Effect of eplerenone in percutaneous coronary intervention-treated post-myocardial infarction patients with left ventricular systolic dysfunction: a subanalysis of the EPHESUS trial
Abstract
Aims
EPHESUS was a multicentre, double-blind clinical trial in which 6632 patients with acute myocardial infarction (AMI) complicated by LV systolic dysfunction (LVSD) were randomized to receive eplerenone (n = 3319) or placebo (n = 3313). A total of 1580 EPHESUS patients were treated with PCI, which is now the standard treatment for AMI. This EPHESUS substudy examined the effects of eplerenone upon cardiovascular outcomes in PCI-treated patients.
Methods and results
EPHESUS patients were divided into PCI-treated and non-PCI-treated cohorts, and the effect of eplerenone upon mortality and other major adverse cardiovascular outcomes was assessed in each cohort. The PCI-treated patients (n = 1580) were younger, and had better renal function and fewer co-morbidities than non-PCI-treated patients (n = 5052). Cardiovascular mortality was significantly lower in PCI-treated patients as compared with non-PCI-treated patients (7% vs. 16%, P < 0.0001). However, the incidence of non-fatal events was similar in PCI-treated and non-PCI-treated cohorts. There was no statistical difference between the PCI-treated and non-PCI-treated cohorts in the primary or secondary outcomes of the trial. Eplerenone administration, compared with placebo, in the PCI-treated cohort did not affect PCI-related clinical outcomes, including recurrence of angina, the occurrence of acute coronary syndromes, or the need for further revascularization.
Conclusions
The beneficial effects of eplerenone in the EPHESUS trial exist for both PCI- and non-PCI-treated AMI patients with LVSD. Eplerenone has minimal, if any, effect upon reducing PCI-related adverse events in the PCI-treated cohort.
Introduction
Acute myocardial infarction (AMI) and consequent LV systolic dysfunction (LVSD) are the leading causes of morbidity and mortality. Mineralocorticoid receptor (MR) antagonism with eplerenone has been shown to improve survival and reduce hospitalization due to cardiovascular events in patients with AMI and LVSD in the landmark EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) trial.1 In this trial, a mean dose of 43 mg of eplerenone produced a significant reduction in all-cause and cardiovascular mortality compared with placebo.1 National and international guidelines therefore strongly recommend using MR antagonists for patients with post-infarct LVSD.2, 3
In contemporary practice, patients with AMI are usually treated with PCI, involving balloon angioplasty and stent implantation.4, 5 However, only a quarter of patients in the EPHESUS trial received PCI for treatment of AMI while others were treated with thrombolysis, coronary artery bypass grafting (CABG), or conservative medical treatment.1 Therefore, application of the EPHESUS data to current practice can be questioned. We aimed to evaluate the impact of eplerenone administration in heart failure among patients managed with PCI.
Experimental data have suggested that eplerenone may improve the PCI outcomes by reducing neointimal proliferation6 and accelerating endothelial regeneration.7, 8 While pre-clinical studies support these hypotheses,9-11 there has been no study investigating this potential benefit of eplerenone in humans. Although the EPHESUS trial did not have a pre-specified aim to examine PCI outcomes, and there was no routine angiographic follow-up, surrogate clinical markers were systematically recorded. The second objective of this study was to evaluate the impact of eplerenone upon PCI-related adverse clinical outcomes, including recurrence of angina, the occurrence of acute coronary syndromes, and the need for repeat revascularization.
Methods
EPHESUS trial
EPHESUS was a large multicentre, double-blind randomized controlled clinical trial that assessed the impact of eplerenone upon clinical outcomes in patients with LVSD after AMI.1 Patients (n = 6632) were randomized to receive eplerenone (n = 3319) or placebo (n = 3313). Eplerenone was initiated at 25 mg/day at ∼7 days after AMI and titrated at 4 weeks to 50 mg/day, if serum potassium was <5 mmol/L. About 24% patients in the EPHESUS trial received PCI as a treatment of AMI while others were treated with thrombolysis (27%), CABG (1%), or conservative medical treatment (48%). Patients were followed-up for an average of 16 months.
EPHESUS PCI substudy
This substudy evaluated the effect of eplerenone administration in the PCI-treated subgroup of patients in the EPHESUS trial. We analysed the PCI-treated (n = 1580) vs. non-PCI-treated (n = 5052) patients. Furthermore, we compared the baseline characteristics and clinical outcomes in PCI-treated patients in the eplerenone- (n = 799) and placebo- (n = 781) treated groups.
Statistical analysis
Data are presented as mean ± SD or proportion and percentage, as indicated. Continuous variables were analysed using the Mann–Whitney test and categorical variables with the χ2 test. Multivariate analyses were performed using the Cox regression method. Hazard ratios (HRs) along with the 95% confidence interval (CI) are presented. P-values <0.05 were considered significant. All statistical analyses were performed using SAS® 9.2 software (SAS Institute, Cary, NC, USA).
Results
The EPHESUS PCI cohort is different from the non-PCI cohort
The baseline demographic and clinical characteristics of PCI-treated (n = 1580) and non-PCI-treated (n = 5052) patients in the EPHESUS trial are shown in Table 1. The PCI-treated patients were younger, and had better renal function, fewer co-morbidities, and higher prescription of evidence-based medication including beta-blockers, ACE inhibitors, and statins. Cardiovascular mortality was significantly lower in PCI-treated patients as compared with non-PCI-treated patients (7% vs. 16%, P < 0.0001), but the incidence of non-fatal events was similar in the two groups (Table 1).
| Characteristic | Non-PCI treated, n = 5052 (76%) | PCI treated, n = 1580 (24%) | P-value | ||
|---|---|---|---|---|---|
| n | Mean ± SD or n (%) | n | Mean ± SD or n (%) | ||
| Demographics | |||||
| Age (years) | 5052 | 65 ± 11 | 1580 | 61 ± 12 | <0.0001 |
| Caucasian | 4589 (91%) | 1395 (88%) | 0.003 | ||
| Male | 3491 (69%) | 1223 (77%) | <0.0001 | ||
| Time from AMI to randomization (days) | 5051 | 7.4 ± 2.9 | 1580 | 6.7 ± 3.0 | <0.0001 |
| Clinical | |||||
| Blood pressure (mmHg) | |||||
| Systolic | 5050 | 120 ± 17 | 1580 | 116 ± 16 | <0.0001 |
| Diastolic | 5050 | 73 ± 11 | 1580 | 70 ± 11 | <0.0001 |
| LV ejection fraction (%) | 5041 | 33 ± 6 | 1576 | 33 ± 6 | 0.85 |
| Previous hospitalization for HF | 452 (9%) | 60 (4%) | <0.0001 | ||
| Symptoms of heart failure | 4347 (87%) | 1232 (79%) | <0.0001 | ||
| Potassium (mmol/L) | 5029 | 4.28 ± 0.45 | 1566 | 4.21 ± 0.43 | <0.0001 |
| Creatinine (µmol/L) | 5026 | 102 ± 29 | 1558 | 95 ± 25 | <0.0001 |
| eGFR (MDRD, mL/min/1.73 m2) | 4888 | 67 ± 19 | 1493 | 74 ± 19 | <0.0001 |
| Medical history | 5052 | 1580 | |||
| Previous MI | 1467 (29%) | 336 (21%) | <0.0001 | ||
| Diabetes mellitus | 1658 (33%) | 484 (31%) | 0.10 | ||
| Heart failure | 853 (17%) | 122 (8%) | <0.0001 | ||
| Hypertension | 3159 (63%) | 848 (54%) | <0.0001 | ||
| Medications | 5052 | 1580 | |||
| ACEI/ARB | 4318 (85%) | 1433 (91%) | < 0.0001 | ||
| Beta-blockers | 3641 (72%) | 1320 (84%) | < 0.0001 | ||
| Diuretics | 3172 (63%) | 812 (51%) | < 0.0001 | ||
| Aspirin | 4377 (87%) | 1493 (94%) | < 0.0001 | ||
| Anticoagulants | 934 (18%) | 174 (11%) | < 0.0001 | ||
| Statins | 2026 (40%) | 1069 (68%) | < 0.0001 | ||
| Outcomes | 5052 | 1580 | |||
| Cardiovascular mortality | 784 (16%) | 106 (7%) | <0.0001 | ||
| Non-fatal events (ACS/revascularization) | 1073 (21%) | 339 (21%) | 0.85 | ||
- ACEI, ACE inhibitor, ACS, acute coronary syndrome; AMI, acute myocardial infarction; eGFR, estimated glomerular filtration rate [four-variable Modification of Diabetes in Renal Disease (MDRD) formula]; HF, heart failure; PCI, percutaneous coronary intervention.
Eplerenone improves outcomes in both the PCI-treated and the non-PCI-treated cohort
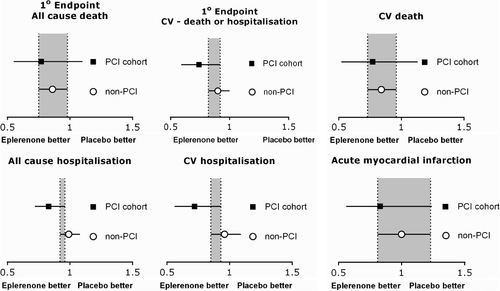
Eplerenone treatment was similarly effective in reducing mortality, cardiovascular events, and hospitalization in both PCI-treated and non-PCI-treated AMI patients with LVSD (Figure 1). On multivariate Cox regression analysis, with eplerenone and PCI as co-variables, both PCI and eplerenone administration were independently associated with better clinical outcomes (Table 2).
| Eplerenone, yes vs. no | PCI, yes vs. no | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Primary outcomes | ||||
| All-cause death | 0.85 (0.75–0.96) | 0.008 | 0.42 (0.35–0.51) | <0.0001 |
| Cardiovascular death or hospitalization | 0.87 (0.80–0.95) | 0.003 | 0.62 (0.55–0.70) | <0.0001 |
| Secondary outcomes | ||||
| All-cause death or any hospitalization | 0.92 (0.86–0.99) | 0.017 | 0.91 (0.84–0.99) | 0.022 |
| Cardiovascular death | 0.83 (0.73–0.95) | 0.005 | 0.41 (0.33–0.50) | <0.0001 |
| Sudden death from cardiac causes | 0.79 (0.64–0.97) | 0.027 | 0.49 (0.36–0.65) | <0.0001 |
| Acute myocardial infarction | 0.82 (0.61–1.11) | 0.20 | 0.34 (0.20–0.56) | <0.0001 |
| Heart failure | 0.80 (0.62–1.04) | 0.099 | 0.35 (0.23 – 0.53) | <0.0001 |
| Stroke | 0.91 (0.54–1.56) | 0.73 | 0.37 (0.16–0.87) | 0.023 |
| Any hospitalization | 0.95 (0.89– 1.02) | 0.18 | 1.00 (0.92–1.09) | 1.00 |
| Cardiovascular hospitalization | 0.91 (0.82– 1.02) | 0.098 | 0.73 (0.64–0.84) | <0.0001 |
| Acute myocardial infarction | 0.96 (0.80–1.16) | 0.67 | 0.81 (0.65–1.02) | 0.069 |
| Heart failure | 0.86 (0.75–0.98) | 0.029 | 0.67 (0.56–0.80) | <0.0001 |
| Stroke | 1.18 (0.87–1.62) | 0.29 | 0.40 (0.25–0.64) | 0.0002 |
| Ventricular arrhythmia | 0.95 (0.65–1.39) | 0.79 | 0.62 (0.37–1.03) | 0.066 |
| Acute myocardial infarction or unstable angina | 1.01 (0.89–1.14) | 0.92 | 0.96 (0.83–1.11) | 0.60 |
- CI, confidence interval; HR, hazard ratio; PCI, percutaneous coronary intervention.
Baseline characteristics of the EPHESUS PCI cohort
The baseline characteristics of the EPHESUS PCI cohort treated with placebo or eplerenone were similar (Table 3). The mean age of the patients was 60 years, with 77% male patients. The two groups were similar in terms of age, gender, ethnicity, LV function, co-morbidities, and prescription of medication at discharge (Table 3). There was no significant difference in adverse events between the eplerenone- or placebo-treated EPHESUS PCI cohort: hyperkalaemia (2.4% vs. 3.3%, P = 0.33), hypokalaemia (1.7% vs. 0.8%, P = 0.10), gynaecomastia in men (0.6% vs. 0.6%, P = 1.00), and renal dysfunction (3.2% vs. 2.0%, P = 0.13).
| Characteristic | Placebo, n = 781 (49%) | Eplerenone, n = 799 (51%) | P-value | ||
|---|---|---|---|---|---|
| n | Mean ± SD or n (%) | n | Mean ± SD or n (%) | ||
| Age (years) | 781 | 61 ± 12 | 799 | 60 ± 11 | 0.05 |
| Caucasian | 688 (88%) | 707 (88%) | 0.81 | ||
| Male gender | 591 (76%) | 632 (79%) | 0.10 | ||
| Blood pressure (mmHg) | |||||
| Systolic | 781 | 116 ± 16 | 799 | 115 ± 15 | 0.43 |
| Diastolic | 781 | 70 ± 11 | 799 | 70 ± 10 | 0.65 |
| LV ejection fraction (%) | 779 | 33 ± 6 | 797 | 33 ± 6 | 0.83 |
| Time from AMI to randomization (days) | 781 | 6.6 ± 3.0 | 799 | 6.8 ± 3.0 | 0.23 |
| Previous hospitalization for HF | 34 (4%) | 26 (3%) | 0.25 | ||
| Symptoms of heart failure | 615 (79%) | 617 (78%) | 0.37 | ||
| Potassium (mmol/L) | 771 | 4.21 ± 0.43 | 795 | 4.21 ± 0.43 | 0.85 |
| Creatinine (µmol/L) | 768 | 94 ± 25 | 790 | 95 ± 25 | 0.23 |
| eGFR (MDRD, mL/min/1.73 m2) | 737 | 74 ± 19 | 756 | 73 ± 19 | 0.45 |
| Medical history | 781 | 799 | |||
| Previous MI | 161 (21%) | 175 (22%) | 0.53 | ||
| Diabetes mellitus | 240 (31%) | 244 (31%) | 0.93 | ||
| Heart failure | 66 (8%) | 56 (7%) | 0.28 | ||
| Hypertension | 423 (54%) | 425 (53%) | 0.70 | ||
| Medications | 781 | 799 | |||
| ACEI/ARB | 708 (91%) | 725 (91%) | 0.95 | ||
| Beta-blockers | 648 (83%) | 672 (84%) | 0.54 | ||
| Diuretics | 406 (52%) | 406 (51%) | 0.64 | ||
| Aspirin | 739 (95%) | 754 (94%) | 0.82 | ||
| Anticoagulant agents | 82 (10%) | 92 (12%) | 0.52 | ||
| Statins | 537 (69%) | 532 (67%) | 0.36 | ||
- Symptoms of heart failure: Killip's class > I.
- ACEI, ACE inhibitor, AMI, acute myocardial infarction; eGFR, estimated glomerular filtration rate [four-variable Modification of Diabetes in Renal Disease (MDRD) formula]; HF, heart failure; PCI, percutaneous coronary intervention.
Eplerenone does not affect PCI-related clinical outcomes
Eplerenone, compared with placebo, in the PCI cohort did not impact upon the incidence of sudden cardiac death, non-fatal MI, or stroke (Table 4). Although there was a statistically significant reduction in events related to heart failure and hospital admissions, there was no effect upon PCI-related adverse outcomes, including recurrence of angina pectoris, unstable angina, AMI, or need for further revascularization (Table 4).
| Eplerenone (n = 799) | Placebo (n = 781) | HR (95% CI) | P-value | |
|---|---|---|---|---|
| Repeat revascularization (PCI/CABG) | 51 (6%) | 44 (6%) | 1.13 (0.75–1.69) | 0.56 |
| Angina pectoris | 24 (3%) | 24 (3%) | 0.97 (0.55–1.70) | 0.90 |
| Unstable angina | 84 (11%) | 82 (10%) | 1.00 (0.73–1.35) | 0.98 |
| Acute myocardial infarction | 45 (6%) | 52 (7%) | 0.83 (0.56–1.24) | 0.37 |
| Sudden cardiac death | 21 (3%) | 30 (4%) | 0.67 (0.39–1.17) | 0.16 |
| Heart failure | 60 (8%) | 86 (11%) | 0.66 (0.47–0.91) | 0.012 |
| Cardiovascular hospitalization | 108 (14%) | 140 (18%) | 0.72 (0.56–0.93) | 0.011 |
- CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio.
Discussion
This substudy analysis of the EPHESUS trial has highlighted that eplerenone is effective in patients with AMI and LVSD whether treated with or without PCI. However, there is no evidence that eplerenone has any impact upon PCI-related outcomes including recurrence of angina, AMI, and repeat revascularization.
Eplerenone improved outcomes in both the PCI-treated and non-PCI-treated population
Mineralocorticoid receptor antagonists are strongly recommended for patients with LVSD and symptoms of heart failure,12, 13 based on morbidity and mortality benefits seen in three landmark trials: RALES, EPHESUS, and EMPHASIS-HF.1, 14, 15 The RALES trial consisted of patients with advanced heart failure, the EPHESUS trial included patients with LVSD after AMI, and the EMPHASIS-HF trial enrolled patients with heart failure and mild (NYHA class II) symptoms.1, 14-16 In RALES, spironolactone produced 30% relative reduction in mortality during an average follow-up of 24 months.14 In EMPHASIS-HF, eplerenone was associated with a 24% reduction in cardiovascular death and a 42% reduction in hospitalization for heart failure.15 Only the EPHESUS trial specifically examined patients with LVSD after an AMI and showed that eplerenone produced 15% reduction in all-cause mortality, 17% reduction in cardiovascular mortality, and 21% reduction in sudden cardiac death.1 In contemporary practice, most patients with AMI receive PCI treatment.4 Our data show that eplerenone confers similar benefit in patients with AMI and LVSD whether or not they are treated with PCI. The outcomes observed in the PCI subgroup were similar to those reported in the full EPHESUS cohort.1 Our analysis shows that PCI itself was associated with a major reduction in adverse outcomes in AMI patients, consistent with numerous other reports.17, 18 However, eplerenone administration was also an independent factor associated with improved outcomes, emphasizing the importance of MR antagonism in LVSD after AMI, including patients treated with primary PCI. Therefore, prescribing eplerenone to patients with AMI and LVSD remains beneficial in patients treated with and without PCI.
Whilst the EPHESUS trial included patients with all forms of AMI [unstable angina, non-ST segment elevation myocardial infarcion (NSTEMI), and STEMI] and there was a delay in commencing eplerenone, a recently reported clinical trial (REMINDER: impact of eplerenone on cardiovascular outcomes in patients post myocardial infarction, NCT-01176968) has shown that early use of eplerenone can improve the outcome of patients presenting with acute STEMI without heart failure.19 Over 1000 patients were randomized (n = 506/group) to initiation of eplerenone or placebo within 12–24 h of STEMI. Eplerenone 25 or 50 mg/day (89% patients received 50 mg/day) produced a significant reduction (HR 0.58, 95% CI 0.45–0.75, P < 0.0001) in the primary endpoint (a composite of cardiovascular mortality, ventricular arrhythmia, clinical or subclinical heart failure at 1 month) at a mean follow-up of 10.5 months.19 ALBATROSS (Aldosterone blockade early after acute myocardial infarction; NCT-01059136) is an ongoing multicentre, open-labelled, randomized trial to assess the effects of MR blockade with a 200 mg i.v. bolus of potassium canrenoate followed by 25 mg/day spironolactone for 6 months in 1600 patients with STEMI or high risk NSTEMI.20
Eplerenone did not affect PCI-related outcomes
Mineralocorticoid receptors are widely distributed in the vascular system, including endothelial cells, endothelial progenitor cells (EPCs), vascular smooth muscle cells (VSMCs), and intact vessels.8, 21, 22 These receptors also influence vascular function and remodelling;6, 23 MR activation has been shown to increase proliferation of VSMCs6 and to impair differentiation and proliferation of EPCs.7 These characteristics suggest that MR activation is a potential stimulus for neointimal proliferation and restenosis. In humans, elevated baseline plasma aldosterone levels directly correlate with risk of restenosis24 and inversely correlate with circulating EPCs and endothelial colony-forming units.8 Mineralocorticoid receptor antagonists reduce atherosclerosis,23, 25 prevent adverse vascular remodelling,26 and attenuate post-angioplasty neointimal proliferation in experimental animals.9, 11, 27
Spironolactone has been shown to inhibit neointimal proliferation after balloon angioplasty of iliac arteries in rabbits.27 However, these results could not be reproduced in a porcine coronary angioplasty model,9, 28 which is the gold standard pre-clinical model for restenosis. Additionally, a single-centre clinical trial failed to show any effect of spironolactone in reducing restenosis.29 Nevertheless, promising experimental data have been obtained with eplerenone.9-11 Our data suggest that, like spironolactone, eplerenone does not appear to affect PCI-related clinical outcomes. Therefore, eplerenone cannot currently be recommended for patients undergoing PCI without co-existing AMI or LVSD.
Study limitations
This study has several limitations. It is a post-hoc analysis with inherent shortcomings of any such study. Patients in the EPHESUS PCI cohort did not have routine angiographic follow-up to document any effect of eplerenone on angiographic restenosis; however, our assessment of clinical events is perhaps more relevant than angiographic outcomes.
Conclusion
The beneficial effects of eplerenone on heart failure events and hospitalization seen in the EPHESUS trial are similar for both PCI-treated and non-PCI-treated AMI patients with LVSD. There is no evidence that eplerenone reduces the risk of recurrent ischaemia-related events including recurrence of angina or the need for repeat revascularization. Use of eplerenone is strongly recommended in AMI patients with LVSD.
Funding
The EPHESUS trial was funded by Pharmacia/Pfizer.
Conflict of interest: I.S. has received grants and honoraria from Novartis, Pfizer, Vifor, and Jansen. B.P. has received honoraria from Pfizer and serves on the advisory boards for Pfizer and Novartis. F.Z. has received honoraria from Pfizer, Novartis, Roche, Servier, AstraZeneca, and Takeda. All other authors have no conflicts to declare.




