B cell activation leads to shedding of complement receptor type II (CR2/CD21)
Abstract
Complement receptor type II (CR2/CD21) is the major receptor for C3d fragments on immune complexes. CD21 also serves as the receptor for Epstein–Barr virus in humans. On mature B cells, CD21 reduces the threshold of BCR signaling together with CD81, Leu13 and CD19, but it also occurs on other cells of the immune system where it performs unknown functions. A soluble form of CD21 (sCD21) is shed from the cell surface and is found in human blood plasma. An as-yet-unknown protease is thought to be responsible for this shedding. Altered levels of sCD21 occur in plasma in certain clinical conditions. We show here by mass spectrometry that sCD21 in human plasma of healthy donors is predominantly a short form of CD21 without the exon-11-encoded sequences. Whereas the N terminus of sCD21 was found unmodified, the C terminus is truncated, implying that only the extracellular portion of CD21 is shed. Peripheral blood B cells, but not T cells, contribute to the plasma CD21-pool. CD21 shedding is induced by stimulation with PMA plus Ca2+ ionophore, or by stimulation of the BCR with anti-IgM+anti-CD40.
Abbreviations:
-
- CaI:
-
Ca2+ ionophore
-
- sCD21:
-
Soluble CD21
1 Introduction
Many proteins are shed from the cell surface by endoproteolytic activities associated with the plasma membrane. The shed portions of surface molecules are called ectodomains. Usually the ectodomains are not ligand-bound but they retain their ability to bind ligand 1. Ectodomain shedding reduces the concentration of cell surface receptors 2. However,soluble ectodomains compete with cell surface receptors for their ligands 3. In addition, the remaining transmembrane/cytoplasmic portion of the molecule may have signaling functions different from those of the full-length molecule because the remaining portion is not controlled by ligand 4. Since the majority of cell surface proteins are resistant to proteolytic release, shedding has to be a highly regulated process, catalyzed by specialized proteases 5. Plasma soluble-receptor concentrations are increased in many disease conditions and are used for therapeutic purposes, e.g. soluble IL-2R, sCD23, sCD27, sCD30, sTNFR, and sIL-6R 6.
The main physiological function of complement receptor II (CR2/CD21) is binding of C3d fragments on immune complexes. It is also known to bind to Epstein–Barr virus (EBV) in humans. CD21 is expressed on mature B cells, T cells, and a number of other cell types 7, 8. Functionally, CD21 participates in the development of normal immune response by severalmechanisms. Ligation of CD21 results in signals critical for normal B cell responses 9–12. While CD21 on B cells and follicular DC is implicated in the recognition and binding of immune complexes, its function in T cells and all other cell types is not known. In human pro- and pre-B cells, the CD21 gene is repressed, by methylation of a CpG island in its promoter, and expression in mature B cells is accompanied by the loss of CpG-methylation 13, 14.
CD21 is shed from the cell surface and appears circulating in plasma. Clinically, altered levels of soluble CD21 (sCD21) in plasma have been correlated with B cell chronic lymphocytic leukemia, common variable immunodeficiency, Brutons's X-lined agammaglobulinemia 15, 16 and EBV-associated malignancies 17. Soluble CD21 activatesmonocytes through binding to membrane CD23. As sCD21 could potentially bind to its ligands in plasma 18, 19, the amount of sCD21 in serum could be a modulator ofimmunity. An unknown protease is thought to be responsible for shedding of CD21 from the membrane. We have previously shown a reduction of surface CD21 in synovial lymphocytes (as compared with peripheral blood lymphocytes) as well as in activated T cells 20, 21. In addition we recently reported the isolation and characterization of sCD21 from human plasma 22. CD21 occurs in two splice variants dependent on the presence of an exon-11-encoded peptide 23–25. It is not known whether both isoforms are shed from the cell surface.
In this work, we characterized sCD21 by mass spectrometry, investigated the source of sCD21 in human plasma and studied the mechanism of CD21 shedding from the lymphocyte surface. PMA plus Ca2+ ionophore (CaI) synergistically induced shedding of CD21 from peripheral blood lymphocytes and Raji B cells. Similarly, cross-linking BCR and CD40 in B cells induced shedding of CD21 paralleled by a decrease in membrane-associated CD21.
2 Results
2.1 Mass-spectrometric protein identification and structure characterization
Purified sCD21 from human plasma was visualized with colloidal Coomassie stain after SDS-PAGE separation. After in gel tryptic digestion, mass-spectrometric analyses were performed. The high-quality mass spectra were subjected to a database search against the Swiss-Prot sequence database and this identified the protein as CD21 (Swiss-Prot entry: P20023) with sufficient confidence (probability-based score: 137) and a sequence coverage of 33%. Close inspection of the mass spectra showed that the most N-terminal peptide was detected with an ion signal at m/z 1367.71 (Table 1). This result shows that the first 20 amino-acid residues of the sequence deposited in the database form the signal peptide that is removed upon membrane-passage of the translation product.
Inspection of the CD21 sequence covered by mass-spectroscopy showed that only tryptic peptides in which no glycosylation sites are located were detected. Hence, one is tempted to speculate that most if not all glycosylation sites are in fact glycosylated in sCD21. Glycosylated peptides in many cases form ion signals with low abundance and thus may not be detectable in the mass fingerprints.
Furthermore, the recorded mass spectra show ion signals at m/z 2584.18 (A646–R666) and at m/z 2872.42 (C644–R666) that are attributed to the presence of the "short isoform" of CD21 lacking exon 11 (Fig. 1). From these results it seems likely that the majority of the present protein belongs to the "short form" of CD21. However, the presence of the "long isoform" of CD21 cannot be ruled out completely. The most C-terminal peptide that was detected included amino acids G768–R781 with m/z 1525.70 (Table 1). This result indicates that the soluble form of CD21 consists of the extracellular portion of the protein.
It should be noted that the peptide ion signal at m/z 1639.89 can be assigned to two peptides, one covering amino acids 34–48, and one consisting of amino acids 1001–1013 (Table 1). However, as no other peptide ion signals covering peptides in the vicinity of the partial sequence 1001–1013 are detected, the assignment of the mass value of m/z 1639.89 to this peptide seems less likely. By contrast, peptide ion signals to both flanking partial sequences of the peptide with amino acids 34–48 were detected in the mass spectra.
|
Sequence range (aa position)a) |
Peptide masses |
|
|---|---|---|
|
|
[M+H]+(calculated)f) |
m/z(observed)g) |
|
21 – 33 |
1367.71 |
1367.70 |
|
34 – 48b) |
1639.91 |
1639.89 |
|
49 – 56 |
977.41 |
977.48 |
|
88 – 103 |
1822.92 |
1822.89 |
|
104 – 119 |
1782.82 |
1782.81 |
|
129 – 142 |
1706.75 |
1706.73 |
|
203 – 214 |
1402.64 |
1402.65 |
|
217 – 225c) |
975.54 |
975.49 |
|
234 – 247 |
1634.73 |
1634.71 |
|
234 – 255 |
2457.16 |
2457.12 |
|
268 – 286 |
2213.06 |
2213.03 |
|
332 – 341 |
1029.51 |
1029.48 |
|
342 – 358 |
1995.97 |
1995.95 |
|
409 – 424 |
1868.89 |
1868.88 |
|
477 – 500 |
2788.38 |
2788.37 |
|
501 – 520 |
2401.13 |
2401.03 |
|
563 – 575 |
1407.75 |
1407.74 |
|
595 – 612 |
2010.06 |
2009.96 |
|
630 – 637d) |
975.46 |
975.49 |
|
638 – 659 |
2590.20 |
2590.15 |
|
644 – 666 |
2872.28 |
2872.42 |
|
646 – 666 |
2584.16 |
2584.18 |
|
660 – 677 |
2096.03 |
2095.96 |
|
667 – 677 |
1185.62 |
1185.60 |
|
716 – 729 |
1566.86 |
1566.81 |
|
764 – 781 |
1942.88 |
1942.86 |
|
768 – 781 |
1525.70 |
1525.70 |
|
1001 – 1013e) |
1639.79 |
1639.89 |
- a) Amino acid positions refer to sequence entry P20023 from the Swiss-Prot database.
- b–e) Ion signals matched two peptide masses.
- f) [M+H]+(calculated): calculated protonated molecular mass of peptide.
- g) m/z(observed): experimentally determined mass to charge value (z=1 in all table entries).
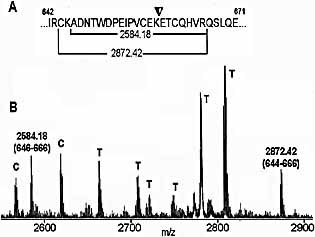
Mass-spectrometric structure characterization of sCD21 obtained from human plasma. (A) Amino acid sequence (single-letter code; amino acid positions refer to sequence entry P20023 from the Swiss-Prot database) of the region in CD21 (I642–E671) in which "long form" and "short form" are different. The junction of exon 9/10- and 12a-derived sequences is indicated by an arrow on top of the sequence. The masses for the tryptic peptides that show the presence of peptides from the "short form" are given in the bracketed sequence stretches. (B) MALDI-TOF mass-spectrometric fingerprint (zoom) obtained after in gel tryptic digestion of the SDS-PAGE-separated protein. Numbers in the mass spectrum give precise m/z values for the detected peptide ion signals and corresponding amino-acid positions are indicated in parentheses. Autoproteolysis products from trypsin are indicated with T. Ion signals labeled with C correspond to contaminants. α-Cyano-4-hydroxy cinnamic acid was used as a matrix.
2.2 Peripheral blood B lymphocytes contribute to the plasma sCD21 pool
Soluble CD21 occurs at about 300 ng/ml in the blood of healthy persons and levels change in pathological conditions. However, it is not clear which class of immune cells shed CD21. To investigate the source of sCD21 in plasma, peripheral blood lymphocytes were isolated from two healthy individuals; B and T lymphocytes were sorted and taken into culture. Cell supernatants were collected after 36 h of incubation and sCD21-concentrations estimated by ELISA (Fig. 2). Only B cells were found to shed CD21. T cells shed very little or no detectable amounts of CD21 under these culture conditions. Thus although T cells outnumber B cells in the peripheral blood and express CD21 they do not appear to contribute to the sCD21 serum pool.
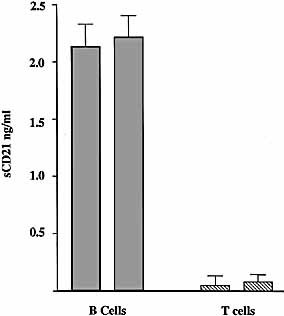
Peripheral blood B lymphocytes, but not T lymphocytes, shed CD21. Peripheral blood lymphocytes were isolated from fresh human blood from two healthy volunteers. Sorted B and T cells (107 cells/ml) were cultured in serum-free medium for 36 h and the concentration of sCD21 in the supernatant was estimated by ELISA. B cells shed about 2–3 ng sCD21 whereas T cells shed very little, or undetectable amounts, under the culture conditions.
2.3 PMA activation of B lymphocytes leads to CD21 shedding
To dissect the mechanism of CD21 shedding we were interested to see if CD21 shedding is associated with lymphocyte activation. Peripheral blood lymphocytes from three healthy individuals were activated with the phorbol ester PMA and CaI for 5 h. Peripheral blood lymphocytes from all the three donors that were tested shed higher amounts of sCD21 when activated with PMA. Therefore, it is tempting to speculate that PMA+CaI activation directly acts on the activity of the protease.
Raji B cells were found to shed at least 10–50-times more CD21 than peripheral blood B cells. Raji B cells also shed more CD21 when activated with PMA+CaI for 5 h (Fig. 3A). The shedding was more pronounced during activation in the presence of PMA+CaI than with PMA or ionophore alone, indicating a synergistic effect of the ionophore on PMA action. A parallel decrease of membrane-associated CD21 was found by cytometric analyses (Fig. 3C).
As PMA-stimulation is pan-specific, we were interested in identifying specific signal transduction mechanism that leads to regulated shedding of CD21. Since CD21 may participate in particular in T cell dependent responses, as suggested by the analysis of CD21-deficient mice 26, 27, we investigated shedding under such conditions. Cross-linking Raji B cells with anti-IgM+anti-CD40 resulted in a similar phenomenon (Fig. 3B and D), suggesting a role of anti-IgM+anti-CD40 stimulation and signal transduction in CD21 shedding.
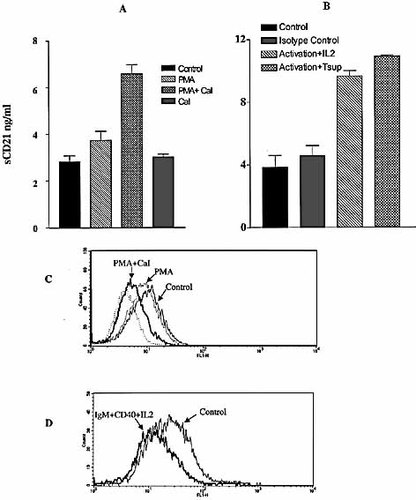
B cell activation induces CD21 shedding. (A) Raji B cells (106/ml) were activated for 5 h by PMA (10 μM) with or without CaI (1 μM), and sCD21 concentrations were estimated in the tissue culture supernatant by ELISA. (B) Soluble CD21 concentrations in supernatants of Raji B cells cross-linked with anti-IgM+anti-CD40 and also given IL-2 (20 U/ml). (C and D) Cytometric analysis of membrane-associated CD21 in Raji B cells activated with PMA+CaI and IgM+CD40+IL-2, respectively.
3 Discussion
In gel enzymatic digestion 28 results in mixtures of peptides that carry structural information from the parent protein. Mass spectrometry in combination with proteolysis is well suited for the study of structural details of proteins 29–31. Here we show that at least the short splice variant of sCD21, without exon 11, circulates in plasma and that the N-terminal amino acid of the molecule is non-modified I21, as anticipated for the processed CD21 lacking the leader peptide. The estimated theoretical half-life of sCD21 according to the N-end rule 32 is 20 h, which is classified as unstable. The transmembrane region is predicted to start from S972.
According to our results, it is likely that the C terminus of the CD21 protein under study is located somewhere between R781 and S972. Peptides of this region may result in ion signals that were not observed, perhaps due to suppression effects. Alternatively, due to the unknown C-terminal cleavage site and, thus, the unknown length of the C-terminal peptide, this C-terminal peptide has not yet been assigned to one of the unidentified ion signals in the spectra. It should be mentioned that mass spectra of peptide mixtures obtained after in gel digestion of complete Raji-CD21 showed peptide ion signals for both the extracellular parts and the intracellular parts of the protein (Glocker and Illges, unpublished observation).
We report on the biochemical characterization of sCD21 and show that shedding is inducible by mitogen (PMA+CaI) or anti-IgM+anti-CD40 stimulation. Among peripheral blood lymphocytes, B cells shed more sCD21 in vitro than T cells. B cells shed about 3 ng/ml in 36 h ex vivo whereas sCD21 in plasma averages to about 150–300 ng/ml. Assuming the cell culture conditions did not change the ability of the lymphocytes to shed CD21 by one or two orders of magnitude, we conclude that circulating B cells and T cells play a minor role in the generation of the sCD21 pool in the blood. However, it might well be that B and/or T cells in lymphoid organs are promoted by the environment to shed the larger amounts to account for the serum pool. Considering the reduced stability of the protein and the low shedding from peripheral blood B cells, circulating B cells may not be the only source of sCD21.
CD21 expression is lost during differentiation of B cells into B-blastoid cells. If the function of CD21 during this process would only require transcriptional silencing, shedding would be obsolete. On the contrary, B cells actively shed CD21 from their surfaces instead of waiting until the CD21 protein's half-life expires after shut-off of transcription 33. This suggests a role of CD21-shedding/sCD21 in initial steps of B cell activation/interaction during an immune response.
PMA activation of Raji B cells induces a series of signal transduction events through protein kinase C (PKC) 34. The activation is augmented by addition of CaI, which makes calcium ions available for the activity of PKC by releasing the intracellular store of Ca2+ from the endoplasmic reticulum. Taking into consideration that PMA activation also mimics signal transduction through the BCR, we rightly suspected and proved that shedding of CD21 can indeed be due to anti-IgM+anti-CD40 activation. Soluble CD21 represents a fully functional part of the molecule without the transmembrane and intracellular portions and potentially could bind to all of its known ligands. The most likely ligands of sCD21 in a local environment, such as a lymph node and/or germinal centers, are iC3b, C3d and CD23. On the basis of the results shown above, it is likely that shedding of CD21 leads to a local accumulation of the sCD21 glycoprotein. Any local increase in sCD21 concentration could competitively inhibit the binding of CD21ligands that activate cells e.g. via the CD23 receptor and could very well contribute to fine regulation of CD21-dependent responses and B cell activation.
Soluble CD21 might therefore be a candidate for therapeutic purposes. Studies in model organisms, such as mice, have already revealed the importance of CD21 in tolerance mechanisms 35 and the germinal center reaction but a function of sCD21 remains less clear. The availability of CD21-deficient mice might be a useful tool to introduce transgenic CD21 to explore the possible functions discussed above.
4 Materials and methods
4.1 Cells, antibodies and reagents
The mature human B cell line, Raji (ATCC, Manassas, USA) was maintained in Iscove's DMEM (Invitrogen, Karlsruhe, Germany) supplemented with 10% FCS (Linaris, Bettingen, Germany), 1000 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37°C in 7.5% CO2. The cells were transferred to serum-free medium (Invitrogen) for 24 h prior to shedding-experiments. Monoclonal anti-CD21 antibody clones BU32 (IgG1) 36 and THB5 (ATCC) were grown in serum-free hybridoma medium (Invitrogen) at 37°C in 7.5% CO2. Peripheral blood B and T cells wereisolated from fresh blood collected from healthy volunteers and cultured in serum-free medium.
4.2 Lymphocyte isolation, sorting and activation
Lymphocytes were purified by ficoll density-gradient centrifugation of fresh human blood. B and T cells were enriched by magnetic sorting as previously described 22. The relative purity of the cells was determined by cytometric analysis using anti-CD4–PE/anti-CD8–FITC as markers for T cells, and anti-CD19–PE/anti-CD21–FITC as markers for B cells. Cells were stained in microtiter plates and loaded into a FACScan (Becton Dickinson, Heidelberg, Germany) using a self-constructed loader as previously described 37. B and T cells (1×107 per ml) were cultured in Iscove's DMEM in tissue culture wells. For Raji B lymphocyte activation, cells were either incubated with PMA+CaI or activated by cross-linking IgM and CD40 in the presence of IL-2 (20 U/ml).
4.3 Purification of sCD21
Soluble CD21 from pooled human plasma was purified to homogeneity by affinity chromatography and density-gradient centrifugation as described before 22 and the purity was checked by 4–12% gradient SDS-PAGE (Invitrogen) and subsequent Western blotting.
4.4 Mass-spectrometric protein characterization
Gel pieces from SDS-PAGE containing sCD21 were excised and subjected to in gel digestion using an Investigator ProGest system (Genomic Solutions Inc., Ann Arbor, MI, USA). Proteins were reduced, alkylated, and digested with trypsin as previously described 28, except that 15 μl of 25 mM NH4HCO3 containing 72 ng of modified sequencing gradetrypsin (Promega, Madison, WI, USA) was used for digestion. After extraction of peptides from the gel, the samples were dried in a vacuum centrifuge. The peptides were resuspended in 5 μl of 60% acetonitrile / 0.1% TFA, and 0.5 μl of this peptide mixture was spotted simultaneously with 0.5 λ matrix (a saturated solution of α-cyano-4-hydroxy cinnamic acid in 35% acetonitrile / 0.1% TFA) on a MALDI target plate. Peptides were analyzed by MALDI-TOF MS using a Reflex III mass spectrometer (Bruker Daltonik, Bremen, Germany), equipped with the SCOUT source and delayed extraction, and operated in positive ion reflector mode. The spectra were first calibrated externally 28 and then internally recalibrated using three peptides arising from trypsin autoproteolysis([M+H]+ 842.50, [M+H]+ 2211.10, and [M+H]+ 3323.77). Tryptic, monoisotopic peptide masses were searched against the MSDB database using the Mascot software 1.8 (Matrix Science, Oxford, GB) setting a mass tolerance of 50 ppm. Spectra were analyzed using the BioTools software, version 2.0 (Bruker Daltonik, Bremen, Germany).
4.5 Quantification of sCD21 by ELISA
A sandwich ELISA was developed to quantitate sCD21 in tissue culture supernatants. The monoclonal antibodies THB5 and biotinylated BU32 were used as capture and revealing antibody, respectively. Titration was performed with purified sCD21 22 and a standard curve was plotted. Briefly, THB5 was coated onto an ELISA plate (TPP, Trassadingen, Switzerland) at a concentration of 5 μg/ml in coating buffer (0.1 M Na2HPO4 / NaH2PO4, pH 9.0) for 12–15 h at 4°C. After two washes with PBS containing 0.1% Tween-20 (PBST), the plate was blocked with 1% milk powder in PBS for 2 h at room temperature. The samples at appropriate dilutions in triplicates were added to the plates along with the standard. The plates were incubated at 4°Cfor 12–15 h, washed twice with PBST and then incubated for 2 h with BU32–biotin at room temperature. After two washes, an appropriate dilution of streptavidin coupled to horseradish peroxidase was added, the plates incubated for 1 h at room temperature, washed twice and H2O2/o-phenylenediamine was added as substrate/coloring agent. Enzymatic reactions were quantified measuring optical density at 492 nm in an ELISA reader (Anthos Microsystems, Krefeld, Germany) and sCD21 concentrations were calculated by extrapolating from the standard graph.
Acknowledgements
We would like to thank Drs Rolf Knippers and Annette Aichem for critical reading of the manuscript and Markus Ulbrich, University of Rostock, for excellent technical support. This work was supported by the Thurgauische Stiftung für Wissenschaft und Forschung, the Ministry of Science and Culture of the state of Baden-Württemberg, the Hans-Hench-Stiftungand the Bundesamt für Bildung und Wissenschaft (BBW), Bern, Switzerland through grants QLG1-CT-2001–01536 and QLG1-CT-2001–01407 to HI.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




