CD55/decay accelerating factor is part of the lipopolysaccharide-induced receptor complex
Abstract
Recently, we described an 80-kDa lipopolysaccharide (LPS)-binding membrane protein to be identical to CD55 [decay accelerating factor (DAF)]. Here, we demonstrate that CD55 is able to contribute to lipopolysaccharide (LPS) signaling. Transfection of Chinese hamster ovary (CHO) cells with human CD55 resulted in a translocation of NF-κB after stimulation with LPS as well as with free lipid A. In addition, interaction of lipid A and CD55 was shown by co-immuno-precipitation of these molecules from CHO-CD55 cells after incubation with lipid A and anti-lipid A monoclonal antibody, as well as by fluorescence resonance energy transfer (FRET) analysis in human monocytes. The comparison of LPS-induced signaling pathways in CHO-CD55 and CHO-CD14 cells revealed that p38, JNK and ERK MAP kinases are activated upon LPS stimulation in both cell lines, and that the activation by LPS can be blocked at the level of Toll-like receptor 4. Finally, through FRET analysis we could demonstrate LPS-induced clustering of CD55 and CD11/CD18 in human monocytes. Our results imply a new functional role of CD55 as a member of a multimeric LPS receptor complex.
Abbreviations:
-
- DAF:
-
Decay accelerating factor
-
- LMP80:
-
80-kDa LPS-binding membrane protein
-
- TLR:
-
Toll-like receptors
-
- FRET:
-
Fluorescence resonance energy transfer
1 Introduction
Lipopolysaccharide, the outer membrane component of Gram-negative bacteria, is a potent stimulator of the innate immune response in mammals 1. The initial step of endotoxin activation of monocytes/macrophages is the binding of LPS to specific cell surface receptors. The main binding receptor for LPS on myeloid cells is the GPI-linked membrane glycoprotein CD14 (mCD14) 2–4. Despite the importance of CD14, the lack of an intracellular domain of the mCD14 molecule clearly implied the existence of a transmembrane signaling moleculetransmitting signals into the cell 5–7.
Recently, members of the human Toll-like receptor (TLR) family 8 were identified as signaling receptors for many pathogen-associated molecular patterns (PAMP) 9–11. Through the discovery of the genetic basis for the LPS hyporesponsiveness in the C3H/HeJ mouse, the essential signaling receptor for LPS has been identified as TLR4 12. These results were confirmed by the generation of a TLR4-knockout mouse showing the same LPS hyporesponsiveness as C3H/HeJ mice 13, as well as by transfection ofthe human TLR4 cDNA into LPS-unresponsive HEK293 cells 14.
Since signaling through TLR4 activates the central immune transcription factor NF-κB 9, its importance during the cellular activation by LPS is unequivocal. However, recent data suggest a more complex scenario when LPS binds and activates cells: Pfeiffer et al. 15 showed that LPS induces a co-clustering of multiple receptors including CD14 and TLR4 on human monocytes. In contrast, Triantafilou et al. 16 identified four molecules, namely hsp70, hsp90, CXCR4 as well as GDF-5, to be involved in the activation of monocytes by LPS in a CD14-independent manner. Thus, we hypothesize that activation of cells by LPS leads to the formation of an LPS-specific receptor cluster that is responsible for the entire biological response towards LPS.
We have recently identified an 80-kDa LPS-binding membrane protein (LMP80) on human cells 17, which interacts with LPS in a LPS-binding protein and sCD14-dependent manner 18. In addition, co-immuno-precipitation analysis revealed an interaction of LMP80 and LPS within the intact cell membrane. We characterized and identified this protein as human CD55 [decay accelerating factor (DAF)] 19. CD55 is a complement regulatory molecule that causes an accelerated decay of the C3- and C5-convertases. It is expressed on nearly all cells, coming into contact with blood stream complement components 20, 21, including monocytes and endothelial cells.
To characterize the potential role of CD55 in LPS-induced signaling events, we transfected CHO cells with human CD55. Here, we show that CD55 transfection imparts LPS responsiveness to CHO cells, which do not express LMP80 19, and which are known to be nearly LPS unresponsive 22. In addition, we show co-clustering of CD55 with other LPS-binding and signaling receptors, indicating a new function for the CD55 molecule in the context of cellular stimulation by LPS. We hypothesize that CD55 is part of a multimeric LPS receptor complex on mammalian cells.
2 Results
2.1 LPS induces translocation of NF-κB in CD55-transfected CHO cells
To evaluate a functional role for CD55 in LPS-induced cell activation, we analyzed the ability of CD55-transfected CHO cells to translocate NF-κB in response to stimulation with LPS (Fig. 1A). Control stimulations were done with CHO cells transfected with the empty expression vector lacking CD55-cDNA. The stimulation of CHO-CD55 cells with LPS resulted in a marked increase of NF-κB translocation, starting at an LPS concentration of 10 ng/ml. This activation of CHO-CD55 cells was observed in all of three established CD55-transfected CHO cell clones as well as in the batch cell line (data not shown). In addition, activation of both cell lines by LPS leads to phosphorylation of IκB-α (Fig. 1C). All CHO-CD55 cells were less sensitive to LPS than CHO-CD14 cells (Fig. 1A, C; 3 and 4D). In contrast to CHO-CD55 and CHO-CD14 cells, control transfectants did not show any NF-κB-response. However, all cell lines showed the same reactivity for murine TNF-α (Fig. 1B).
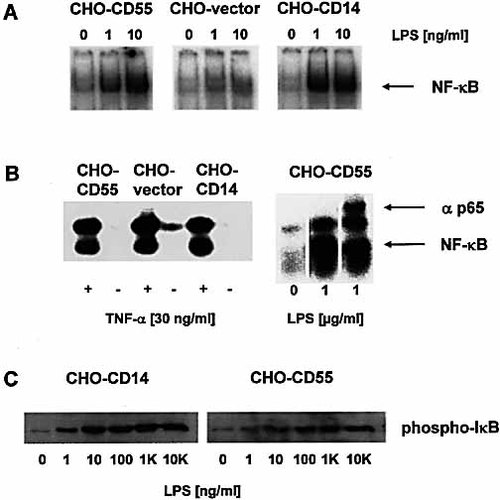
LPS-induced activation of NF-κB in transfected CHO cells. CHO vector control cells, CHO-CD14 and CHO-CD55 cells were stimulated with different concentrations of LPS (S. enterica serovar Friedenau) (A), or 30 ng/ml of murine TNF-α (B) for 30 min. Supershift analysis reveals the involvement of p65 (B). NF-κB translocation was determined by EMSA. In CHO-CD14 and CHO-CD55 cells, LPS also induces the phosphorylation of IκB-α (C), determined by Western blot.
2.2 Activation of CHO transfectants by LPS and lipid A preparations
Experiments with LPS types from various Gram-negative bacterial strains revealed that CD55-transfected cells respond in general to all LPS preparations and even to purified free lipid A with the translocation of NF-κB (Fig. 2A). To exclude that bacterial membrane compounds, which may contaminate bacterial LPS and free lipid A preparations 23, are responsible for the activation observed, we performed experiments with a synthetic hexa-acylated E. coli type lipid A (compound 506). We found a dose-dependent activation of NF-κB in CHO-CD55 cells by compound 506, starting at a concentration of 50 ng/ml (Fig. 2B).
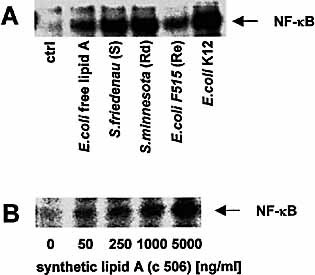
NF-κB activation in CD55-transfected cells by different LPS preparations. NF-κB activation in CD55-transfected CHO cells after stimulation with 100 ng/ml of different LPS preparations (A), or increasing amounts of synthetic E. coli lipid A (compound 506) (B). NF-κB translocation was determined by EMSA.
2.3 Activation of mitogen-activated protein kinases after LPS stimulation
It has been demonstrated that the activation of MAP kinases plays a crucial role in the activation of human monocytes by LPS 24–26, which is also seen in CHO cells expressing CD14 27. Therefore, we investigated the effect of LPS stimulation on the phosphorylation of the three major MAP kinases p38, JNK and ERK in CHO-CD55 in comparison to CHO-CD14 transfectants.
Both cell lines showed increased phosphorylation of p38 in a dose-dependent manner. Consistent with the results from the NF-κB experiments, CHO-CD14 cells appeared to be more sensitive to LPS than CHO-CD55 cells (Fig. 3, top panels). Basically, the same result was obtained when JNK and ERK phosphorylation was analyzed: again, CHO-CD14 cells responded to lower LPS doses than CHO-CD55 cells (Fig. 3, middle and lower panels).
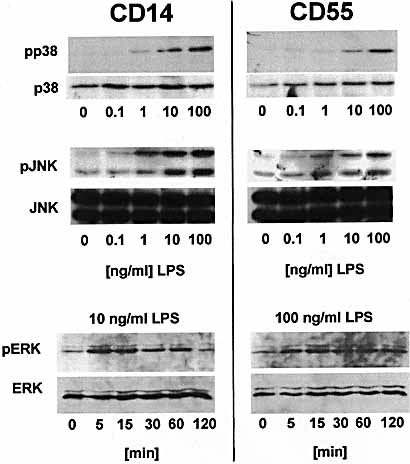
Activation of MAP kinases after LPS stimulation in CHO-CD14 and CHO-CD55 cells. Cells were stimulated with indicated amounts of LPS (S. enterica serovar Friedenau) for 15 min (p38, JNK) or indicated time points (ERK). Subsequently, whole cell lysates were subjected to SDS-PAGE and blotted onto PVDF membranes. The amount of total MAP kinase content in the lysates was detected by immunoblotting with indicated antibodies against total protein levels (lower panels). Phosphorylation of indicated MAP kinases was detected by using phospho-specific antibodies (upper panels).
2.4 Effect of LPS antagonists on the activation of CHO-CD55 and CHO-CD14 cells
Since we could only detect quantitative, but not any qualitative differences in the signaling pathways induced by LPS in CHO-CD14 compared to CHO-CD55 cells, it seemed likely that both cell lines signal through TLR4. We wanted to confirm our hypothesis by using TLR4-specific LPS antagonists. The species specificity of LPS antagonists and lipid A partial structures is a well documented feature in human, mouse as well as hamster cells 28. Recently, it has been shown that this species-specific activity depends on the origin of the TLR4 molecule, indicating a direct interaction of these antagonists with TLR4 29, 30. The synthetic tetra-acylated lipid A precursor, compound 406 (also known as lipid IVa), is a well established LPS antagonist in human cells 31–34, but also activates mouse cells, CHO-CD14 cells 28 and CHO-CD55 cells (Fig. 4b). This strongly hints towards TLR4 being the signal transducer in CHO-CD55. The structure of the LPS antagonist B1233 differs from compound 406 (Fig. 4a) by the replacement of the hydroxyl groups at the C-6′-position and the 3′-acyl chain by MeO groups, and the ester linkage of the fatty acyl side chains at the 3- and 3′-positions by ether linkages. The 3- and 3′-acyl groups are shortened to C10, and a double bond has been introduced in the 2′-acyl chain, which has been extended to C18. With the exception of a hydroxy group instead of a 3-keto group at the 2-acyl chain, B1233 is identical to B1287, an LPS antagonist capable of blocking LPS responses in CHO-CD14 and CHO-CD11b cells 35.
Preincubation of CHO-CD14 as well as CHO-CD55 cells with compound B1233 completely blocked the LPS-induced translocation of NF-κB (Fig. 4c). However, it appears that the CD55-transfected cells are more sensitive to the LPS antagonist than the CD14-expressing cells. In order to further strengthen the hypothesis that TLR4 is the signal transducer in CHO-CD55 cells, we transiently transfected CHO-CD14 as well as CHO-CD55 cells with a plasmid encoding for a dominant-negative version of murine MyD88 (DN-MyD88) 36 together with an NF-κB-dependent luciferase reporter construct. In both cell lines, co-expression of DN-MyD88 severely impairs the LPS-induced activation of NF-κB as compared to cells transfected with the empty vector, whereas the TNF-induced NF-κB activation is not significantly changed (Fig. 4D).
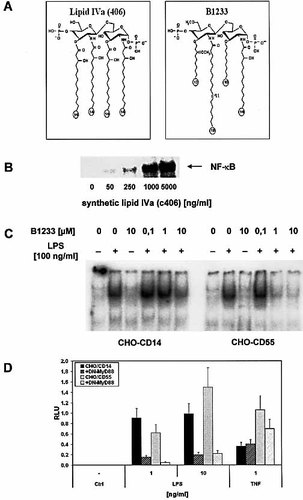
Structure and function of the LPS antagonists compound 406 and B1233 in CHO-CD55 cells. Compared to compound 406, the hydroxyl groups at the C-6′-position and the 3′-acyl chain have been replaced by MeO groups, and the ester linkage of the fatty acyl side chains at the 3- and 3′-positions has been replaced by ether linkages. In addition, the 3- and 3′-acyl groups are shortened to C10 and a double-bond has been introduced in the 2′-acyl chain, which has been extended to C18 (A). NF-κB activation in CD55-transfected CHO cells after stimulation with increasing amounts of the human lipid A antagonist compound 406 (B). Inhibition of NF-κB translocation in CHO-CD55 and CHO-CD14 cells. Cells were preincubated with indicated amounts of B1233 for 30 min before stimulation with LPS from S. enterica serovar Friedenau (100 ng/ml) (C). NF-κB translocation was determined by EMSA. CHO-CD14 and CHO-CD55 cells were transiently transfected with or without a DN-MyD88 construct and subsequently stimulated with indicated amounts of LPS or murine TNF (D). LPS- and TNF-induced NF-κB activation was determined by a dual luciferase assay and expressed in relative light units (RLU) of the mean ± SD induced/constitutive luciferase activity of duplicate wells.
2.5 Interaction of free lipid A with CD55 and CD14 as determined by co-immunoprecipitation from CHO-CD55 or CHO-CD14 cells
The results presented above suggest an interaction of lipid A with CD55 in the membrane of CHO-CD55 cells as a prerequisite for activation of the cells. To experimentally prove this hypothesis, co-immunoprecipitation experiments were performed with lipid A and anti-lipid A mAb using CHO-CD55 or CHO-CD14 cells. Cell membranes were treated with lipid A in the presence of human serum, and immunoprecipitation of lipid A-binding proteins was performed with the anti-lipid A mAb A6. Subsequently, the precipitates were analyzed for the presence of CD55 or CD14, respectively. As shown in Fig. 5, CD55 could be co-immunoprecipitated from the cell membranes of CHO-CD55 cells, whereas CD14 was co-immunoprecipitated from the cell membranes of CHO-CD14 cells. In addition to the CD55 or CD14 bands, further slower moving bands are detectable. These bands represent fragments of the anti-lipid A precipitation antibodies, which were also detected by the secondary enzyme-conjugated goat anti-mouse antibody in Western blot. To control the specificity, co-immunoprecipitation was also performed by the use of LPS plus anti-lipid A mAb, or lipid A plus an isotype control mAb. In both controls neither CD55 nor CD14 was found to be co-precipitated with lipid A.
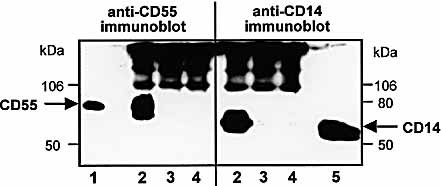
Co-immunoprecipitation of CD55 or CD14 from CHO-CD55 or CHO-CD14 cells, respectively. Cell membranes from CHO-CD55 or CHO-CD14 cells were incubated with lipid A (1 μg/ml) in the presence of 10% human serum, and co-immunoprecipitation was performed with monoclonal anti-lipid A mAb. For the detection of CD55 or CD14, the precipitates were electrophoresed under nonreducing conditions and blotted. CD55 and CD14 detection was performed with anti-CD55 mAb or anti-CD14 mAb and a peroxidase-conjugated goat anti-mouse Fc-fragment-specific secondary mAb. Lane 1: Detergent extract of CHO-CD55 cell membranes as CD55 positive control, lane 2: Co-immunoprecipitation with lipid A and anti-lipid A mAb, lane 3: Co-immunoprecipitation with lipid A and a control isotype mAb, lane 4: Co-immunoprecipitation with LPS and anti-lipid A mAb, lane 5: Detergent extract of CHO-CD14 cell membranes as a CD14 positive control.
2.6 CD55 is in close proximity to CD14 and CD11/CD18 upon stimulation of human MNC
To give further evidence for a participation of CD55 in the LPS receptor complex in natural LPS-responsive cells, we investigated if CD55 clusters with other LPS-binding receptors upon stimulation with LPS or Lipid A. We used fluorescence resonance energy transfer (FRET) analysis under two different settings. First, in a whole blood assay (Fig. 6A), we demonstrated LPS-specific clustering of CD55 with CD11b, another receptor that has been shown to interact with LPS 37. Secondly, using isolated human mononuclear cells, FRET analysis indicated low, but significant, close proximity of lipid A with CD55 (Fig. 6B). As a positive control, the same experiments were performed with CD14 and showed a much stronger signal. However, close proximity of CD13, a molecule with a similar distribution on monocytes as CD14, and Lipid A was not detectable.
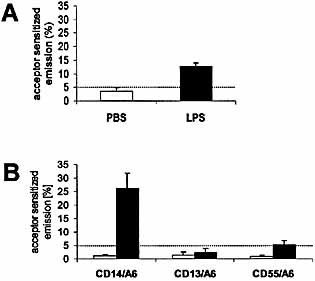
FRET analysis of LPS- and lipid A-induced receptor clustering. Heparinized whole blood was incubated with PBS (open bar) or 40 ng/ml of LPS (black bar) for 30 min, and energy transfer was measured between CD11b and CD55 (A). Isolated human MNC were stimulated with PBS (open bar) or 1 μg/ml synthetic lipid A (compound 506, black bar) for 30 min, and energy transfer was measured between anti-lipid A (A6) and either CD13, CD14 or CD55 (B). Energy transfer values are expressed in terms of FRET efficiency (see Sect. 4). Data are the mean ± SD of five experiments.
3 Discussion
We have recently described an 80-kDa LPS-binding membrane protein (LMP80), which is found on nearly all human cells 17, and could be identified as the CD55/DAF molecule 19. Here, we provide evidence for a new functional role of CD55 contributing to LPS signaling.
We found that LPS induces NF-κB translocation as well as phosphorylation of IκB-α in CD55-transfected CHO cells in a dose-dependent manner at a minimum concentration of 10 ng/ml. Compared to CHO-CD14, CHO-CD55 cells are about one log less sensitive to LPS (Fig. 1). Supershift analysis revealed that the NF-κB complex in CHO-CD55 cells contained the NF-κB-p65 subunit (Fig. 1B), which is also known to be functionally involved in the activation of human monocytes after LPS stimulation 38. Translocation of NF-κB in CHO-CD55 cells was observed after stimulation with various LPS types prepared from different smooth or rough bacterial strains (Fig. 2A). Furthermore, the lipid A moiety was identified as the stimulatory principle of the LPS molecule for the activation of CD55-transfected cells (Fig. 2B).
When we analyzed the involvement of various signaling pathways in the LPS-induced activation of CHO-CD55 in comparison to CHO-CD14 cells, only quantitative differences of the responses in the different cell types could be seen (Fig. 3). All three MAP kinases p38, JNK and ERK were activated by LPS.
The tetra-acylated lipid A partial structure, compound 406, which is a strong LPS antagonist in human cells, was still active in CHO-CD55 cells (Fig. 4B), and thus, exhibits the same species-specificity as observed in CHO-CD14 cells 28. This finding provided the first evidence that TLR4 is also involved in the LPS signaling of CHO-CD55 cells. Therefore, we wanted to prove the assumption that LPS engages the same signaling receptor in both cell types by blocking the activation at the level of TLR4. Data from species-specificity experiments led to the conclusion that physical contact between TLR4 and Lipid A or lipid A partial structures must exist, and that the inhibition of LPS activation takes place at the level of TLR4 29, 30. B1233 was designed to be a potent antagonist in CHO cells. As shown in Fig. 4C, B1233 completely blocks LPS responses in CHO-CD14 as well as in CHO-CD55 cells. Moreover, in both cell lines the LPS-induced translocation of NF-κB is drastically reduced in the presence of a dominant version of MyD88, strongly supporting our hypothesis that TLR4 is the principle signal transducer not only in CHO-CD14 but also in CHO-CD55 cells (Fig. 4D). Thus, in both cell types LPS/lipid A is transferred to TLR4, where its signaling can be blocked by B1233. However, we cannot rule out the possibility that B1233 is also able to bind to CD55 and to block the activation of CHO-CD55 cells by lipid A at the level of CD55.
In previous studies, we have already demonstrated the physical association of LPS or free lipid A to LMP80/CD55 by co-immunoprecipitation analysis in monocytes and erythrocytes 18. After the identification of CD55 as a mediator of LPS-induced cell activation, we were interested in the interaction of CD55 with LPS on the cell membrane of CHO-CD55 cells. The results obtained with CHO-CD55 cells confirmed our previous experiments showing co-immunoprecipitation of CD55 also in cell membranes of CHO-CD55 cells with lipid A and anti-lipid A mAb. In addition, control experiments showed co-immunoprecipitation of CD14 from CHO-CD14 cells.
We extended these studies by using FRET technology in order to investigate close proximity of lipid A and CD55 in natural LPS-responsive cells, namely human monocytes (Fig. 6B). Based on this experiment, we cannot distinguish between direct binding of lipid A to CD55 or binding of lipid A to CD14, and subsequently induced co-clustering of CD14 and CD55. Nonetheless,these experiments indicate that CD55 physically interacts with lipid A on LPS-responsive cells.
HEK293 express a high level of CD55 on their surface, yet, are LPS-unresponsive unless transfected with TLR4 and MD-2 (H. Heine, unpublished results). Thus, CD55 by itself is not able to transmit LPS signals. The question about the mechanism by which CD55 contribute to LPS signaling has to be raised. In particular, it may be asked if additional membrane molecules are involved.
It is unquestionable that CD14 is the major binding receptor for LPS on monocytes/macrophages, and that TLR4 represents an essential membrane signal transducing element for LPS. However, thereare several membrane proteins described which may act as accessory LPS receptor molecules. The members of the β-integrin family CD11a,b,c/CD18 are known to serve as mediators of LPS-induced activation of cells 37. Although it has been shown that signaling through the integrins is not required for activation of NF-κB 39, recent data from Perera et al. 40 clearly demonstrate that in addition to TLR4, CD11/CD18 as well as CD14 are required for certain LPS responses. Interestingly, Triantafilou et al. 16 demonstrated a CD14-independent LPS-inducible receptor cluster on human cells, consisting of hsp70, hsp90, CXCR4, and GDF-5. Finally, data from Pfeiffer et al. 15 indicate that LPS stimulation of human monocytes leads to an clustering of membrane molecules including CD14, TLR4, CD11b, CD81, various Fc receptors, and also CD55, forming an LPS receptor complex.
In this context, it should be considered that CD55 together with other GPI-linked molecules is assembled within large insoluble glycolipid-enriched domains on the cell surface, which are associated with src family protein tyrosine kinases and other signal transducing elements 41–47. These microdomains (also called rafts) are believed to play a role in ligand-induced signaling in various cell types including T cells 48. Cross-linking of GPI-anchored molecules, including CD55, by mAb results in cellular activation of various inflammatory cells. This indicates that these membrane microdomains are, indeed, the loci of signal transduction mediated by GPI-anchored proteins like CD55 and CD14 49–54.
Although the contribution of each of the various receptors to the overall response towards LPS is not at all understood, these data clearly favor the existence of an LPS-induced receptor cluster in lipid rafts consisting of many different receptor molecules. In this paper, we provide evidence that LPS physically interacts with CD55 in the cell membrane of natural LPS-responsive cells andthat CD55 is part of a multimeric LPS receptor complex (Figs. 5; 6A, B).
In summary, our results indicate a new function of CD55/DAF, namely the enhancement of LPS-induced signal transduction. The dissection of the different signal pathways engaged by CD55 and other receptors that are part of the LPS receptor complex, however, remains to be elucidated.
4 Materials and methods
4.1 Reagents and antibodies
Unless otherwise indicated all fine chemicals were purchased from Sigma (Deisenhofen, Germany), Serva (Heidelberg, Germany), Merck (Darmstadt, Germany) or Boehringer Mannheim (Mannheim, Germany). The anti-NF-κB p65 polyclonal antibody and the anti-phospho-JNK mAb were obtained from Santa Cruz Biotech. (Santa Cruz, CA). All other anti-MAP-kinase polyclonal antibodies as well as the phosphor-IκB Ab were from NEB (Schwalbach, Germany). IgG 1 isotype control antibodies were purchased from Sigma. The anti-CD55 mAb BRIC 110 was from Biozol (Eching, Germany). Anti-CD14 mAb, clone MEM-18, was a kind gift from V. Horejsi (Institute of Molecular Genetics, Prague, Czech Republic) 55. Anti-lipid A mAb A6, which recognizes the phosphorylated carbohydrate backbone of lipid A independently of the acylation pattern, was used as hybridoma serum-free supernatant 56. This mAb was kindly provided by L. Brade (Borstel, Germany). Horseradish peroxidase-conjugated goat anti-mouse as well as FITC-conjugated goat anti-mouse mAb were from Dianova (Hamburg, Germany). Normal human sera were obtained from healthy volunteers. LPS derived from various Gram-negative strains was kindly provided by H. Brade (Borstel, Germany). LPS was prepared by the hot phenol-water procedure, purified by repeated ultracentrifugation, and converted into the sodium salt form by electrodialysis 57. Unless otherwise stated, LPS from S. enterica serovar Friedenau has been used. All LPS preparations were solubilized in pyrogen-free distilled water and showed no TLR2 transmittable activity. Synthetic bisphosphorylated hexa-acyl lipid A (E. coli type), also known as compound 506, and tetra-acylated compound 406, alsoknown as lipid IVa or lipid A precursor Ia, were synthesized as described previously 58, 59. B1233, a synthetic lipid A antagonist, was a kind gift of EISAI Research Institute (Andover, MA). Murine TNF-α was obtained from Sigma (Deisenhofen, Germany).
4.2 Cell culture
CHO-K1 cells (DSM ACC110) from the DSMZ (Braunschweig, Germany), were transfected with either pCEP4, pCEP4-CD14 (a kind gift of D. Golenbock, Worcester, USA), or pCEP4-CD55 as described elsewhere 19, and were cultured at 37°C in a humidified atmosphere containing 5% CO2. Cells were grown in Ham's F12 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 400 μg/ml hygromycin.
4.3 Cell stimulation
Before stimulation, CHO cells were seeded into 6-well plates (1×106/well) and allowed to adhere overnight in Ham's F12 supplemented with 2% human serum. Cells were stimulated with various amounts of LPS or lipid A for 1 h in Ham's F12 containing 2% human serum.
4.4 Transient transfection
For transient transfection, CHO-CD14 and CHO-CD55 cells were seeded into 24-well dishes at a density of 50,000 cells/well 1 day prior transfection. The expression plasmid coding for a dominant-negative murine MyD88 36 was a kind gift from Dr. T. K. Means (Boston University School of Medicine, Boston, MA); the plasmid coding for an NF-κB-dependent expression of firefly luciferase, ELAM-Luc, 60 was a kind gift from Dr. C. Kirschning, Technical University of Munich (Munich, Germany). The internal control expression plasmid for constitutive expression of Renilla luciferase (pRL-TK) was from Promega (Mannheim, Germany). The following day, cells were transiently transfected with Polyfect (Qiagen, Hilden, Germany) with either 2 μg of either DN-MyD88 or empty vector DNA/well, together with 500 ng/well of the ELAM-Luc plasmid and 25 ng/well of the pRL-TK plasmid according to the manufacturers' protocol. After 36 h of incubation, cell were stimulated with LPS and murine TNF for an additional 6 h, followed by the analysis of induced luciferase activity with the Dual-luciferase reporter assay system (Promega).
4.5 Preparation of nuclear extracts and electrophoretic mobility shift assay
The preparation of nuclear extracts was performed as described elsewhere 61. The oligonucleotide containing the NF-κB-binding sequence of the murine immunoglobulin κ light chain enhancer 62 was obtained from MWG-Biotech (Ebersberg, Germany). The oligonucleotide (1.25 pmol) was end-labeled in the presence of [γ-32P]dATP using T4-polynucleotidkinase (Boehringer Mannheim, Mannheim, Germany) for 30 min at 37°C. Unincorporated nucleotides were removed using a Nick-S-column (Pharmacia, Freiburg, Germany). Labeled oligonucleotides (7.5 fmol) were used in the DNA-binding reaction containing 4 μg of crude nuclear extract. The binding reaction also contained 1 μg poly/dI-dC, 1 μg poly/dAdT, 4% Ficoll, 1 mM dithiothreitol, 2 mM MgCl2, 0.03% NP40, and 60 mM KCl. Reactions were incubated for 20 min at 4°C and separated by electrophoresis in 4% polyacrylamide gels containing 0.5× TBE (45 mM Tris-borate, 1 mM EDTA). Gels were run at 200 V for 1.5 h, sealed, exposed overnight to a phosphoscreen and analyzed with a PhosphorImager (Molecular Dynamics, Krefeld, Germany). For electrophoretic mobility shift assay (EMSA), probes where incubated with 1 μl of the anti-NF-κB p65 antibody immediately after incubation with the labeled oligonucleotide.
4.6 Preparation of cell lysates for Western blot analysis
CHO cells (1×106) were stimulated and washed twice with cold PBS. Lysis was performed in 150 μl of electrophoresis buffer by shaking for 20 min. Lysates were mixed extensively and cleared by centrifugation (20,000×g, 30 min). Each probe (15 μl) was subjected to SDS-PAGE under reducing conditions on a 10% gel, followed by blotting of the proteins onto PVDF membranes using a wet blotting apparatus for 1 h at 2 mA/cm (constant). PVDF membranes were blocked for 2 h at room temperature with TBS containing 5% nonfat dry milk (TBS/M). Membranes were incubated over night with anti-MAP-kinase mAb, anti-phospho-MAP-kinase mAb or anti-phospho-IκB, respectively, in concentrations described in the manufacturers' protocol. Immunoblots were washed four times in TBS/T, incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit mAb (1:2,000) in TBS/M, and developed with the ECL system (Amersham Buchler, Braunschweig, Germany).
4.7 Co-immunoprecipitation of CD55 and CD14
Co-immunoprecipitation of CD55 and CD14 from cell membranes were performed as described previously 19. Cell membranes from CHO-CD55 or CHO-CD14 cells were incubated with 1 μg lipid A and 50 μl serum in 1 ml PBS containing 1 mM PMSF, 2 mM EDTA for 60 min at 4°C on a shaker. The membranes were washed three times (30 min, 36,000×g, 4°C) with PBS containing 1 mM PMSF and 2 mM EDTA, and were solubilized for 30 min at 4°C in 500 μl solubilizing buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 1 mM PMSF, 2 mM EDTA, 1% CHAPS). After centrifugation (30 min, 36,000×g, 4°C), the supernatants were diluted with 500 μl detergent-free solubilization buffer and incubated for 60 min at 4°C with 200 μl of the monoclonal anti-lipid A antibody A6. For precipitation of the antibody-protein complexes, 50 μl of protein A agarose was added and shaken overnight at 4°C. On the next day, beads were washed three times with solubilizing buffer containing0,5% CHAPS. Elution of proteins was achieved by adding 50 μl SDS-PAGE sample buffer to the precipitates and by boiling for 5 min. Immunoprecipitates or a detergent extract of membrane proteins were subjected to SDS-PAGE under nonreducing conditions on a 9% gel and blotted onto nitrocellulose membranes. For detection of CD55 or CD14, anti CD55 mAb, clone BRIC 110 (1:2,000), or anti-CD14 mAb, clone MEM-18 (1:2,000), were used. After subsequent incubation with horseradish peroxidase-conjugated goat-anti-mouse mAb, the ECL system (Amersham Buchler, Braunschweig) was applied.
4.8 FRET analysis
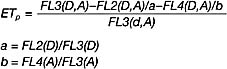 (1)
(1)Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 367, Project C5). We thank L. Brade and H. Brade (Research Center Borstel, Borstel, Germany) for providing the LPS preparations and anti-lipid A mAb. We also thank Eisai Research Institute, Andover, USA, for providing us the lipid A antagonist B1233. V. Horejsi (Institute of Molecular Genetics, Prague, Czech Republic) kindly provided anti-CD14 mAb, clone MEM-18. The pCEP4-CD14 expression vector was a kind gift of D. Golenbock (Boston, USA). We gratefully thank I. Goroncy and K. Klopfenstein for technical assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




