Tissue-specific regulation of the human acute-phase serum amyloid A genes, SAA1 and SAA2, by glucocorticoids in hepatic and epithelial cells
Abstract
The human acute-phase protein serum amyloid A (A-SAA), encoded by the SAA1 and SAA2 genes, is dramatically induced by pro-inflammatory mediators during the acute-phase response to infection or injury. Circulating A-SAA is predominantly synthesized by the liver. However, other tissues are the source of locally produced A-SAA. Here, we establish that the qualitative and kinetic aspects of SAA1 and SAA2 transcription following treatment of HepG2 hepatoma cells and KB epithelial cells with glucocorticoids and cytokines are quite distinct. Untreated HepG2 cells do not express A-SAA mRNA and glucocorticoids, when administered alone, fail to induce either SAA1 or SAA2. In contrast, untreated KB cells constitutively express SAA1 mRNA. Following cytokine stimulation, both A-SAA genes are rapidly up-regulated to similar extents. As in the hepatoma cell line, co-stimulation of KB cells with glucocorticoids places SAA1 at a transcriptional advantage over SAA2. Interestingly, SAA1 can be significantly induced by glucocorticoids alone in KB cells. The effects of glucocorticoids on SAA1 in both cell lines is glucocorticoid receptor-dependent. Differential regulation of A-SAA expression in these cell lines may reflect different temporal and spatial requirements for A-SAA synthesis in response to different inflammatory challenges.
Abbreviations:
-
- SAA:
-
Serum amyloid A
-
- A-SAA:
-
Acute-phase SAA
-
- Dex:
-
Dexamethasone
-
- GR:
-
Glucocorticoid receptor
-
- GRE:
-
Glucocorticoid responsive element
-
- APR:
-
Acute-phase response
-
- APP:
-
Acute-phase protein
1 Introduction
Challenges such as infection and injury provoke a range of physiological changes collectively known as the acute-phase response (APR) 1. These changes affect vascular permeability, temperature regulation, clotting and the biosynthetic profile of the liver. The APR is initiated, coordinated and sustained by pro-inflammatory cytokines, in particular IL-1β, TNF-α and IL-6. In the absence of a continued challenge, homeostasis is restored by the combined actions of anti-inflammatory cytokines, cytokine antagonists and glucocorticoids (GC) 2.In addition to their role in suppressing inflammation, GC are potent inducers of gluconeogenesis. GC act either directly, via binding to glucocorticoid receptors (GR) and the subsequent engagement of glucocorticoid response elements (GRE) in the regulatory regions of genes, or indirectly, via interactions with other transcription factors 3.
Acute-phase serum amyloid A (A-SAA) is a major acute-phase protein (APP) which can be up-regulated by as much as 1,000-fold during the APR 4. Circulating A-SAA is primarily hepatic in origin; however, A-SAA is also synthesized in a wide range of other organs 5–7. In sections of histologically normal tissue, A-SAA mRNA and protein is predominantly localized to the epithelium 8. In tissue culture studies the synthetic GC dexamethasone has been shown to induce A-SAA transcription in KB epithelial cells and aortic smooth muscle cells, to enhance cytokine-driven A-SAA transcription in HepG2 and other hepatic cell lines, and to be required for cytokine-driven A-SAA transcription in THP-1 monocytic cells 6, 9, 10.
A-SAA is the precursor of AA, an insoluble degradation product deposited in major organs in secondary amyloidosis, a progressive and fatal disease that is an occasional consequence of chronic or episodic inflammatory conditions 11. A-SAA has also been found in the brain amyloid plaques of Alzheimer's disease 12 and in the foam cells, smooth muscle cells and endothelial cells of atherosclerotic lesions 5, indicating a possible role in the etiology of diverse clinical conditions in which inflammatory processes are involved.
The principal host defense function of A-SAA remains the subject of debate. However, A-SAA has been shown to displace ApoA1 as the predominant apolipoprotein in HDL3 particles 13 and may modify lipid transport to facilitate tissue repair; such a function may promote long term pro-atherogenic changes in chronically inflamed individuals. A-SAA has also been shown to haveimmune-related properties: it is chemotactic for monocytes and T cells 14, 15, induces the release of IL-8 from neutrophils 16, and, indirectly, promotes the killing of Candida albicans by neutrophils 17. A-SAA has recently been shown to form channels 18, 19, suggesting that it may act directly as an anti-microbial agent by depolarizing bacterial membranes.
A-SAA protein is the product of two highly homologous genes, SAA1 and SAA2, which are >90% identical in the regions encompassing their proximal promoters, coding sequences and mRNA untranslated regions 20. Both promoters contain binding sites for the transcription factors NF-κB and C/EBP which confer responsiveness to IL-1β, TNF-α and IL-6 in hepatic cells 21. These sites are identical in sequence and are analogously positioned in each gene. Using A-SAA promoter luciferase reporter constructs, we have previously shown that the cytokine-driven induction of SAA1, but not SAA2, in HepG2 hepatoma cells is enhanced by the addition of GC 22. However, GC alone have no effect on either promoter in this cell line 22. Here, we present data establishing that there are significant qualitative and kinetic differences in SAA1 and SAA2 gene expression between hepatic and epithelial cells following treatment with cytokines and GC.
2 Results
2.1 Transcriptional regulation of transfected SAA1 and SAA2 promoters by cytokines and glucocorticoids in an epithelial cell line
Numerous reports have documented the induction of A-SAA mRNA, accompanied by an increase in A-SAA protein synthesis, after treatment of various hepatic cell lines with cytokines. It has further been shown that the cytokine-driven induction of A-SAA mRNA in such cell lines can be enhanced by co-treatment with dexamethasone 9, 23, 24. However, the detection methods employed in the above studies were based on Northern blotting and the use of hybridization probes that cannot distinguish between the products of the SAA1 and SAA2 genes. Consequently, none of the published reports have assessed the magnitude or kinetics of induction of the individual SAA1 and SAA2 mRNA. In a recent study using an RT-PCR assay that could discriminate between the two A-SAA mRNA, we were able to establish that dexamethasone alone could not induce either SAA1 or SAA2 transcriptional activity in HepG2 cells, an observation which was in agreement with previous studies using Northern blotting. However, published reports that dexamethasone alone could induce A-SAA mRNA accumulation in KB epithelial cells 9 suggested that there is tissue specificity in the mechanisms governing A-SAA transcriptional regulation. We therefore characterized and compared the kinetics of A-SAA mRNA isoform induction in response to cytokines and GC in both KB and HepG2 cells.
KB epithelial cells were subjected to single and various combination treatments with 10 ng/ml IL-1β, 10 ng/ml IL-6 and 50 nM dexamethasone for 2, 4, 6, 8, 12 and 24 h following transfection with pGL3-SAA1pt (Fig. 1A) or pGL3-SAA2pt (Fig. 1B). The SAA1 and SAA2 promoters both responded moderately to IL-1β alone, and more modestly to IL-6 alone; for each promoter the addition of IL-1β plus IL-6 was synergistic. In KB cells, as is the case for HepG2 cells 22, IL-1β-induced and combination IL-1β/IL-6 readouts from the SAA1 promoter, but not the SAA2 promoter, could be greatly enhanced by dexamethasone at all time points. However, the two cell lines exhibited markedly different responses to dexamethasone alone. The SAA1 promoter could be up-regulated by dexamethasone alone in KB cells, a response which is not observed in the hepatoma cell line 22. Interestingly, the magnitude of the dexamethasone-mediated transcriptional readout from the SAA1 promoter in KB cells was even greater than that driven by IL-1β. The SAA2 promoter is not transcriptionally activated in response to dexamethasone alone. This result, together with the above observation that exogenous IL-6 can induce SAA2 transcription, indicates thatdexamethasone does not act merely by stimulating the production of biologically active amounts of IL-6, or other cytokines, in KB cells.
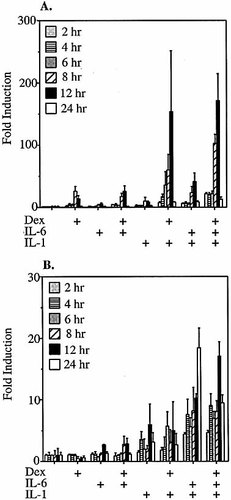
Time course of cytokine/dexamethasone induction of SAA1 and SAA2 promoter luciferase reporter constructs in KB epithelial cells. KB cells transfected with pGL3-SAA1pt (A) or pGL3-SAA2pt (B) luciferase reporter constructs were treated with medium only, dexamethasone (dex; 50 nM), IL-1β (10 ng/ml), IL-1β plus dex, IL-6 (10 ng/ml), IL-6 plus dex, IL-1β plus IL-6, or IL-1β plus IL-6 plus dex. Cells were harvested 2, 4, 6, 8, 12 and 24 h after treatment, and relative luciferase values were calculated and compared to those derived from untreated controls.
2.2 Comparison of the transcriptional regulation of the endogenous SAA1 and SAA2 genes by cytokines and glucocorticoids in cultured epithelial and hepatoma cells
The responses of the endogenous A-SAA genes, in HepG2 and KB cells, to treatment with cytokines and/or dexamethasone for 0, 1, 3, 6, 9, 12, 24 and 48 h were evaluated by direct analysis of extracted RNA. Proportional RT-PCR was performed using a single primer-pair that binds to common complementary regions of both SAA1 and SAA2 cDNA. The primers generate products from the SAA1 and SAA2 mRNA that differ in size by 26 nucleotides, thereby allowing the relative levels of SAA1 and SAA2-derived transcripts to be assessed. In HepG2 cells treated with IL-1β (Fig. 2A), the percentages of total A-SAA product derived from SAA1 mRNA ranged from 47% at the 6 h (at which the SAA1 and SAA2 products are first observed) to 36% and 34% at 24 h and 48 h, respectively (Fig. 2F). This indicates that SAA2 has a transcriptional advantage over SAA1 that becomes established at a ratio of approximately 2:1 by 24 h. In contrast, co-administration of dexamethasone to IL-1β–treated HepG2 cells (Fig. 2B) reverses the ratio of RT-PCR products, clearly favoring those derived from SAA1 mRNA. At 3 h, SAA1 mRNA constitutes >80% of the total mRNA, suggesting that dexamethasone, in the presence of IL-1β, confers a marked early transcriptional advantage to the SAA1 gene. This transcriptional advantage is maintained at a slightly lower level from 9–48 h, such that the ratio of SAA1:SAA2-derived products is approximately 2:1. Thus, in the context of cytokine induction dexamethasone effects a switch in the relative transcriptional activities of SAA1 and SAA2.
Low levels of SAA1-derived product could be detected in untreated KB cells (Fig. 2C–E), but not in untreated HepG2 cells (Fig. 2A, B). This observation in the former cell type is in accord with in situ hybridization studies by Urieli-shoval et al. 8, which documented the presence of A-SAA mRNA in normal (i.e. uninflamed) human epithelium. Taken together, the above findings suggest that the SAA1 gene has a low level of constitutive activity in epithelial cells. In KB cells treated with IL-1β alone (Fig. 2C), there is a clear induction of SAA1-derived product as early as 1 h post-stimulus. By 3 h, products derived from both SAA1 and SAA2 mRNA are evident. And through 48 h, the ratio is approximately equal (Fig. 2G), indicating equivalent transcriptional activity of SAA1 and SAA2. In contrast, when dexamethasone is also present (Fig. 2D), SAA1 has a 2:1 transcriptional advantage over SAA2 (Fig. 2G), i.e. the impact of dexamethasone in the context of IL-1β induction is similar to that in HepG2 cells.
No A-SAA-derived PCR product was detected in HepG2 cells treated with dexamethasone alone (data not shown), whereas in KB cells treated with dexamethasone alone only the SAA1-derived product was detected (Fig. 2E). These results are consistent with those generated using isoform-specific promoter reporter constructs and underscore the striking differences in the kinetics of cytokine and GC induction of SAA1 and SAA2 transcription between HepG2 and KB cells (Fig. 2F, G).
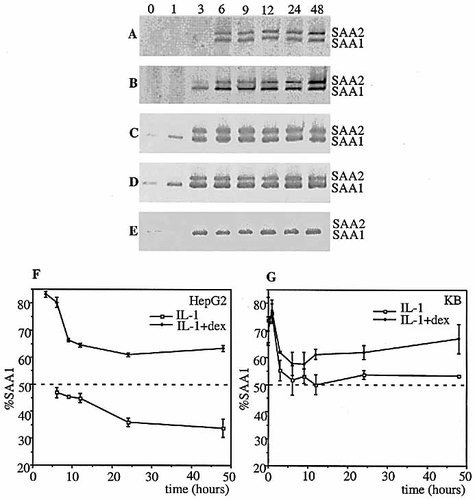
Time course of cytokine/dexamethasone induction of SAA1 and SAA2 mRNA in hepatic and epithelial cells. SAA RT-PCR of RNA from HepG2 cells treated with 10 ng/ml IL-1β (A), 10 ng/ml IL-1β plus 50nM dex (B), and from KB cells treated with IL-1β (C), IL-1β plus dex (D) or dex (E) for 0, 1, 3, 6, 9, 12, 24 and 48 h. Percentages of PCR products derived from SAA1 and SAA2 mRNA relative to total A-SAA mRNA in HepG2 cells (F) or KB cells (G) treated with IL-1β or IL-1β plus dex.
2.3 HepG2 and KB cells do not have biologically significant levels of constitutive or dexamethasone activated NF-κB
It has been reported that many cancer cell lines have constitutively active NF-κB (reviewed in 25). To confirm that the transcriptional responses of the SAA1 gene described above are determined by properties intrinsic to its promoter, rather than by a particular capacity (i.e. that is not shared by SAA2) to respond to constitutively active NF-κB, and/or dexamethasone-dependent (i.e. IL-1β–independent) NF-κB activation, we performed a series of experiments using the NF-κB inhibitor curcumin (diferuloylmethane). Curcumin has been shown to prevent the up-regulation of various NF-κB inducible genes via its inhibition of the pre-requisite activation of IKK 26. HepG2 and KB cells were transfected with the PathDetect® NF-κB reporter plasmid, pNF-κB-Luc (Stratagene), which has five NF-κB responsive sequences located in an artificial promoter upstream of luciferase. They were treated with medium only, curcumin (50 mM), IL-1β, or IL-1β plus curcumin for 4 h (Fig. 3A, B). The addition of curcumin alone did not reduce the low level of constitutive transcriptional readout in either cell line. However, in both HepG2 and KB cells, 50 mM curcumin was sufficient to completely abolish the IL-1β–driven component of transcriptional readout. These data indicate that any constitutively active NF-κB present in the cell lines under test is biologically insignificant with respect to the engagement of targets in promoters that have been optimized to respond to this transcription factor.
The above experiment did not eliminate the trivial explanation that the capacity of the SAA1 promoter to respond to dexamethasone may be due to GC being able to activate NF-κB via an undefined mechanism. KB cells were, therefore, transfected with pGL3-SAA1pt and treated with medium only, dexamethasone alone, or dexamethasone plus IL-1β in the presence or absence of curcumin (Fig. 3C). There was no significant difference in transcriptional readout from cells treated with medium only and with curcumin. As established above (Fig. 1A, 2E), the administration of dexamethasone alone causes transcriptional up-regulation of the SAA1 promoter. However, this was unaffected by the addition of curcumin, strongly suggesting that the mechanism of GC-mediated induction in KB cells is independent of NF-κB. The addition of dexamethasone plus IL-1β resulted in a marked and synergistic activation of the SAA1 promoter. This synergy was completely abolished by the co-administration of curcumin, which yielded a readout equivalent to that observed following treatment with dexamethasone alone. Thus, in contrast to the GC-driven component, the IL-1-driven component of the synergistic response appears to be entirely NF-κB-dependent.
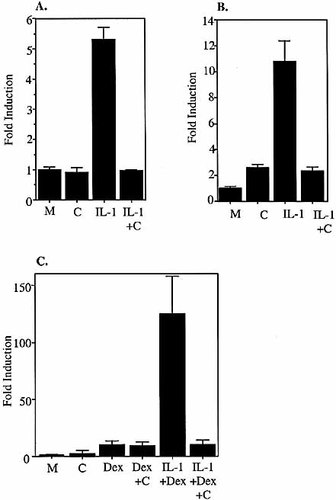
Inhibition of NF-kB. HepG2 cells (A) and KB cells (B) were transfected with NF-κB-Luc and treated with medium only, curcumin (50 mM), IL-1β (10 ng/ml), IL-1β plus curcumin. (C) KB cells were transfected with pGL3-SAA1pt and treated with medium only, curcumin (50 mM), dex (50 nM), dex plus curcumin, IL-1β (10 ng/ml) plus dex or IL-1β plus dex plus curcumin. Cells were harvested 4 h after treatment, and relative luciferase values were calculated and compared to untreated controls.
2.4 Onset of SAA1 and SAA2 promoter induction by cytokines and glucocorticoids in cultured epithelial cells
To study the kinetics of A-SAA induction at very early time points post-stimulus, KB cells were transfected with pGL3-SAA1pt (Fig. 4A) or pGL3-SAA2pt (Fig. 4B) and subjected to single and various combination treatments with cytokines and dexamethasone for 15, 30, 45, 60, 90 and 120 min. The induction of both promoters by IL-6 began to become apparent at 30 min and became significant at 60 min (p=0.02 for SAA1, p=0.01 for SAA2). In HepG2 cells, it has previously been shown that the SAA2 promoter is induced more rapidly following IL-6 treatment than following IL-1β treatment 25. In contrast to the later time points shown in Fig. 1, there was no significant induction of either promoter by IL-1β alone, and the transcriptional readout in response to the combined treatment of IL-1β plus IL-6 was not significantly different from that observed in response to treatment with IL-6 alone at these early time points. The increase in transcriptional readout from the SAA1 promoter in response to treatment with GC was evident as early as 60 min, and reached significance by 90 min (p=0.006).
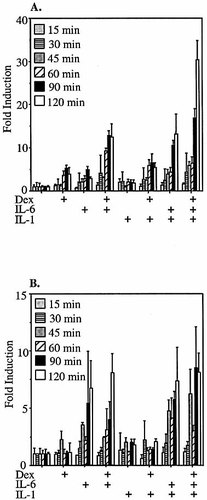
Early time course of cytokine/dexamethasone induction of SAA1 and SAA2 promoter luciferase reporter constructs in KB epithelial cells. KB cells transfected with pGL3-SAA1pt (A) or pGL3-SAA2pt (B) luciferase reporter constructs were treated with medium only, dex (50 nM), IL-1β (10 ng/ml), IL-1β plus dex, IL-6 (10 ng/ml), IL-6 plus dex, IL-1β plus IL-6, or IL-1β plus IL-6 plus dex. Cells were harvested 15, 30, 45, 60, 90 and 120 min after treatment, and relative luciferase values were calculated and compared to untreated controls.
2.5 SAA1 glucocorticoid responsiveness is dose-dependent
In KB cells transfected with the SAA1 promoter luciferase construct and treated with increasing amounts of dexamethasone (from 10 nM to 1 μM), there was a clear dose response even in the absence of cytokines (Fig. 5A). In contrast, in HepG2 cells transfected with pGL2-SAA1pt, even very high concentrations of dexamethasone alone failed to activate the SAA1 promoter (Fig. 5B). However, in the presence of 10 ng/ml IL-1β plus 10 ng/ml IL-6, the GC-mediated enhancement of transcriptional activity was clearly dose-dependent (Fig. 5B).
It has been reported that HepG2 cells have low endogenous levels of GR 26. To eliminate the possibility that the lack of SAA1 response to GC alone in HepG2 cells was due to insufficient GR, or to treatment with doses of dexamethasone that failed to reach a threshold needed to activate GR in this cell type, HepG2 cells were co-transfected with a GR expression construct 27 and pGL2-SAA1pt, and treated with the highest concentration (1 μM) of dexamethasone (Fig. 5C). Even this superphysiological dose failed to induce transcription in the absence of cytokines. However, in HepG2 cells transfected as above and treated with the same dexamethasone concentration in the presence of fixed concentrations of IL-1β plus IL-6, there was an enhanced response, indicating that the lack of SAA1 response to GC alone could not be attributed to inadequate levels of GR.
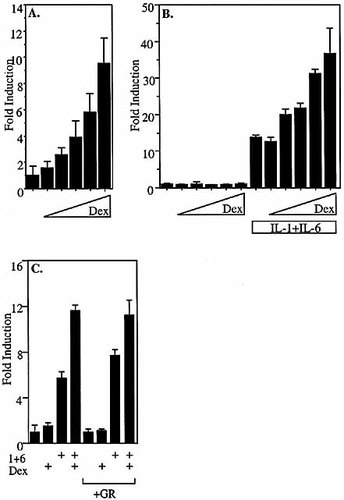
glucocorticoid responsiveness is dose-dependent. (A) KB cells transfected with pGL3-SAA1pt were treated with increasing amounts of dex (25 nM, 50 nM, 100 nM, 500 nM, 1 μM). Cells were harvested 6 h after treatment, and relative luciferase values were calculated and compared to those derived from untreated controls. (B) HepG2 cells transfected with pGL2-SAA1pt were treated with increasing amounts of dex (10 nM, 50 nM, 100 nM, 500 nM, 1 μM) in the presence or absence of 10 ng/ml IL-1β plus 10 ng/ml IL-6. (C) HepG2 cells co-transfected with pGL2-SAA1pt and CMX-GR or empty vector were treated with 1 μM dex in the presence or absence of 10 ng/ml IL-1β plus 10 ng/ml IL-6. Cells were harvested 4 h after treatment, and relative luciferase values were calculated and compared to those derived from untreated controls.
2.6 SAA1 glucocorticoid responsiveness is GR and GRE-dependent
To confirm that the GC responsiveness of the SAA1 promoter in KB cells occurs via direct interaction of GR with the previously identified GRE positioned –208 to –194 bp upstream of the transcription start site, experiments were carried out using the GR antagonist RU486 (mifepristone) and cells transfected with native and mutant promoter constructs. KB cells were transfected with the wild-type SAA1 promoter luciferase construct and treated with 50 nM dexamethasone and/or 100 nM RU486 for 6 h (Fig. 6A). RU486 has been shown to exhibit partial agonist activity that is dependent on which corepressors are present 28. There was a slight, but non-significant, increase in transcriptional readout in the presence of RU486 (p=0.2). Treatment with dexamethasone resulted in an approximately 10-fold increase in transcriptional activity, an effect that was blocked by the addition of RU486. This establishes that GC-mediated induction of the SAA1 promoter in KB cells occurs via the GR.
We have previously documented the sequence and position of the GRE in SAA1 that is solely responsible for the GC-mediated enhancement of cytokine-driven transcription in HepG2 cells. When key residues in this sequence are mutated, the in vitro binding of GR is greatly reduced, and the enhancement of cytokine induction of the SAA1 promoter in HepG2 cells is completely abolished (data not shown). KB cells were transfected with a SAA1 promoter luciferase construct (GREM) in which critical residues in the GRE had been mutated. This construct exhibits a level of transcriptional activity in response to IL-1β and IL-6 that is the same as that of the native SAA1 promoter (Fig. 6B). However, in IL-1β plus IL-6-treated KB cells, there is no dexamethasone-mediated enhancement of transcriptional activity; nor does the mutant promoter respond to dexamethasone alone. Taken together, these data indicate that the GRE at positions –208 to –194 in the SAA1 promoter is functionally active in epithelial cells and is an absolute requirement for SAA1 engagement by GC in all cell types in the presence or absence of concomitant cytokine stimulation.
Kumon et al. 29 recently proposed the involvement of a C/EBP site in the GC-mediated up-regulation of SAA1 in human aortic smooth muscle cells. Here, we have shown that the up-regulation of SAA1 by GC in epithelial cells occurs exclusively via a GRE between positions –208 and –194 bp, the site which is also responsible for GC enhancement of cytokine induction of SAA1 transcription in HepG2 cells. Furthermore, we have also established that a SAA1 promoter luciferase reporter construct with a mutated C/EBP site is subject to the same GC-mediated enhancement of cytokine induction in HepG2 cells as the native SAA1 promoter (data not shown). It is, therefore, possible that the decrease in GC responsiveness observed by Kumon et al. 29 following deletion of the region from –252 to –174 bp of the SAA1 promoter was, in fact, due to coincident deletion of the GRE together with the C/EBP site.
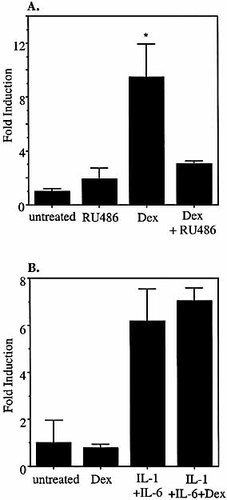
SAA1 glucocorticoid responsiveness is GR and GRE-dependent. (A) KB cells transfected with pGL3-SAA1pt were treated with 50 nM dex, 100 nM RU486 and 50 nM dex plus 100 nM RU486. (B) KB cells transfected with GREM were treated with 50 nM dex, 10 ng/ml IL-1β plus 10 ng/ml IL-6, or 10 ng/ml IL-1β plus 10 ng/ml IL-6 plus 50 nM dex. Cells were harvested 6 h after treatment, and relative luciferase values were calculated and compared to those derived from untreated controls.
3 Discussion
Historically, A-SAA was identified as an APP synthesized primarily by the liver, and consequently, most studies of the transcriptional regulation of the A-SAA genes have been carried out in cells of hepatic origin. In the past decade, however, A-SAA has been shown to be expressed by many different non-hepatic cell types including monocytes, adipocytes, synoviocytes, epithelial cells, endothelial cells and smooth muscle cells 5–7, 9, 10.
A variety of functions have been proposed for A-SAA, including several involving important aspects of immune function. For example, A-SAA induces chemotaxis of immune cells and the release of cytokines 14–16. A-SAA can form channels in membranes and may, therefore, have direct bactericidal properties 19, in addition to its ability to enhance neutrophil anti-microbial activity 17. Furthermore, the hepatic transcription of A-SAA in fish has been shown to be quantitatively correlated to pathogen load 30. Each of the above functions may be required locally or systemically according to the nature of the stimulus. We hypothesize that the timing, magnitude and site of A-SAA response is determined by both the extent and the location of pathogen challenge. Generally, low-level constitutive expression of A-SAA by epithelial cells, a cell type at the boundary between organism and environment, should be sufficient to protect against ongoing exposure to potential opportunistic pathogens. However, once an overt infection or injury occurs, the release of inflammatory mediators may provoke a rapid, local epithelial A-SAA response, as part of the process of neutralizing the challenge before it can involve other sites. Finally, if the pathogen load exceeds a threshold beyond which it can no longer be effectively countered by local processes, the consequent systemic production of cytokines and GC would mobilize a vigorous APR including the induction of hepatic A-SAA transcription to generate very high circulating concentrations of A-SAA. This last stage of defense likely has a significant short-term survival advantage that takes precedence over the possible long term pro-atherosclerotic and pro-amyloidigenic costs of A-SAA over-production. Nevertheless, biological mechanisms that preclude the aberrant activation of the hepatic A-SAA genes, thereby limiting adverse events attributable to elevated circulating concentrations of A-SAA, would clearly represent an effective evolutionary adaptation. The data reported here, broadly support the multi-step mobilization of A-SAA outlined above.
In liver cells, the lack of constitutive A-SAA expression and the inability of the SAA1 gene to respond to GC alone minimize the possibility of substantial hepatic A-SAA synthesis (leading to high circulating A-SAA concentrations) in the absence of an authentic inflammatory stimulus. This barrier to expression is desirable in an organ that is otherwise subject to phenotypic modulation by GC. Low blood glucose stimulates the release of GC that act on the liver to promote gluconeogenesis (the synthesis of glucose from amino acids and lipids). A-SAA expression under such conditions (i.e. in the absence of inflammation) would be subject to transcriptional repression that could only be overcome by cytokine stimulation, thereby permitting efficient cytokine-driven SAA1 and SAA2 transcription and also rendering the SAA1 GRE available for engagement by the GR to further enhance expression.
The differences in constitutive expression of SAA1 between cells of epithelial and hepatic origin is not due to differences in constitutively active NF-κB. Nor is the dexamethasone-driven induction of SAA1 in KB cells in the absence of exogenous cytokines attributable, even in part, to activation of NF-κB in addition to activation of GR. This suggests the possibility that additional, as yet unidentified, transcription factors may contribute to the regulation of SAA1 and may in particular participate in its constitutive expression in epithelialcells and its repression in hepatic cells. There is circumstantial evidence suggesting several potential mechanisms that may act singly or together in hepatoma cells, both to prevent the constitutive expression of SAA1 and SAA2 and to block induction of these genes in response to GC alone. The rat SAA1 promoter is transcriptionally repressed by YY-1, which in unstimulated hepatic cells is constitutively bound to its target site adjacent to the NF-κB element. YY-1 is displaced by the binding of activated NF-κB, thereby allowing the promoter to become transcriptionally active 31. The role of YY-1 in the regulation of human hepatic A-SAA expression has not yet been examined. However, there are potential YY-1 sites near to the NF-κB sites of the SAA1 and SAA2 genes. Another candidate repressor is the nuclear receptor PPARα that can act as both an activator and a repressor and regulates many genes involved in lipid regulation including that encoding the HDL-associated protein ApoAI. PPARα agonists up-regulate the expression of ApoAI in vivo, which increases circulating HDL 32. A-SAA displaces ApoAI from HDL under acute-phase conditions 13. The PPARα agonist fenofibrate has been shown in vivo to block the endotoxin-mediated up-regulation of A-SAA in mice 33. In addition, the PPARα agonist Wy14,643 can inhibit the IL-6 induction of A-SAA mRNA in HepG2 cells 34. Althoughthe action of PPARα with respect to the human A-SAA promoters in untreated hepatic cells has not yet been investigated, it is attractive to speculate that PPARα prevents hepatic A-SAA expression under non-acute-phase conditions.
The data presented in this paper establish that the A-SAA genes are subject to regulatory constraints that differ according to cell type. Future studies will determine the impact of such tissue-specific mechanisms on different host defense functions under a range of inflammatory conditions.
4 Materials and methods
4.1 Plasmids
The human A-SAA promoter luciferase reporter constructs pGL3-SAA1pt and pGL3-SAA2pt were generated by digestion of pGL2-SAA1pt and pGL2-SAA2pt 22 with Nco1 and Mlu1, and ligation of approximately 700 bp of upstream regulatory sequence and 5′UTR of the respective genes into pGL3 (Promega). The PathDetect® NF-κB reporter plasmid, pNF-κB-Luc, was purchased from Stratagene. A luciferase reporter construct containing the SAA1 promoter with a mutated GRE (GREM) was generated by site-directed mutagenesis using the primers 5′-CATCTTTTGGGCTCAGGTTGC-3′ and 5′-AGCCCAAAAGATGTGCCTGG-3′. The constitutive human Glucocorticoid Receptor-α expression plasmid, CMX-GR 27, was a gift of Dr Ron Evans, The Salk Institute, La Jolla, CA.
4.2 Cell culture and transient transfection
Human HepG2 hepatoma cells and KB epithelial cells (ATCC) were cultured in DMEM containing 10% FCS, gentamycin, sodium pyruvate and non-essential amino acids (Gibco BRL). Cells were seeded into 24-well plates, 24 h prior to transfection using FuGENE® (Roche Biomolecular) as previously described 35. Cells transfected with A-SAA promoter luciferase reporter constructs together with renilla control plasmid, were incubated for 16–20 h before replacement of culture medium with fresh medium alone or fresh medium containing 10 ng/ml cytokine(s) and/or 50 nM dexamethasone and/or 100 nM RU486 (Mifepristone). IL-1β was purchased from Peprotech. IL-6 was obtained from Astra Zeneca. Dexamethasone, curcumin and RU486 were from Sigma.
4.3 Luciferase assays
Cells were harvested at various times post-treatment, washed in PBS and resuspended in Passive Lysis Buffer (Promega). Lysates were assayed for luciferase and renilla activity using the LLR and Stop and Glo reagents (Promega) in a dual injection luminometer (Turner Designs). Each treatment was carried out in triplicate and the mean ratio of luciferase to renilla activity and standard deviations were calculated. The ratios are expressed relative to untreated controls and are representative of three independent experiments.
4.4 RT-PCR
Total RNA was prepared using RNeasy columns (Qiagen) from HepG2 and KB cells treated for up to 72 h under various experimental conditions. A-SAA RT-PCR was as described previously 22. PCR samples were run on 9% polyacrylamide minigels, stained with ethidium bromide and visualized using a UVP imaging system and Labworks 4.0 software.
Acknowledgements
This work was supported by a research grant from Marligen Biosciences Inc.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




