Tumor-associated fibroblasts recruit blood monocytes into tumor tissue
Abstract
Tumor-associated fibroblasts (TAF) and tumor-associated macrophages are the main stromal components in desmoplastic breast tumors. These host cell types were extensively studied individually with regard to tumor development and progression but little is known about their reciprocal interactions. To elucidate the role of TAF in the recruitment of monocytes (MO) we designed a 3D co-culturesystem of multicellular fibroblast spheroids of different origin, co-cultured with MO suspensions from healthy donors. Spheroids of tumor-derived but not of normal fibroblasts were extensively infiltrated by MO. A linear correlation between number of infiltrated MO and number of MO applied per spheroid was shown, indicating a distinct migratory MO subpopulation (≈15%) within the peripheral blood MO pool. Our data imply that MO migration into fibroblastic tumor areas may partially result from high expression of CCL2 (monocyte chemotactic protein-1), which was regulated by endogenousIL-6 as shown by neutralization experiments. The effect of CCL2 on MO migration was inhibited by CCL2 neutralizing antibody in tumor-derived fibroblast conditioned media in a Boyden chamber migration assay but not in spheroid culture. While this phenomenon needs further evaluation, our data clearly support the concept of fibroblasts as "sentinel cells" relevant for tumor progression.
Abbreviations:
-
- BRAK:
-
Breast and kidney-expressed chemokine
-
- ECM:
-
Extracellular matrix
-
- MCP-1:
-
Monocyte chemotactic protein-1
-
- MCS:
-
Multicellular spheroid
-
- MO:
-
Monocytes
-
- TAF:
-
Tumor-associated fibroblasts
-
- TAM:
-
Tumor-associatedmacrophages
1 Introduction
Tumor-associated macrophages (TAM) represent a major component of the leukocyte infiltrate of many solid tumors. They derive from blood monocytes (MO) that migrate into tumor tissue and undergo a process of differentiation determined by the tumor microenvironment. TAM are often characterized by lack of immunological activity, exhibiting a phenotype that rather contributes to tumor growthand propagation than to immunological control 1–3.
In ductal breast carcinomas, a high macrophage (MΦ) content correlates with poor prognosis and clinical outcome 3–5. In this tumor entity, TAM are predominantly located within the peritumoral stroma surrounding tumor cell islets (Fig. 1a). This stromal compartment also comprises endothelial cells and fibroblasts. Indeed, fibroblasts are the most abundant host stromal cell type in desmoplastic tumors. Desmoplasia as a tumor-induced fibrotic host response associated with a modified, collagen-rich extracellular matrix (ECM)composition is most frequently described in squamous cell carcinomas, pancreatic tumors, as well as colorectal and breast malignancies. Systematic investigations of fibroblast-tumor cell interactions over the past 15 years demonstrated that tumor-associated fibroblasts (TAF) actively affect tumor growth and progression via diverse mechanisms. Direct cell-cell interactions, release of auto-/paracrine factors, and the complex ECM network are all modulated by TAF and may directly and indirectly support tumor cell proliferation and migration 6–10.
An impact of TAF on anti-tumor immune response is hypothesized, but experimental attempts to gain deeper insight into the interaction between TAF and immune cells are rudimentary. With respectto other pathological conditions such as tissue damage and injury, fibroblasts are well known to modulate the functional status of immune competent cells. They are capable of regulating the local cellular and cytokine milieu and adjusting kinetics and components of the inflammatory infiltrate to the type of damage 11. Lack of deactivation of fibroblasts contributes to persistence of the inflammatory infiltrate. Hence, in chronic inflammation processes fibroblasts as producers of various paracrine immune modulators, including peptide growth factors, cytokines/chemokines, and low-molecular weight inflammatory mediators, are discussed as ‘sentinel cells’ that contribute to leukocyte migration and local immune response 12.
With regard to immune reactions, cancer and chronic inflammation processes show some analogies 13, 14. We therefore propose an active role of TAF in the immunological host response, and have designed an experimental set-up to investigate interactions between fibroblasts and MO in vitro in a three-dimensional (3D) format under well-defined conditions. According to a 3D co-culture model of urothelial tumor cell lines and MO 15, 16, fibroblasts were grown as multicellular spheroids (MCS) and co-cultured with suspensions of MO from healthy donors. Fibroblasts in spheroid culture were in general cell-cycle arrested and expressed high levels of multiple, but distinct, ECM molecules 17. The model system allowed MO migration into fibroblast spheroids of normal (skin/breast) and pathological (breast carcinoma) origin to be monitored, and also represents a valuable tool for examining the impact of TAF on MO-to-MΦ/TAM differentiation. Our results indicate a fundamental participation of TAF in the recruitment of MO into tumor tissue.
2 Results
2.1 MO migration into fibroblast spheroids
Immunohistochemical staining of N1 fibroblast spheroids after 40 h in co-culture with peripheral blood MO (Fig. 1b) confirmed the previous finding that normal skin fibroblast spheroids are poorly invaded by MO 15. In contrast, spheroids of fibroblasts derived from a poorly differentiated invasive ductal breast tumor (PF1) were excessively infiltrated by MO (Fig. 1c).
To quantify and verify distinct migration of MO into spheroids of fibroblasts of different origin including normal skin (N1, Kaufmann), normal breast (PFN2), and different ductal breast carcinomas (PF27, PF28, PF32, PF36, PF37), spheroids were dissociated 40 h after addition of defined MO suspensions and analyzed via flow cytometry. Staining with FITC-conjugated AS02 antibody enabled us to define regions in the dot plot diagram of side scatter and fluorescence signals to distinguish fibroblasts and MO (R1 and R2) (Fig. 2a). For each fibroblast spheroid type, MO from different donors were applied and the proportion of fibroblasts:MO was determined. Results presented in Fig. 2b verify that normal skin and breast fibroblasts are infiltrated by a limited number of MO contributing to less than 25% of the total spheroid cell count. Infiltration accounted to 6±1% in N1 (n=6) and 24±4% in Kaufmann (n=3) skin fibroblasts, and 18±8% in PFN2 breast fibroblast spheroids (n=3). The MO proportion in spheroids of breast tumor-derived fibroblasts was significantly higher (p<0.001; non-parametric Mann-Whitney-U test): 52±10% in PF27 (n=7), 53±4% in PF28 (n=8), 48±8% in PF32 (n=9), 41±7% in PF36 (n=5), and about 49% (n=2) in PF37 spheroids. In addition, one fibroblast type (PF32M) isolated from an area of a breast tumor biopsy specimen showing fibrocystic mastopathy, but no carcinoma, was analyzed and showed a similar infiltration rate of 45±10% (n=5).
With PF28 spheroids, MO from eight different healthy donors were applied to assess a donor-dependent MO migratory activity. The data presented in Fig. 2c imply that MO migration is relatively independent of the donor. However, it has to be taken into account that the number of migrated MO, while prominent with respect to the amount of fibroblasts in co-culture is rather small relative to the number of MO applied per spheroid.
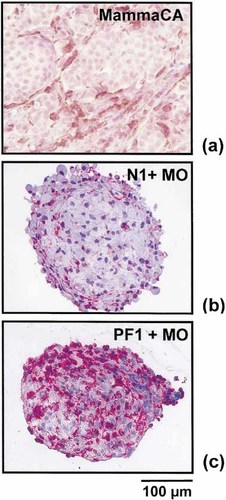
Localization of TAM in ductal invasive breast tumors and of MO migrated into fibroblast spheroids. (a) Representative 5-μm section of a paraffin-embedded invasive ductal carcinoma (G=3) stained immunohistochemically for CD68 to show the typical distribution of TAM within the tumor stroma surrounding tumor cell islets. Peroxidase-based reaction; DAB for color development; hematoxylin counterstain. (b, c) Frozen sections (5 μm thick) of N1 skin and PF1 breast tumor-derived fibroblast spheroid, respectively, 40 h after addition of 1×104 MO from a healthy donor stained for CD14. N1 fibroblasts showed poor MO infiltration; PF1 spheroids were extensively infiltrated. APAAP technique; fast red as chromogen; hematoxylin counterstain.
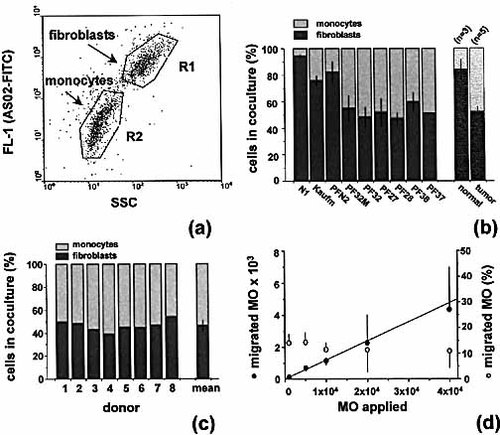
Blood monocytes migrate into 3D cultures of tumor-derived fibroblasts. (a) Representative dot plot diagrams showing FL-1 fluorescence versus 90° scatter signals of a mixed cell suspension isolated from PF28 spheroids 40 h after addition of 1×104 MO. The cell suspension was stained with the FITC-conjugated ‘fibroblast-specific’ antibody AS02. (b) Proportion (± SD) of fibroblasts and MO in spheroid co-cultures 40 h after addition of MO as determined by FACS analysis according to (a). Fibroblast types: normal skin N1 and Kaufmann; normal breast PFN2; breast tumor-derived PF27, PF28, PF32, PF36, and PF37; PF32M fibroblasts derived from carcinoma-free fibrocystic mastopathy area of PF32 tumor biopsy. Mean values were also averaged for normal (n=3) as compared to tumor-derived fibroblast types (n=5). (c) Proportion of fibroblasts and MO in PF28 spheroids 40 h after application of 1x104 MO from 8 different healthy donors. (d) Number of migrated MO as a function of MO applied per PF28 spheroid and proportion of MO (subpopulation) migrated from the total blood MO pool.
2.2 Indication for a defined migratory MO subpopulation
In a separate series of experiments different MO concentrations from one donor (n=3) were added to PF28 fibroblast spheroids. After 40 h in co-culture, spheroids were processed and analyzed flow cytometrically. The absolute numbers of migrated MO per spheroid were calculated from the total cell count/spheroid and the proportion of fibroblasts:MO in the dissociated single-cell suspensions. A positive linear correlation (r=0.994) between number of migrated MO and number of MO applied per fibroblast spheroid was recorded for MO concentrations ranging between 103 and 4×104 per fibroblast MCS (Fig. 2d). This was verified with a second fibroblast MCS type (PF36). Calculation of the proportion of migrated MO from the MO number applied per spheroid revealed a constant fraction of 12.5–15%, indicating a defined MO subpopulation in the blood MO pool capable of invading fibroblast spheroids (Fig. 2d).
From the limited extracellular space in fibroblast MCS that can be infiltrated by MO a saturation process was expected. In an additional series of experiments with 2×104–1.6×105 MO added per spheroid, the number of migrated MO did not further increase for MO concentrations above 4×104/MCS. The maximum MO proportion in fibroblast MCS accounted to 60–70% of the total cell count.
2.3 Cytokine expression in fibroblasts of different origin
To elucidate the potential role of paracrine factors in the process of MO migration into fibroblast spheroids of different origin, we first examined cytokine release from fibroblast monolayer cultures. Release rates were analyzed relative to the number of cells at the time of supernatant removal. These data indicated a profoundly higher cellular level of CCL2 and IL-6 production in tumor-derived (IL-6 and CCL2: >0.1 pg/cell in 96 h, n=3) as compared with normal skin and breast fibroblasts (IL-6 and CCL2: <0.02 pg/cell in 96 h, n=3) for both subconfluent and confluent conditions. Since fibroblasts in 3D culture are cell cycle arrested 17 and the regulation of cell quiescence may differ for 2D and 3D cultures 18, cellular release rates in non-proliferating fibroblast spheroids were analyzed for further verification.
CCL2 release clearly differed for normal and tumor-derived fibroblasts in MCS. Here, cellular release rates in normal skin and breast fibroblasts were 5×10–3–5×10–2 pg/cell in 96 h. Tumor-derived fibroblasts and fibroblasts derived from a mastopathy of a breast tumor patient showed an enhanced CCL2 secretion with highly variable release rates ranging from 0.1 to 0.75 pg/ cell over the 96-h period (Fig. 3a). For IL-6 a similar expression pattern was observed. An IL-6 release between 7×10–3 and 2×10–2 pg/cell in 96 h was measured for normal fibroblasts, while the lowest release rate for tumor-derived fibroblasts was 7×10–2 pg/cell (Fig. 3b). In addition to IL-6 and CCL2, granulocyte-MΦ colony stimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF) were determined in culture supernatants of fibroblast MCS spheroids. M-CSF release from fibroblasts was in general low (5×10–3–2×10–2 pg/cell in 96 h) and did not essentially differ for the fibroblast types investigated. GM-CSF was below the detection level of the assay (<7.8 pg/ml) in all supernatants reflecting a 96-h release rate of less than 4.5×10–3 pg/cell.
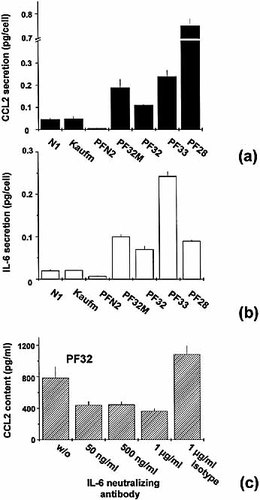
Endogenous expression and regulation of CCL2 in tumor-derived fibroblasts. Spontaneous cellular release of (a) CCL2 and (b) IL-6 of different fibroblast types in 3D culture over a period of 96 h as determined by ELISA. Fibroblast types: normal skin N1 and Kaufmann; normal breast PFN2; breast tumor-derived PF28, PF32, PF33; PF32M fibroblasts derived from carcinoma-free fibrocystic mastopathy area of a PF32 tumor biopsy specimen. An analogous difference in the cytokine release rates was recorded in monolayer cultures. (c) Neutralization of endogenous IL-6 by an anti-IL-6 mAb (0.05–1 μg/ml) leads to a significant reduction in the CCL2 level in supernatants of confluent tumor-derived PF32 monolayer cultures with an incubation period of 24 h (p<0.01; non-parametric Mann-Whitney-U test).
2.4 Neutralization of endogenous IL-6 in fibroblast cultures
Since qualitatively analogous differences in the cellular cytokine release rates of different fibroblast types were recorded in monolayer and spheroid culture, confluent PF32 monolayer cultures were used to evaluate the existence of an autocrine IL-6–CCL2 regulatory loop in tumor-derived fibroblasts. Here, addition of a neutralizing anti-IL-6 mAb (50 ng/ml–1 μg/ml) led to a significant decrease in the CCL2 release as compared to untreated (and isotype-treated) controls as documented in Fig. 3c. With 1 μg/ml anti-IL-6 antibody, the CCL2 level was reduced by more than 50% after 24 h of incubation. We reproducibly recorded an ambiguous increase in CCL2 accompanying treatment with the isotype antibody (1 μg/ml).
2.5 Neutralization of endogenous CCL2 in fibroblast cultures
Two experimental approaches were applied to evaluate the impact of fibroblast-derived CCL2 on MO migration. Conditioned medium collected from two tumor-derived fibroblast spheroid types (PF27, PF32) were applied in a Boyden chamber assay. The absolute number of migrated MO per visual field and per well was highly variable for different experiments and MO donors and also varied within one experiment (Fig. 4a). Therefore, the migration data for three MO donors were averaged following normalization of each individual experiment, i.e. the mean proportions of migrated MO relative to the respective conditioned, antibody-free medium (set to 100%) were calculated and averaged (Fig. 4b). As shown in Fig. 4a–c, MO migration induced by PF27 or PF32 conditioned media declined in a dose-dependent manner by neutralization of CCL2; 10 μg/ml neutralizing antibody reduced the migration to the baseline activity in DMEM.
A 2.5-fold higher anti-CCL2 antibody concentration was used for neutralizing CCL2 in PF27 and PF32 fibroblast spheroid cultures. However, flow cytometric analyses revealed that neither 10 nor 25 μg/ml antibody affected MO migration into these spheroid types (PF32: n=3; PF27: n=2) (Fig. 5).
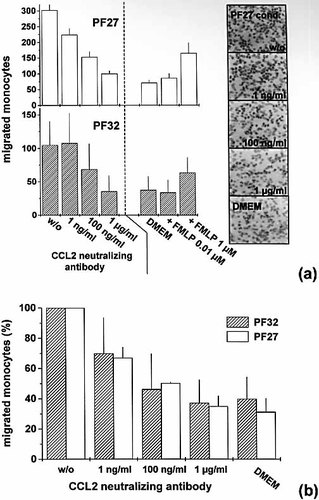
Neutralization of CCL2 in conditioned medium of tumor-derived fibroblast spheroids PF27 and PF32 reduces the migratory activity of MO in the Boyden chamber assay. (a) Individual experiments showing number of migrated MO towards PF27 and PF32 conditioned media with and without CCL2 neutralizing antibody (1 ng/ml–1 μg/ml); FMLP served as positive, unconditioned DMEM as negative control. Isotype controls (10 μg/ml) do not affect MO migration (data not shown). Columns show mean values (± SD) of three to six wells per condition with four high-power fields (magnification ×400) evaluated per well. Representative Hemacolor (Merck) stained membranes/spots are shown on the right- hand side. (b) Average migratory activity (%) of MO from three independent donors in DMEM and in PF27 or PF32 conditioned medium spiked with CCL2 neutralizing antibody relative to the antibody-free conditioned control (=100%). Columns show averaged mean values and SD of three independent experiments for each fibroblast type according to (a).
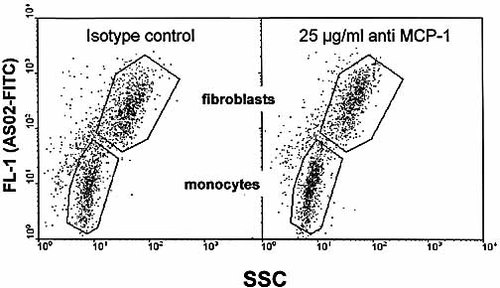
Incubation of fibroblast spheroids with CCL2 neutralizing antibody is not accompanied by reduced MO infiltration as representatively shown in flow cytometric dot plot diagrams according to Fig. 2. Identification of PF32 fibroblasts and MO from spheroid co-cultures incubated with either an isotype control or the anti-CCL2 antibody (25 μg/ml). Antibodies were applied 2 h prior to the addition of MO and cell populations were analyzed after 40 h in spheroid co-culture.
3 Discussion
3.1 Spheroid co-cultures as model system to study MO–fibroblast interactions
Heterologous in vitro systems are required to gain deeper insight into the complex mechanisms of MO migration and MO-to-TAM differentiation in tumor tissues. 3D tumor spheroid–MΦ co-cultures were first applied by Hauptmann et al. 19, who showed different MΦ populations to support tumor proliferation and migration. Later, MO-to-MΦ/TAM differentiation phenomena were investigated in bladder cancer spheroids. Here, J82 tumor spheroids were strongly infiltrated by MO and MΦ, and down-regulation of several maturation-associated antigens and inhibition of the shift in the cytokine repertoire usually observed during MO-to-MΦ differentiation was recorded. An analogous effect associated with suppression in the cytotoxic/cytostatic activity of TAM was neither recorded in monolayer co-cultures nor in well-differentiated urothelial cancer spheroids 15, 16, indicating that this model system well resembles the in vivo situation. Indeed, while ignoring extravasation, one of the major advantages of the spheroid model is the consecutive progression of immune cell migration and differentiation/activation, which cannot be considered autonomous processes 20. In desmoplastic breast tumors, TAM are most frequently found in tumor-adjacent fibroblastic stroma. We therefore applied spheroid co-cultures to evaluate the effect of fibroblasts on MO migration and showed that tumor-derived in contrast to normal skin and breast fibroblast spheroids are profoundly and reproducibly infiltrated by MO. We conclude that spheroid co-cultures of TAF and MO are a valuable tool to further study the impact of TAF on TAM phenotype.
3.2 MO subpopulation migrating into fibroblast MCS
Our results imply that a relatively constant subpopulation of the blood MO pool (12–15%) is capable of infiltrating TAF spheroids. Indeed, human peripheral blood MO are a phenotypically and functionally diverse population 21. Characteristics defining MO subpopulations include size and granularity, expression of surface antigens and/or a variable potential for secretingparacrine factors (e.g. 22). Heterogeneity of MO with respect to migratory activity was first described in the early 1980s with an MO population of 20–40% found to respond to different chemotactic stimuli in vitro 23. A neutrophil-like subpopulation showing lack of HLA-DR but high expression of diverse ECM-binding integrins was identified later to be predominantly recruited to sites of acute inflammation 24. More recently, differential expression of chemokine receptors accompanied by divergent chemotactic responsiveness in MO subpopulations was described 25. Further studies with the spheroid model will therefore aim to define the MO subpopulation that migrates into TAF spheroids.
3.3 Fibroblast-derived CCL2 and MO migration
TAF express an altered phenotype associated with multiple modifications in vivo and in vitro, e.g. in the production of ECM and ECM-modulating molecules 6, 7, 9, 10. Phenotypic alterations under diverse other pathological conditions include the fibroblast cytokine expression profile, e.g. rheumatoid arthritis fibroblasts were shown to express a cytokine pattern that differed from normal synovial fibroblasts but was stable in culture 26. Brouty-Boyé et al. 27 were the first to report differential expression of chemokines and CD40 in fibroblasts of different origin including normal and tumorous hematopoietic, lung, and breast tissues. In contrast to this study, our results showing high spontaneous CCL2 secretion in breast-tumor derived fibroblasts indicate some relevance of this CC chemokine for MO migration into fibroblastic tumor areas. This is supported by the observation of elevated CCL2 levels in extracts of malignant breast tumors correlating with high MΦ content 28 and the demonstration of CCL2 in both tumor and stromal compartments in breast tumor tissue by in situ hybridization 29. Similarly, fibroblasts from scleroderma lesions showed high expression of CCL2 30 and promoted MO migration through endothelial monolayers in a transwell assay 31. Our observations with the Boyden chamber using CCL2-neutralizing antibody indicate that CCL2 indeed is a potent paracrine chemotactic factor in TAF-conditioned media. However, the same antibody was ineffective in fibroblast spheroids. This can be interpreted as follows: (a) CCL2 was not sufficiently blocked in fibroblast MCS, although the amount of neutralizing antibody was in excess (×100) of the supernatant CCL2 level. CCL2 can bind to ECM components 32, which may lead to local accumulation in fibroblast spheroids and/or to a masking of the CCL2 antibody binding site; also, antibody distribution in 3D cultures may be inadequate for CCL2 blockade. (b) MO migration into fibroblast spheroids is rather independent of CCL2 and other paracrine factors; direct MO–fibroblast or MO–matrix interactions are key players in this scenario. This will be the subject of future studies, which will also consider the new finding of CXCL14 (BRAK) -secreting fibroblasts to be involved in MO recruitment and maturation in homeostatic human dermis 33. Such a mechanism could explain the basic level of MO infiltration in normal skin and breast fibroblast spheroids.
3.4 Regulation of CCL2 expression in fibroblasts
CCL2 expression in diverse fibroblasts can be induced by cytokines. IL-1β and IFN-γ synergistically stimulated CCL2 and MCP-2 expression in human diploid fibroblasts 34. IL-1β and TNF-α were capable of inducing CCL2 secretion in isolated pancreatic periacinar myofibroblasts 35, primary lung fibroblasts 36, and cultured interstitial renal fibroblasts 37. In human skin fibroblasts, autocrine IL-6 was demonstrated to up-regulate CCL2 with evidence for involvement of an IL-6–sIL-6Rα–gp130 signal transduction pathway 38. High spontaneous expression of IL-6 in breast-tumor derived fibroblasts and reduced CCL2 release in TAF monolayer cultures following blockade of endogenous IL-6 in the present study indicate that this autocrine loop is a general mechanism with particular relevance in tumor stroma, which should be considered as potential stroma-related therapeutic target in desmoplastic tumors.
3.5 The role of fibroblast-derived cytokines/chemokines in tumors
While only a small panel of cytokines in supernatants of tumor-derived fibroblast has been analyzed systematically, not only CCL2 but also IL-6, an anti-inflammatory cytokine with potential pro-inflammatory properties 39, showed stronger expression in TAF as opposed to normal fibroblasts. This ‘abnormal’ cytokine profile well fits into the concept of fibroblasts as immune-regulating ‘sentinel cells’ in tumors. IL-6 is described to promote differentiation of CD14+ MO to CD14+CD1a– MΦ accompanied by inhibition of thedendritic cell differentiation pathway 40. However, this effect is antagonized by TNF–α, IL-1β, CD40 ligand, and TGF-β1, some of which may also be produced by TAF. Accordingly, besides recruitment of leukocytes, the role of increased CCL2 expression in breast tumor stromal fibroblasts is hard to predict. CCL2 is known to affect expression of adhesion molecules and cytokines in MO, thus altering their activation status 41, and it also displays a variety of other functions relevant in tumor progression, e.g. its angiogenic potential 5, 42. CCL2 is discussed in tissue fibrosis 43, 44, where it enhances fibroblast synthesis of TGF-β1 and collagen 45 but also of TIMP-1 and MMP-1 46. We therefore intent to prove if IL-6-regulated CCL2 expression in tumor-derived fibroblasts affects TGF-β release or activation associated with stimulation in collagen type I production. This regulatory pathway may essentially contribute to: (a) the establishment of a TGF-β(1) induced desmoplastic reaction, (b) alterations in ECM and ECM-modulating molecules that contribute to an invasion-promoting stromal environment, and (c) an immune suppression in tumor-associated immune cells.
4 Materials and methods
4.1 Fibroblast isolation and routine culturing
Primary fibroblasts from normal breast (PFN2), and invasive ductal breast carcinomas (PF1, PF27, PF28, PF32, PF33, PF36, PF37) were cultured from fresh resected breast reduction or tumor surgery specimens as described earlier 17. In brief, biopsy material was washed, sliced into 1–4-mm3 sections, transferred into culture flasks, and covered with supplemented DMEM after attachment (medium supplements: 20% FCS, 25 mM glucose, 1% sodium pyruvate, 1% L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin; Sigma-Aldrich, Steinheim or PAN Biotech, Aidenbach, Germany). After sufficient outgrowth in a humidified atmosphere (5% CO2 in air, 37°C) fragments were removed, cells were enzymatically harvested (0.05% trypsin/0.02% EDTA in PBS, PAN Biotech), and transferred into DMEM with reduced glucose (5 mM) and serum (10%). Stock cultures of morphologically and immunohistochemically characterized fibroblasts 17] were frozen in liquid N2 using 90% FCS and 10% DMSO. Fibroblasts outgrown from skin of healthy adult donors according to an analogous protocol (N1, Kaufmann) were kindly provided by the Department of Clinical Chemistry, University of Regensburg. All fibroblast types were subsequently recultured from frozen stocks in complete DMEM with 10% FCS. For preparation of single-cell suspensions subconfluent monolayers were enzymatically dissociated and cell counts were determined with a CASY1 cell counter (Schärfe System, Reutlingen, Germany). Effects caused by cell aging/senescence were avoided using fibroblasts with cumulative population doublings of 50 or less.
4.2 Monocyte isolation and culturing
Peripheral blood mononuclear cells (MNC) from healthy donors were obtained by leukapheresis followed by density gradient centrifugation over Ficoll/Hypaque. MO were isolated from MNC by countercurrent centrifugal elutriation in a J6M-E centrifuge (Beckmann, Munich, Germany) using a large-volume chamber (50 ml), a JE-5 rotor at 2,500 rpm, and a flow rate of 111 ml/min in Hanks' balanced salt solution supplemented with 8% autologous human serum. Purity of elutriated MO (≥90%) was determined by morphological criteria and immune phenotyping (for details see 47).
4.3 Spheroid mono- and co-culture
Fibroblast spheroids were cultured in liquid overlay (e.g. 15, 17) using agarose-coated 96-well plates (100 μl of 1.5% agarose/well, Sigma Aldrich) and single-cell suspensions from subconfluent fibroblast monolayer cultures. For MCS initiation, 5×103 skin fibroblasts or 4×103 breast fibroblasts were inoculated per well in 200 μl supplemented DMEM (10% FCS). After 4 days, spheroids were 350–400 μm in diameter, consisted of ≈1.5×102–2×102 cells, and showed no central necroses. Fibroblast MCS were co-cultured with MO by replacing 100 μl of the supernatant with 100 μl of a MO suspension (1×104 MO per spheroid) at day 4 in culture. Each experiment was carried out with MO from 3 or more donors. In a separate set of experiments different MO concentrations from the same donor were added (1×103–1.6×105 MO per spheroid). MCS were harvested after defined times in co-culture (24 or 40 h), washed carefully with PBS (6×) to remove non-migrated MO from the surface, and processed for immunohistochemistry or flow cytometric analysis.
4.4 Immunohistochemistry
Spheroid co-cultures and respective fibroblasts MCS controls were shock-frozen in liquid N2 using Jung tissue embedding medium (Leica, Nussloch, Germany) and stored at –80°C. Embeddedmaterial was serially sliced into 5–6-μm cryostat sections, mounted on glass slides coated with poly-L-lysine (Sigma, Deisenhofen, Germany) and fixed in ice-cold acetone for 15–20 min.Sections were air-dried and eventually stored over night at –20°C. Immunohistochemistry was performed according to either a standard alkaline phosphatase anti-alkaline phosphatase (APAAP) technique(monoclonal mouse IgG, DAKO) and Fast red chromogen (Biogenex, San Ramon, USA) for detection or a peroxidase-DAB method. Samples were routinely counter-stained with hematoxylin. Migrated MO were identified with primary mouse anti-human mAb against CD11c (clone BU15, Immunotech, Prague, Czech Rep., 1:20, working concentration: 10 μg/ml) and CD14 (Beckman Coulter; 1:20, working concentration: 5 μg/ml).
4.5 Flow cytometric analysis
Spheroid co-cultures were dissociated by incubation with a 0.25% trypsin/0.1% EDTA solution in PBS at 37°C for 2 min and mechanic means (gentle pipetting). Enzyme activity was stopped by addition of supplemented DMEM. Cell concentrations in single-cell suspensions were determined as described above and the number of cells/spheroid were documented. Cells were washed twice in PBS containing 1% FCS and 0.25% sodium azide (wash buffer), incubated with a FITC-conjugated ‘fibroblast-specific’ antibody (AS02-FITC, Dianova, Hamburg, Germany, 1:5, working concentration: 40 μg/ml; 48) for 30 min at 4° C, washed again, and analyzed on a FACSCalibur (Becton Dickinson, San José, CA) with laser excitation of 488 nm. Forward scatter (FSC), side scatter (SSC), and FL-1 fluorescence signals were recorded. Regions/gates were defined in the SSC vs. FL-1 dot plot diagrams for MO–fibroblasts discrimination.
4.6 Cytokine release from fibroblasts
Supernatants of fibroblast MCS were collected at day four in culture, i.e. 96 h after initiation, filtered through a 0.22-μm filter, aliquoted, and stored at –80°C. Spheroids werecounted, enzymatically dissociated after medium removal, and cell counts per spheroid were determined as described above. Concentrations of IL-6, CCL2 (MCP-1), M-CSF and GM-CSF in supernatants weredetermined via commercial ELISA kits according to the manufacturer's instructions (IL-6: Coulter-Immunotech, Krefeld; MCP-1: Biozol, Eching; M-CSF and GM-CSF: R&D Systems, Wiesbaden, Germany).The cytokine release rates (96 h) were measured in pg/ml and referred to cell number per spheroid. Supernatants were also collected from subconfluent and confluent monolayer cultures after an incubation time of 96 h. Here, CCL2 and IL-6 contents were recorded relative to the number of cells at the time of medium removal.
4.7 Neutralization of endogenous IL-6
Neutralization experiments were performed exclusively with fibroblast monolayer cultures to guarantee homogenous distribution of neutralizing antibody. Tumor-derived PF32 fibroblasts (3.2×103 in 200 μl supplemented DMEM per well) were seeded in 96-well plates. After 72 h, medium was removed, cells were washed with PBS (3×), and 200 μl complete DMEM containing neutralizing mouse anti-human-IL-6 mAb (R&D Systems) were added. Three antibody concentrations were applied: 50 ng/ml, 500 ng/ml, 1 μg/ml; a mouse IgG1 isotype control (1 μg/ml, R&D Systems) and antibody-free, complete DMEM served as negative controls. After 24 h, supernatants were collected, processed, and stored as described above. Three independent experiments were performed. CCL2 concentrations were determined (pg/ml) using commercial ELISA kits (R&D Systems).
4.8 Neutralization of endogenous CCL2
Conditioned media collected from spheroids of two tumor-derived fibroblast types (PF27, PF32) was applied in a Boyden chamber assay (48-well, Neuroprobe, Bethesda, MA) with a polycarbonate membrane (pore size: 5 μm; Osmonics Inc., Minnetonka, MN). Conditioned media (27 μl) with or without CCL2 neutralizing mAb (clone 24822.111, R&D Systems; concentration range: 0.1 ng/ml–10 μg/ml) were placed into the wells of the lower chamber; 5×104 MO in 50 μl fresh, supplemented DMEM were added to each well of the upper chamber. N-Formyl-L-methionyl-leucyl-L-phenylalanine (FMLP, Sigma, Taufkirchen, Germany; 0.01–1 μM in DMEM) served as positive, unconditioned DMEM as negative control. Isotype controls (10 μg/ml, R&D Systems) do notaffect MO migration. The Boyden chamber was incubated for 2 h under standard culture conditions. After careful removal of non-migrated cells, membranes were air-dried, fixed in 70% MeOH, and stained with the Hemacolor staining kit (Merck, Darmstadt, Germany). Membranes were transferred onto glass slides and migrated MO were counted microscopically in four high-power fields (magnification ×400) per well. A minimum of three wells per condition was analyzed. Each experiment was carried out with MO from three different healthy donors.
For potential neutralization of CCL2 in spheroid co-cultures, fibroblast spheroids (PF32, PF27) were initiated according to the protocol in Sect. 4.3. For each condition 48–96 spheroids were prepared. After 96 h, 100 μl of the medium were replaced by fresh supplemented DMEM with or without neutralizing CCL2 antibody (final concentration range: 1–25 μg/ml) or with an IgG1 isotype control (final concentration: 25 μg/ml). After a pre-incubation interval of 2 h, 100 μl of the supernatant of each well and experimental condition were collected and used for the preparationof MO suspensions with a concentration of 1×105 MO/ml. Of this MO suspension, 100 μl was then re-added per well and spheroid. After 40 h of incubation, co-cultures were processedand analyzed by flow cytometry according to Sect. 4.6.
Acknowledgements
The technical assistance of Mr. Frank van Rey and Mrs. Marit Hoffmann is gratefully acknowledged. Deutsche Forschungsgemeinschaft (grants Ku 917/2–1 to 2–4)and Bayerisches Staatsministerium für Wissenschaft, Forschung und Kunst.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




