlung von Autoimmunerkrankungen e.V. and a grant from the Münchener Medizinische Wochenschrift.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH





