Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study
Abstract
Aims
Patients with advanced cancer have been shown to suffer from abnormal cardiac function and impaired exercise capacity that may contribute to their impaired quality of life. As tachycardia is considered as a sign of potential early cardiac damage, we sought to determine whether resting heart rate and other ECG-derived variables have prognostic value.
Methods and results
From 2005 to 2010, we enrolled 145 patients with histologically confirmed cancer (36 colorectal, 72 pancreatic, and 37 non-small cell lung cancer patients) and 59 healthy controls. During a mean follow-up of 27 months, 82 patients (57%) died from any cause. The mean resting heart rate of healthy subjects was 70 ± 13 b.p.m., and that of cancer patients was 79 ± 14 b.p.m. (P< 0.0001). As a sensitivity analysis, we excluded control subjects taking a beta-blocker, but resting heart rate remained increased in cancer patients vs. controls (P < 0.0001). Resting heart rate ≥75 b.p.m. [hazard ratio (HR) 1.84, 95% confidence interval (CI) 1.16–2.94; P = 0.01] significantly predicted survival in univariable analyses and remained an independent predictor of survival in a multivariate model (HR 1.67, 95% CI 1.01–2.78; P = 0.04). Furthermore, the heart rate stayed significant in a second model that included age and sex as well.
Conclusion
The present study is the first to show that resting heart rate independently of haemoglobin and tumour stage predicts survival in patients with advanced colorectal, pancreatic, and non-small cell lung cancer, and may therefore represent a therapeutic target.
Introduction
Worldwide, >2.4 million cases of colorectal, pancreatic, and lung cancer are diagnosed each year, accounting for 24% of all tumour diagnoses (excluding skin cancer).1 Even though mortality rates in colorectal cancer have started to improve in the last decade,2 many people still have a vastly reduced life expectancy. One-year mortality rates in colorectal, lung, and pancreatic cancer patients have been estimated as 24, 68, and 79%, respectively.3 In addition, high mortality rates and patients' symptoms materially affect the course of the disease. Dyspnoea, fatigue, weakness, lack of energy, pain, and early satiety each occur in >50% of patients with advanced cancer and reduce patients' quality of life, and may impact on their ability to tolerate effective cancer therapy.4
An important question that has arisen over the last years is whether the heart and the vasculature play a role in both symptom development and high mortality rates of patients with advanced cancer. Indeed, considerable overlap has been reported between the symptoms of patients with heart failure and those with advanced cancer,5 suggesting that research into this field is promising. Previous work from our group has shown that patients with colorectal cancer demonstrate reduced exercise capacity as assessed by spiroergometry and that mildly, but significantly reduced LVEF, reduced heart rate variability, and reduced lean mass may be pathophysiologically important.6 In addition, cancer patients tend to present with higher cardiac output and a maximal rate of pressure rise during an isovolumic contraction than healthy control subjects, possibly adding additional stress to cardiac function in cancer.7 In patients with cardiovascular disease, we know that an elevated resting heart rate is a valid marker of all-cause mortality8, 9 as well as for outcomes in cardiac rehabilitation.10 Furthermore, a higher heart rate in patients with increased cardiovascular risk predicts worsening of renal function11 and cognitive decline.12 With high mortality and morbidity rates among patients with advanced colorectal, lung, and pancreatic cancer, cardiovascular perturbations may, if proven relevant, provide a novel point of intervention, which necessitates a thorough understanding of the respective pathophysiology.
Using data from three prospectively recruited cohorts of patients with the aforementioned cancers, we hypothesized that resting heart rate is elevated in these patients and that elevated heart rate is associated with poor survival.
Methods
Patient recruitment
Between February 2005 and April 2010, we recruited patients with colorectal cancer (n = 37) or pancreatic cancer (n = 91) from the Department of Haematology and Oncology, and patients with non-small cell lung cancer (NSCLC) (n = 41) from the Department of Pneumology and Infections, both at Charité Medical School, Campus Virchow-Klinikum, Berlin, Germany. Of 169 consecutively enrolled cancer patients and 73 healthy control subjects, a total of 145 cancer patients and 59 controls underwent our specific resting ECG protocol with 10 min supine rest and the specific Welch Allyn ECG module and were included in this analysis.
Only patients with a histologically confirmed diagnosis were eligible for enrolment. Exclusion criteria included: (i) another cancer diagnosis in the past 5 years; (ii) <18 years old; (iii) known inflammatory disease or signs of ongoing infection; (iv) COPD; (v) life expectancy of <2 months; and (vi) clinical signs or symptoms of heart failure or other significant cardiovascular diseases except hypertension.
Our cardiovascular investigation in cancer patients and control subjects included a medical examination, a resting ECG, and an exercise test to exclude ischaemia. The ECGs were conducted with the ‘CardioPerfect’ software (Welch Allyn, Skaneateles, New York, USA). Patients had to be on stable medication for at least 4 weeks before being studied.
The patients' chemotherapy was administered throughout the study as scheduled before inclusion. The patients were followed up prospectively by regular interrogation of electronic hospital databases. Control subjects were recruited intermittently from the medical staff at the Charité Medical School and from the patients' relatives, and we aimed for a ratio of cancer patients to controls of three to one. The controls were allowed antidiabetic medication or antihypertensive drugs. No controls with known cardiac ischaemia were included. Table 1 lists the baseline medication of both cancer patients and control subjects. Our study complies with the Declaration of Helsinki, written informed consent was given by all subjects, and the study was approved by the local ethics committee.
| Measurement | Controls (n = 59) | Cancer patients (n = 145) | Colorectal cancer (n = 36) | NSCLC (n = 37) | Pancreatic cancer (n = 72) |
|---|---|---|---|---|---|
| Aspirin, n (%) | 0 | 11 (8) | 3 (8) | 7 (19) | 1 (1) |
| ACE inhibitor or ARB, n (%) | 8 (14) | 33 (23) | 9 (25) | 15 (41) | 9 (13) |
| Beta-blocker, n (%) | 7 (12) | 25 (17) | 4 (11) | 14 (38) | 7 (10) |
| Spironolactone, n (%) | 0 | 2 (1) | 1 (3) | 1 (3) | 0 |
| Diuretics, n (%) | 0 | 15 (10) | 3 (8) | 11 (30) | 1 (1) |
| Anticoagulants, n (%) | 0 | 18 (12) | 1 (3) | 4 (11) | 13 (18) |
| Lipid-lowering drugs, n (%) | 7 (12) | 16 (11) | 3 (8) | 9 (24) | 4 (6) |
| Antidiabetics, n (%) | 0 | 4 (3) | 3 (8) | 1 (3) | 0 |
| Insulin, n (%) | 0 | 6 (4) | 3 (8) | 2 (5) | 1 (1) |
| Proton pump inhibitors, n (%) | 1 (2) | 30 (21) | 11 (31) | 16 (43) | 3 (4) |
| Opioids, n (%) | 0 | 10 (7) | 1 (3) | 6 (16) | 3 (4) |
| Antidepressants, n (%) | 0 | 4 (3) | 3 (8) | 1 (3) | 0 |
- NSCLC, non-small cell lung cancer.
Blood collection
Venous blood was collected in the morning from an antecubital vein after 10 min of supine rest. The participants were allowed to have a small breakfast. According to our local laboratory standard operating procedure, we collected data on clinical chemistry parameters, made a complete blood count, and the coagulation was analysed.
Electrocardiograms
All patients had a 12-lead ECG performed after 10 min of supine rest. ECGs were analysed using Welch Allyn software and adjudicated by two medical reviewers. We recorded the heart rate in beats per minute (b.p.m.). PQ, QRS, and QTc duration were measured in milliseconds.
Statistical analyses
All data are presented as mean ± SD. We used the Kolmogrov–Smirnov test to assess normal distribution. We used analysis of variance (ANOVA) with Fisher's post-hoc test, unpaired Student's t-test, simple regression, and χ2 test as appropriate. Hazard ratios (HRs) with the 95% confidence interval (CI) for risk factors and significance levels are given for results of Cox proportional hazards survival analyses (log-rank test). Kaplan–Meier cumulative survival curves were constructed for illustrative purposes. We conducted statistical analyses using the Stat View 5.0 software (SAS Institute Inc., Cary, NC, USA). A P-value <0.05 was considered statistically significant in all analyses.
Results
Study population
Among the 145 cancer patients in this study, 21% were classified as UICC (Union Internationale contre le Cancer) tumour stage I or II, and 79% as more advanced stages III or IV; the precise distribution across UICC stages I/II/III/IV was 11/10/32/46%. Patients characteristics are shown in Table 2. Comparisons of patients vs. controls as well as of patient subgroups showed no significant differences for age or gender distribution. A total of 78 patients (54%) underwent surgical tumour resection and 62 (43%) had been previously diagnosed as having metastatic disease. When our follow-up was censored in February 2014, 82 (57%) patients had died [colorectal cancer: 5 (14%); NSCLC: 23 (62%); pancreatic cancer: 54 (75%)]. Mean follow-up was 27.4 months for all patients (maximum 93 months).
| Measurement | Controls (n = 59) | Cancer patients (n = 145) | P-value | Colorectal cancer (n = 36) | NSCLC (n = 37) | Pancreatic cancer (n = 72) | P-valuea |
|---|---|---|---|---|---|---|---|
| Age (years) | 60 ± 11 | 59 ± 10 | 0.6 | 60 ± 12 | 60 ± 9 | 59 ± 9 | 0.7 |
| Male sexb | 54 | 56 | 0.8 | 36 | 60 | 64 | 0.02 |
| BMI (kg/m2) | 26.3 ± 3.7 | 24.5 ± 4.6 | 0.006 | 26.3 ± 5.3†† | 25.0 ± 4.7† | 23.3 ± 3.8 | 0.005 |
| Haemoglobin (g/dL) | 14.0 ± 1.6 | 11.7 ± 1.6 | <0.0001 | 12.1 ± 1.4† | 12.2 ± 1.9 | 11.2 ± 1.4** | 0.003 |
| Leucocytes (× 109/L) | 6.4 ± 1.6 | 5.7 ± 2.5 | 0.046 | 6.1 ± 1.9 | 6.3 ± 3.2 | 5.1 ± 2.3* | 0.03 |
| Platelets (× 109/L) | 229 ± 62 | 257 ± 134 | 0.1 | 251 ± 82 | 249 ± 90 | 265 ± 171 | 0.8 |
| Sodium (mmol/L) | 141 ± 2 | 140 ± 3 | 0.005 | 140 ± 3** | 138 ± 4 | 140 ± 3*** | 0.001 |
| Potassium (mmol/L) | 4.2 ± 0.4 | 4.2 ± 0.5 | 0.3 | 4.1 ± 0.3 | 4.3 ± 0.5 | 4.1 ± 0.5 | 0.2 |
| Creatinine (mg/dL) | 0.89 ± 0.17 | 0.82 ± 0.25 | 0.04 | 0.80 ± 0.19 | 0.84 ± 0.24 | 0.81 ± 0.28 | 0.8 |
| GOT (U/L) | 28 ± 7 | 34 ± 24 | 0.049 | 31 ± 30 | 28 ± 16 | 40 ± 24* | 0.03 |
| GPT (U/L) | 26 ± 14 | 43 ± 60 | 0.04 | 30 ± 44† | 30 ± 23 | 57 ± 75* | 0.03 |
| GGT (U/L) | 29 ± 18 | 125 ± 196 | 0.001 | 84 ± 120† | 72 ± 104 | 176 ± 250** | 0.01 |
| SA (g/L) | 40 ± 5 | 35 ± 6 | <0.0001 | 34 ± 8 | 37 ± 4 | 35 ± 6 | 0.2 |
| UICC 1 (%) | – | 11 | 3 | 5 | 19 | ||
| UICC 2 (%) | – | 10 | 25 | 5 | 5 | ||
| UICC 3 (%) | – | 32 | 44 | 24 | 30 | ||
| UICC 4 (%) | – | 46 | 28 | 65 | 46 |
- Values are means ± SD or %.
- P-values are deterrmined using the unpaired t-test (bexcept χ2 if needed).
- a Analysis of variance only in the three tumour groups (bexcept χ2 if needed).
- * P < 0.05 vs. patients with NSCLC;
- ** P < 0.01 vs. patients with NSCLC;
- *** P < 0.001 vs. patients with NSCLC.
- † P < 0.05 vs. patients with pancreatic cancer
- †† P < 0.01 vs. patients with pancreatic cancer.
- BMI, body mass index; GGT, γ-glutamyltransferase; GOT, glutamic oxaloacetic transaminase; GPT, glutamate pyruvate transaminase; NSCLC, non-small cell lung cancer; SA, serum albumin; UICC, Union Internationale contre le Cancer.
No significant difference was noted between cancer patients and controls with regard to age and gender distribution (Table 2). The body mass index (BMI) was higher in control subjects, and the liver enzymes were elevated in the tumour patients. Systolic and diastolic blood pressure was highest among the control subjects, and haemoglobin and creatinine were significantly lower in the tumour patients (Table 2).
Electocardiography analyses
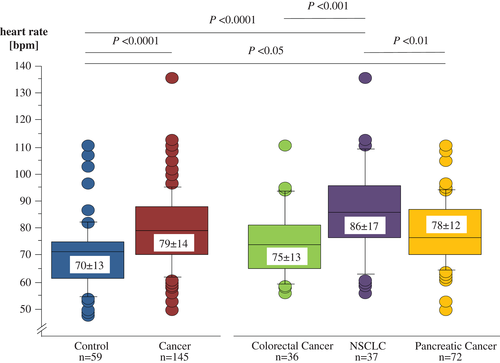
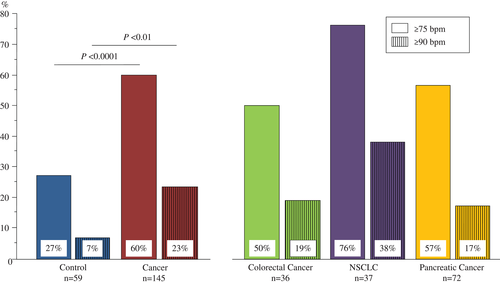
In our ECG analysis, we found that all except one subject were in sinus rhythm (Table 3). The one exception was a patient with NSCLC who showed normofrequent AF. Resting heart rate was significantly lower in controls than in all cancer patients combined (Figure 1). Considering specific cut-off points, we found that 27% of the control subjects presented with a resting heart rate ≥75 b.p.m., whereas this was the case in 60% in cancer patients (P < 0.0001). In addition, 7% of control subjects had a resting heart rate ≥90 b.p.m. compared with 23% of patients with cancer (P < 0.01; Figure 2). Furthermore, we analysed whether any relevant parameter in cancer patients was associated with a heart rate <75 or ≥75 b.p.m. (Table 4); age (P = 0.005) and systolic blood pressure (P = 0.04) were found to be lower in patients with increased heart rate compared with patients with heart rate <75 b.p.m.
| Measurement | Controls (n = 59) | Cancer patients (n = 145) | P-value | Colorectal cancer (n = 36) | NSCLC (n = 37) | Pancreatic cancer (n = 72) | P-valuea |
|---|---|---|---|---|---|---|---|
| Sinus rhytmb | 59 (100) | 144 (99) | 0.5 | 36 (100) | 36 (97) | 72 (100) | 0.2 |
| Heart rate (b.p.m.) | 70 ± 13 | 79 ± 14 | <0.0001 | 75 ± 13*** | 86 ± 17 | 78 ± 12** | <0.001 |
| Heart rate ≥75 b.p.m.b | 16 (27) | 87 (60) | <0.0001 | 18 (50)* | 28 (76) | 41 (57) | 0.06 |
| Heart rate ≥90 b.p.m.b | 4 (7) | 33 (23) | <0.01 | 7 (19) | 14 (38) | 12 (17)* | 0.04 |
| Systolic BP (mmHg) | 132 ± 17 | 125 ± 18 | 0.01 | 125 ± 14 | 119 ± 19 | 127 ± 19* | 0.1 |
| Diastolic BP (mmHg) | 80 ± 11 | 75 ± 10 | 0.002 | 77 ± 8 | 74 ± 12 | 75 ± 10 | 0.3 |
| Atrial fibrillationb | 0 | 1 (1) | 0.5 | 0 | 1 (3) | 0 | 0.2 |
| PQ duration (ms) | 156 ± 20 | 152 ± 22 | 0.3 | 155 ± 22 | 150 ± 23 | 152 ± 22 | 0.6 |
| QRS duration (ms) | 94 ± 11 | 93 ± 13 | 0.7 | 93 ± 13 | 92 ± 13 | 93 ± 13 | 0.9 |
| QTc duration (ms) | 421 ± 20 | 419 ± 22 | 0.6 | 420 ± 25 | 419 ± 22 | 419 ± 22 | 1 |
| Left Sokolow–Lyon index (mV) | 2.3 ± 0.7 | 2.0 ± 0.7 | 0.003 | 2.0 ± 0.7 | 1.9 ± 0.7 | 2.0 ± 0.6 | 0.7 |
| Complete LBBBb | 1 (2) | 3 (2) | 0.9 | 1 (3) | 1 (3) | 1 (1) | 0.8 |
| Incomplete LBBBb | 5 (8) | 7 (5) | 0.3 | 0 | 2 (5) | 5 (7) | 0.3 |
| Complete RBBBb | 0 | 1 (1) | 0.5 | 0 | 1 (3) | 0 | 0.2 |
| Incomplete RBBBb | 2 (3) | 1 (1) | 0.1 | 0 | 0 | 1 (1) | 0.6 |
- Values are means ± SD or number (%).
- P-values are deterrmined using the unpaired t-test (bexcept χ2 if needed).
- a Analysis of variance only in the three tumour groups (bexcept χ2 if needed).
- * P < 0.05 vs. patients with NSCLC;
- ** P < 0.01 vs. patients with NSCLC;
- *** P < 0.001 vs. patients with NSCLC.
- BP, blood pressure; LBBB, left bundle branch block; NSCLC, non-small cell lung cancer; RBBB, right bundle branch block.
| Measurement | Heart rate <75 b.p.m. (n = 58) | Heart rate ≥75 b.p.m. (n = 87) | P-value |
|---|---|---|---|
| Age (years) | 62 ± 9 | 58 ± 10 | 0.005 |
| Male sexa | 31 (53) | 50 (57) | 0.6 |
| BMI (kg/m2) | 24 ± 4 | 25 ± 5 | 0.9 |
| Systolic BP (mmHg) | 128 ± 18 | 122 ± 18 | 0.04 |
| Diastolic BP (mmHg) | 75 ± 8 | 75 ± 11 | 0.7 |
| Sodium (mmol/L) | 140 ± 3 | 140 ± 4 | 0.3 |
| Potassium (mmol/L) | 4.2 ± 0.4 | 4.1 ± 0.5 | 0.5 |
| Creatinine (mg/dL) | 0.85 ± 0.24 | 0.79 ± 0.25 | 0.2 |
| Haemoglobin (g/dL) | 11.5 ± 1.5 | 11.8 ± 1.6 | 0.4 |
| Platelets (× 109/L) | 254 ± 160 | 260 ± 115 | 0.8 |
| Leucocytes (× 109/L) | 5.7 ± 2.8 | 5.6 ± 2.4 | 0.7 |
| GGT (U/L) | 117 ± 172 | 132 ± 207 | 0.7 |
| Operationa | 38 (66) | 54 (62) | 0.7 |
| Chemotherapya | 43 (74) | 63 (72) | 0.8 |
| Anticoagulationa | 8 (14) | 10 (11) | 0.7 |
| Aspirina | 5 (9) | 6 (7) | 0.7 |
| ACE inhibitor or ARBa | 15 (26) | 18 (21) | 0.5 |
| Beta-blockera | 11 (19) | 14 (16) | 0.7 |
| Spironolactonea | 0 | 2 (2) | 0.2 |
| Diureticsa | 4 (7) | 11 (13) | 0.3 |
| Anticoagulantsa | 8 (14) | 10 (11) | 0.7 |
| Lipid-lowering drugsa | 5 (9) | 11 (13) | 0.4 |
- Values are means ± SD or number (%).
- P-values are deterrmined using the unpaired t-test (aexcept χ2 if needed).
- BMI, body mass index; GGT, γ-glutamyltransferase.
As a sensitivity analysis, we excluded control subjects taking a beta-blocker, but resting heart rate remained increased in cancer patients vs. controls (P < 0.0001). Additionally, only 25 (17%) of the patients used beta-blockers at baseline, compared with 7 (12%) healthy controls (P = 0.3). In contrast, no significant differences were found with regard to the distribution of PQ, QRS, and QTc duration between patients and controls or between patient subgroups (all P > 0.2; Table 3). Likewise, no difference was detected with regards to the prevalence of left or right bundle branch block patterns, whether complete or not (all P > 0.1). The left Sokolow–Lyon index was higher in healthy controls compared with cancer patients.
Impact of chemotherapy
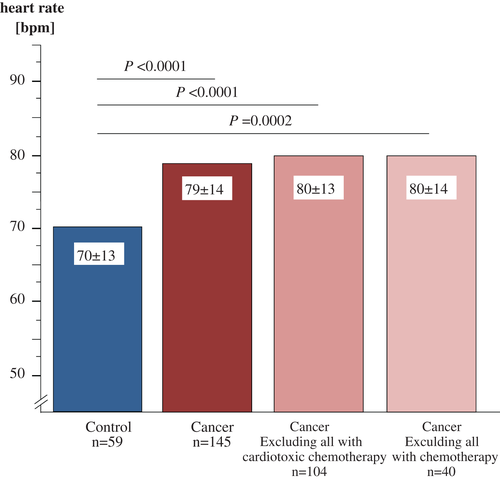
Chemotherapy was administered to 105 (73%) patients, and cardiotoxic chemotherapy13 was administered to 41 patients (28%). Detailed information about the distribution of chemotherapy regimens can be found in the Supplementary material online, Table S1. Resting heart rate was similarly increased in patients with and without any chemotherapy as well as in patients with and without cardiotoxic chemotherapy (Figure 3). As a sensitivity analyses, we performed univariable survival analyses for resting heart rate vs. survival in these subgroups. The results were similar to the results found in the overall study population. Specifically, in 105 cancer patients receiving any chemotherapy, 65 deaths were observed and the HR for resting heart rate ≥75 b.p.m. was 1.81 (95% CI 3.04–1.0; P = 0.02). In 41 cancer patients receiving any cardiotoxic chemotherapy, 16 deaths were observed and the HR for resting heart rate ≥75 b.p.m. was 1.78 (95% CI 4.76–0.67; P = 0.25). In 40 cancer patients receiving no chemotherapy at all, 17 deaths were observed, and the HR for resting heart rate ≥75 b.p.m. was 2.36 (95% CI 7.25–0.77; P = 0.13).
Predictors of survival
Using univariable Cox-proportional hazards analysis, we found, that resting heart rate as a continuous variable (HR per b.p.m. 1.014, 95% CI 1.000–1.028; P = 0.048), BMI, type of tumour, UICC tumour stage, surgical intervention yes/no, chemotherapy administered yes/no, haemoglobin, serum potassium, and administering anticoagulants all predicted survival (all P < 0.05; Table 5). The use of beta-blockers did not predict survival (HR 0.88, 95% CI 0.51–1.55, P = 0.7).
| Measurement | Univariable | Multivariable model 1 | Multivariable model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | χ2 value | P-value | HR | 95% CI | χ2 value | HR | 95% CI | χ2 value | |
| Age (per year) | 0.99 | 0.97–1.01 | 0.7 | 0.4 | 0.99 | 0.96–1.01 | 1.0 | |||
| Sex (male vs. female) | 1.28 | 0.83–1.99 | 1.2 | 0.3 | 1.17 | 0.73–1.88 | 0.4 | |||
| Heart rate ≥75 b.p.m. (yes vs. no) | 1.84 | 1.16–2.94 | 6.6 | 0.01 | 1.67 | 1.01–2.78 | 4.1* | 1.66 | 1.00–2.76 | 3.8* |
| BMI (per kg/m2) | 0.95 | 0.90–1.00 | 4.6 | 0.033 | 1.00 | 0.94–1.06 | 0.02 | 1.00 | 0.94–1.06 | 0.02 |
| Diagnosis (CC vs. NSCLC vs. PC) | – | – | 18.8 | <0.0001 | – | – | 8.9* | – | – | 8.1* |
| Tumour stage (UICC 1 vs. 2 vs. 3 vs. 4) | – | – | 9.9 | 0.020 | – | – | 1.6 | – | – | 2.1 |
| Operation (no vs. yes) | 2.42 | 1.57–3.74 | 15.8 | <0.0001 | 1.83 | 1.01–3.30 | 4.0* | 1.96 | 1.07–3.61 | 4.7* |
| Chemotherapy (no vs. yes) | 0.58 | 0.33–1.00 | 3.9 | 0.048 | 0.83 | 0.44–1.60 | 0.3 | 0.83 | 0.44–1.56 | 0.4 |
| Haemoglobin (per mg/dL) | 0.84 | 0.73–0.97 | 5.9 | 0.015 | 0.90 | 0.76–1.07 | 1.4 | 0.90 | 0.76–1.06 | 1.7 |
| Potassium (per mg/dL) | 0.57 | 0.33–0.98 | 4.3 | 0.038 | 0.65 | 0.37–1.14 | 2.2 | 0.62 | 0.35–1.10 | 2.7 |
| Anticoagulation (yes vs. no) | 2.16 | 1.25–3.75 | 7.6 | 0.006 | 1.35 | 0.72–2.55 | 0.9 | 1.35 | 0.72–2.53 | 0.9 |
- Hazard ratios are presented for continuous, binomially, and multinomially distributed variables.
- * P < 0.05.
- Model 1 includes all clinically relevant significant univariate predictors; Model 2 includes all clinically relevant significant univariate predictors plus age and sex.
- BMI, body mass index; CC, colorectal cancer; CI confidence interval; HR, hazard ratio; NSCLC, non-small cell lung cancer; PC, pancreatic cancer; UICC, Union Internationale contre le Cancer.
In addition, we performed receiver operating characteristic (ROC) analysis for heart rate vs. survival. The area under the curve was 59.3% (95% CI 50.9–76.4), with best cut-offs for the highest product of sensitivity and specificity at >72 and >74 b.p.m.. For further analyses, we selected a cut-off of ≥75 b.p.m. that had a sensitivity of 68% (95% CI 57–78%) and a specificity of 51% (95% CI 38–64%) in these analyses. This cut-off also showed strong significant association with survival in univariable analysis (Table 5).
To investigate further the impact of resting heart rate on survival of the cancer patients, we performed a multivariable survival analysis. In our first model, we included all significant predictors of mortality that were found: resting heart rate ≥75 b.p.m., BMI, type of tumour, the UICC tumour stage, whether surgical interventions were performed or chemotherapy administered, haemoglobin, serum potassium, and administering anticoagulants. Heart rate remained an independent predictor of mortality (Table 5). In a second model, in order to eliminate any possible bias by gender or age, we furthermore included age and gender of patients, and also in this model heart rate was found to be a valid predictor of survival (Table 5).
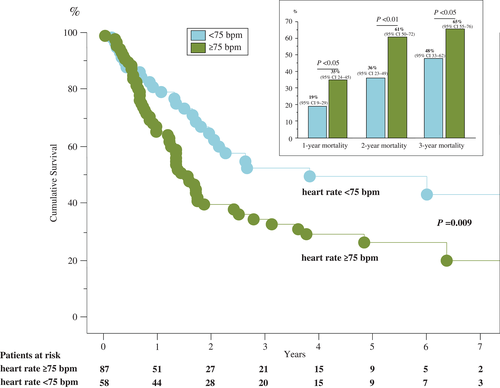
Furthermore, we were able to show that patients with a resting heart rate ≥75 b.p.m. had a significantly higher 1-, 2-, and 3-year mortality (Figure 4). The Kaplan–Meier survival curve emphasizes the underlying survival benefit for cancer patients with a heart rate <75 b.p.m. Both graphs separate early and stay independently apart for the whole follow-up time. Mortality rates with 95% CIs as well as patients at risk over 7 years of follow-up are shown in Figure 4.
Discussion
The present study documents that patients with colorectal carcinoma, NSCLC, and pancreatic tumour have increased resting heart rates compared with healthy control subjects of similar age and gender distribution. Increased heart rates independently predict mortality in these patients. A higher heart rate was generally not associated with other parameters, except for an association with lower age and blood pressure. Overall, cancer patients showed higher values of blood pressure and liver enzymes, whereas their BMI and haemoglobin levels were significantly lower.
Our analyses showed that resting heart rate in advanced cancer patients was the strongest predictor of overall survival in an ECG. Other important predictors of survival were the BMI, diagnosis, tumour stage, surgery, chemotherapy, anticoagulants, haemoglobin, and serum potassium. The use of beta-blockers did not predict survival, but it has to be considered that patients and healthy controls in our study did not differ in the use of beta-blockers. Whether or not patients received any chemotherapy, or whether or not such chemotherapy (if provided) was a known cardiotoxic medication, made no difference to the main result that a resting heart rate ≥75 b.p.m. predicts poorer survival in patients with cancer.
Many studies such as the Framingham Study14 and the Malattie Cardiovascolari Aterosclerotiche, Istituto Superiore di Sanità (MATISS) Project15 have shown that resting heart rate independently predicts overall mortality in the general population. There is an ongoing debate, with conflicting data, on whether heart rate in the healthy population is also sufficient to predict cancer mortality. A significant correlation between cancer mortality and resting heart rate was first shown in 1981 by a publication regarding three Chicago-based epidemiological studies with slightly varying criteria conducted in 8916 healthy men. Two of these three studies documented that higher heart rates were significantly associated with higher lung cancer and colon cancer mortality, by including 1233 men and 5784 men, respectively, between age 40 and 64 and baseline heart rate at rest of 72 b.p.m. (60–83) and 76 b.p.m. (64–88) with a total of 18 and 5 years of follow-up. The third study, including 1899 younger men (40–55 years) with a resting heart rate of 79 b.p.m. (68–90) and a follow-up of 17 years, however, failed to show a significant relationship.16
Since then, many other epidemiological studies have been published examining the question at hand, with some underlining the above-mentioned phenomenon and finding significant associations.17, 18 Hence, the Paris Prospective Study I, which was conducted in 6101 healthy, working men with a follow-up of 25 years, showed relative risks for cancer mortality of up to 2.4 (95% CI 1.9–2.9), when the highest and lowest quartile of heart rates (heart rate <60 or >73 b.p.m.) were compared.19 At the same time, other studies such as the Cardiovascular Occupational Risk Factor Determination in Israeli Industry (CORDIS) study (3527 men, 8 years of follow-up, mean age of 43 at study entry) failed to show a significant relationship between resting heart rate and cancer mortality, even though the CORDIS study showed a weak trend towards an association of higher heart rates with cancer mortality (adjusted relative risk 1.1, 95% CI 0.8–1.5). The authors themselves speculated that this was associated with only a few chosen endpoints, which led to wide CIs, and the follow-up was much shorter compared with the above-mentioned study.20, 21 Similarly, a study conducted by Mensink and Hoffmeister in 1827 men and 2929 women with a follow-up of 12 years failed to show significance but again a tendency towards elevated cancer mortality in healthy people was observed.22
While the role of the baseline heart rate in the healthy population and its impact on cancer mortality remains unclear, many factors can influence the heart rate of cancer patients after the disease is established. Chemotherapy is one of the most obvious factors23 that has a strong impact on the heart and can be associated with the development of hypo- and hypertension, ischaemia, oedema, anaemia, thrombo-embolism, arrhythmia, pericarditis, and even heart failure.24 Therefore, chemotherapy is also associated with an elevated resting heart rate, especially during the time of therapy.25, 26
Senkus and Jassem even hypothesized that tachycardia might be the first sign of cardiac damage.27 Other known factors that can contribute to higher heart rate of cancer patients are chest radiation therapy, which can also damage the heart,28 tobacco smoke,29 and development of depression.30 Lower heart rates are generally associated with high physical fitness31 and therefore some studies examined the relationship of heart rate and physical performance in cancer patients. In breast cancer patients, it was shown that after 15 weeks of training (four times per week walking or resistance training, for a total of ∼3 h) resting heart rate significantly decreased by 5 b.p.m. (from 89 to 84 b.p.m.), compared with the control group without exercise.32 Other studies have confirmed the survival benefits of exercise training in cancer patients, with reduction of all-cause mortality of up to 49%.33-35
Despite all the aforementioned research, the fact that a high heart rate is an independent prognostic factor in cancer patients has not been reported before. However, high resting heart rates have been identified as a marker for mortality in the general population and in other diseases. It was shown, for instance, that in the general population an increase in resting heart rate of 15 b.p.m. is associated with higher cardiovascular mortality (HR 1.32, 95% CI 1.08–1.60 for healthy women; HR 1.24, 95% CI 1.11–1.40 for healthy men).36 Tachycardia is even associated with the development of heart failure and cardiomyopathy.37 At the same time, resting heart rate has been proven to be not only a valid marker for outcome, but also a valid therapeutic target. There are two types of drugs that significantly reduce heart rate, namely beta-blockers and the If-channel inhibitor ivabradine. For four beta-blockers (bisoprolol,38 metoprolol succinate,39 carvedilol,40 and nebivolol41) as well as for ivabradine,42-44 reductions in event rates for death and hospitalizations have been shown in studies of heart failure patients.45 Not the beta-blocker dose but how sufficiently the heart rate was lowered predicted survival.46 Furthermore, it was shown that patients with a resting heart rate ≥75 b.p.m. benefited even more from therapy with ivabradine, as all-cause mortality was found to be reduced (HR 0.83, 95% CI 0.72–0.96; P < 0.02),47 without causing worsening of renal function.48 It is noteworthy that this is the same cut-off point our study found as a valid predictor of mortality. In addition, beta-blockers are also widely used in CAD, where they have been shown to reduce long-term mortality significantly.49 None of these drugs has been prospectively tested in cancer patients. Therefore, both beta-blockers and ivabradine may be new therapeutic options for cancer patients with high resting heart rates.
It was not possible for us to assess the cause of death, because in most cases the clinical picture of patients with advanced cancer in the last 2 weeks before death is very complex. It is often accompanied by severe pain, signs of infection, severe limitation of exercise capacity (usually requiring full bed rest), and strong pain medication and sedation. The cause of death in cancer is generally not well investigated, and publications on this subject are rare. Final cause of death seems to vary greatly between cancer types.50 Most frequent causes of death are infection, with multiorgan failure, cardiovascular causes, and advanced cachexia.51 In the present study, we focused on all-cause mortality to avoid any bias of classification. We recognize that heart rate may be a particularly strong predictor of death in some patient subgroups, but not in others, depending on the clinical circumstances leading to death. Considering that median survival of our patient cohort is 24 months, we do not claim to have shown yet that high heart rate predicts a certain kind of death 2 years later, but we document here that high heart rate significantly predicts the presence of an overall poor prognostic state in patients with advanced cancer. It has not yet been investigated whether there is a possibility to improve symptoms or lower mortality of cancer patients by administering beta-blockers or ivabradine. From a basic science standpoint, beta-adrenergic regulation is not only an important factor for the commencement, but is even considered to promote metastasis and growth of tumours through extensive interaction between epinephrine, norepinephrine, and α- and β-adrenergic receptors.52
Until now there have only been retrospective epidemiological studies investigating the effects of beta-blockers that were given to cancer patients mostly prior to the cancer diagnosis. Therefore, no evaluation is possible of to what extent the patients would benefit from additional beta-blocker or ivabradine therapy started after the diagnosis of cancer is established. In epidemiological studies, the evidence remains unclear as to what extent antihypertensive drugs have cancer protective impact. Some studies found data supporting the hypothesis that beta-blockers have a protective effect against cancer formation,53, 54 while others did not.55, 56 A recent meta-analysis tried to summarize the available data from 12 studies conducted over the last 20 years in >20 000 people and found that beta-blockers are associated with improved survival in cancer patients (HR 0.79, 95% CI 0.67–0.93; P = 0.004) and a longer survival without disease (HR 0.69, 95% CI 0.53–0.91; P = 0.009).57 Another approach is the peri-operative treatment with beta-blockers, which two studies are prospectively investigating at present in colorectal (NCT 00888797) and breast (NCT 00502684) cancer. Results are not expected before the end of 2016.
In order to put our data into context, we analysed the 61 original clinical studies published in Lancet Oncology from December 2015 to May 2016 (75% of which were intervention studies). In none of these publications were baseline blood pressure values or baseline heart rates reported. Twenty-one publications (34%) reported blood pressure changes during the study, one study (2%) reported on the presence of tachycardia, and five (8%) on the presence of AF during the study; however, 54 (89%) reported detailed tumour stage at baseline. Clearly, heart rate is not frequently considered a relevant characteristic of patients with cancer. We suggest that this should be different.
Our study demonstrates that heart rate and fatal outcomes of cancer patients are strongly associated. Through a combination of factors determined by the cancer itself, the aggressive therapy, and other cofactors (including the stress response of the host's body to cancer disease), the heart of cancer patients may be strongly and adversely affected. Resting heart rate in the cancer patients studied here was significantly elevated. This was the case in all cancer subgroups, but most prominent in patients with NSCLC. The results suggest that resting heart rate of patients with colorectal, pancreatic, or non-small cell lung cancer could be used as a predictor for poor survival. To understand this phenomenon fully, more research is needed, but new therapeutic approaches appear possible.
Acknowledgements
We thank the patients, physicians, investigators, and supporting staff who were involved in this study.
Conflict of interest: M.S. reports being on the advisory board of Roche, Baxalta, and Sirtex, and receiving travel support from Bayer and Amgen, outside the submitted work. S.D.A. reports receiving consulting fees from Servier and speaking fees from Helsinn. S.vH. reports receiving consulting honoraria from Vifor, Thermo Fisher Scientific, Chugai, Respicardia, Professional Dietetics, Solartium Dietetics, Novartis, and Pfizer. All other authors have no conflicts to disclose.
Authors' contributions: M.S.A. was responsible for creating a database, data analyses, statistical analyses, and writing the first draft of the manuscript. N.E. helped in statistical analyses. M.S. was responsible for recruitment of patients. B.H., J.S., H.R., S.D.A., U.L., and W.H. were responsible for guiding, planning, and interpretation of results. S.vH. was chief investigator of the study and was responsible for study design, statistical analyses, and clinical supervision. All authors were involved in reviewing and critical revision of the manuscript and approved the final manuscript. All agreed to the submission of the final manuscript.




