Fifteen-year trends in the management of cardiogenic shock and associated 1-year mortality in elderly patients with acute myocardial infarction: the FAST-MI programme
Abstract
Aims
Alhough cardiogenic shock (CS) after acute myocardial infarction (AMI) is more common in elderly patients, information on the epidemiology of these patients is scarce. This study aimed to assess the trends in prevalence, characteristics, management, and outcomes of elderly patients admitted with CS complicating AMI between 1995 and 2010, using data from the FAST-MI programme.
Methods and results
We analysed the incidence and 1-year mortality of CS in four nationwide French surveys carried out 5 years apart from 1995 to 2010, including consecutive AMI patients over 1-month periods. Among the 10 610 patients, 3389 were aged ≥75 years, of whom 9.9% developed CS. The prevalence of CS decreased in elderly patients from 11.6% in 1995 to 6.7% in 2010 (P = 0.02). Over the 15-year period, the characteristics of elderly patients with CS changed, with more diabetes, hypertension, and hypercholesterolaemia. The use of PCI increased markedly in elderly patients with and without CS, reaching 51% and 59%, respectively, in 2010. In addition, medical therapy also evolved, with more patients receiving antithrombotic agents, beta-blockers, and statins. Over time, 1-year mortality decreased by 32% among elderly patients with CS but remained high (59% in 2010). ST-segmet elevation myocardial infarction and previous AMI were independent correlates of increased 1-year death, while study period was associated with decreased mortality (2010 vs, 1995: hazard ratio 0.40, 95% confidence interval 0.27–0.61, P < 0.001), along with early use of PCI.
Conclusion
Cardiogenic shock in elderly patients with AMI remains a major clinical concern. However, 1-year mortality declined in these patients, a decrease potentially mediated by broader use of PCI and the improvement of global patient management.
Introduction
Cardiogenic shock (CS) remains the leading cause of death after hospitalization or in patients hospitalized with acute myocardial infarction (AMI). However, its prognosis has markedly improved, mainly due to early reperfusion therapy.1, 2
The elderly (≥75 years) constitute an increasing proportion of patients presenting with AMI,3, 4 and age has been reported as a risk factor for CS in AMI patients.5-7 However, only a few studies have reported outcomes of elderly patients with AMI complicated by CS.4, 8-10 Thus, in this population, information on the prevalence, determinants, and prognostic factors of CS is scarce.
USIK 1995, USIC 2000, FAST-MI 2005, and FAST-MI 2010 are four prospective observational studies conducted in 1995, 2000, 2005, and 2010 in most French centres taking care of AMI patients; they included consecutive ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) patients over a 1-month period. Each survey assessed the characteristics, management, and outcome of patients admitted to hospital with AMI over a 1-month period.11-14
Accordingly, we assessed trends in prevalence, key features, and 1-year mortality of elderly patients with CS complicating AMI in these four nationwide French surveys from 1995 to 2010.
Methods
Study population
Patients aged ≥75 years (n = 3389) were selected among the 10 610 patients of the four nationwide French registries USIK 1995, USIC 2000, and FAST-MI (2005 and 2010).
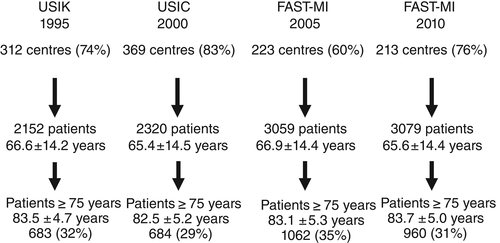
Methods of these registries have been described in detail elsewhere.11-14 In brief, the primary objectives were to evaluate MI management in a ‘real-life’ setting and to assess short- and long-term outcomes of patients admitted to intensive care units (ICUs) for MI. Patients were recruited consecutively from ICU departments over a period of 1 month (November 1995 and 2000, and October 2005 and 2010). Participation in the study was offered to all French institutions, university teaching hospitals, general and regional hospitals, and private clinics with ICUs authorized to receive acute coronary syndrome (ACS) emergencies. In each centre, a physician was in charge of the registry and provided a full list of all patients admitted to the unit. The number of participating centres was 312 in 1995, 369 in 2000, 223 in 2005, and 213 in 2010, and 2152, 2320, 3059, and 3079 patients, respectively, were included. The percentage of participating centres compared with all centres taking care of AMI patients in France was 74% in 1995, 83% in 2000, 60% in 2005, and 76% in 2010.
Inclusion criteria were (i) men or women, >18 years old; (ii) patients admitted within 48 h after symptom onset in an ICU for an AMI15-17 characterized by increased troponin or creatine kinase (CK)-MB associated with at least one of the following elements: symptoms compatible with myocardial ischaemia, appearance of pathologic Q waves, or ST–T changes compatible with myocardial ischaemia (ST-segment elevation or depression, T-wave inversion); and (ii) consent to take part in the study. Patients who died very soon after admission and for whom cardiac markers were not measured were included if they had compatible signs or symptoms associated with typical ST-segment changes.
Exclusion criteria were (i) refusal to participate; (ii) MI admitted >48 h after symptom onset; (iii) iatrogenic MIs, defined as MIs occurring within 48 h of a therapeutic procedure (bypass surgery, coronary angioplasty, or any other medical or surgical intervention); (iv) ACS diagnosis invalidated in favour of another diagnosis; and (v) patients with unstable angina and no increase in cardiac biomarkers.
The study was conducted in compliance with good clinical practice, French law, and the French data protection law. The data files of the four studies were declared to and authorized by the French data protection committee (Commission Nationale Informatique et Liberté).
Participation in the protocol did not change the therapeutic approach of the cardiologist in any way.
Definitions
Cardiogenic shock was defined as systolic blood pressure <90 mmHg in the absence of hypovolaemia and associated with cyanosis, cold extremities, changes in mental status, persistent oliguria, or congestive heart failure.15-18 The definition of CS remained the same during all periods studied, and was such that patients with classic signs and symptoms of this clinical syndrome would be included.
Data collection
Baseline characteristics and clinical complications at the time of admission were collected prospectively.
Also, we collected data on use of cardiac procedures and presence and type of reperfusion therapy in STEMI (with PCI, intravenous fibrinolysis, or coronary artery bypass grafting). In 2000, 2005, and 2010, data on the timing of fibrinolytic treatment (pre-hospital or in-hospital), and the use of inotropes and intra-aortic balloon pump (IABP) were collected. Medications used in the first 48 h and discharge medications were recorded.
Outcome
Mortality was assessed at 30 days and at 1 year for each cohort in elderly patients both with and without CS. One-year follow-up was obtained directly by the physician in charge of the study in each centre for the 1995 and 2000 surveys. For the 2005 and 2010 surveys, 1-year follow-up was centralized at the French Society of Cardiology, and dedicated research technicians contacted both the physicians and the patients themselves, after checking the patients' vital status in municipal registers. The rate of patients lost to follow-up at 1 year was 2.7% in 1995, 8.3% in 2000, 0.3% in 2005, and 0.9% in 2010.
Statistical analysis
Statistical analysis was performed using IBM SPSS 23.0 (IBM SPSS, Inc., Chicago, IL, USA). For quantitative variables, mean and standard deviations were calculated. Discrete variables are presented as percentages. Comparisons between periods were performed with χ2 or Fisher's exact tests for discrete variables, as appropriate, and Kruskal–Wallis tests or analysis of variance (ANOVA) for continuous variables, followed by Mann–Whitney or Student's t-tests to compare the 1995 and 2010 cohorts. Temporal trends for continuous variables were estimated using the Jonckheere Terpstra test. Odds ratios (ORs) or hazard ratios (HRs) are presented with their 95% confidence interval (CI).
Binary logistic multivariate analyses were performed separately in elderly and in younger (<75 years) patients to determine correlates of the development of CS.
Thirty-day and 1-year mortality were calculated for each study period, according to the presence of CS in elderly patients, using the Kaplan–Meier method, and comparisons were made using log-rank tests.
When comparing continuous variables between the four periods, we used ANOVA followed by Student's t-tests comparing 1995 and 2010, when appropriate.
One-year survival was also assessed after performing a landmark analysis on patients surviving at 30 days.
Correlates of 1-year mortality were determined using a multivariate stepwise Cox backward model. Variables included in the final multivariate models were selected ad hoc, based on their physiological relevance and potential to be associated with outcomes; thus, we included variables likely to influence the outcome negatively (age ≥75 years, history of heart failure, history of diabetes, history of prior AMI, history of stroke, history of peripheral artery disease) or positively (history of hypertension, current smoking) as well as sex, type of MI (STEMI vs. NSTEMI) and time period (1995, 2000, 2005, and 2010) (Model 1).
In addition, to assess the potential role of PCI in CS patients, we repeated the multivariate model including the use of early PCI, maximal CK level, early statin, beta-blockers, and ACE inhibitors (Model 2). For confirmation purposes, we also used a model excluding patients dying on day 1 and 2 (because these patients might have died before PCI could actually be performed), and a model including only variables with a P-value <0.15 in univariate analyses.
Variance inflation factors were calculated for testing potential colinearity of the variables, and goodness of fit was assessed by Hosmer–Lemeshow tests.
For all analyses, a P-value <0.05 was considered significant.
Results
Trends in prevalence of CS according to age
Among 10 610 patients, the total study sample consisted of 3389 elderly patients with AMI (31.9%), of whom 9.9% (n = 335) developed CS (Figure 1).
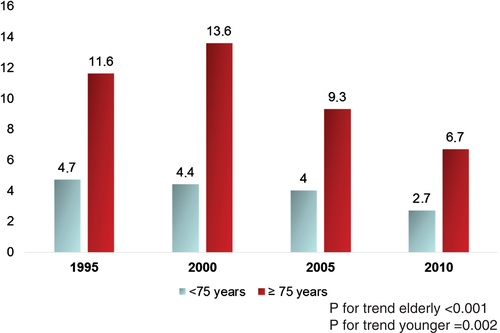
The occurrence of CS was higher in elderly compared with younger patients in each study period and the prevalence of CS decreased over time in both groups, from 11.6% to 6.7% in the elderly group (P < 0.001) and from 4.7% to 2.7% in younger patients (P = 0.002) (Figure 2).
In elderly patients, the prevalence of shock decreased in all age groups (75–79 years, from 7.7% to 4.5%; 80–84 years, from 10.6% to 6.1%; ≥85 years, from 17.0% to 10.1%) (Supplementary material online, Figure S1).
Trends in key features and management of elderly patients according to the presence or absence of cardiogenic shock
Detailed results are shown in Table 1. Over the 15-year period, the characteristics of AMI patients with CS changed, with more diabetes mellitus, hypertension, and hypercholesterolaemia. The proportion of STEMI was lower in 2005 and 2010, reflecting the higher proportion of patients with NSTEMI after the widespread use of troponin measurements. Similar changes were observed among elderly patients without CS.
| Characteristics | 1995 | 2000 | 2005 | 2010 | P-valuea | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS (n = 79) | No CS (n = 604) | CS (n = 93) | No CS (n = 591) | CS (n = 99) | No CS (n = 963) | CS (n = 64) | No CS (n = 896) | P for trend CS | P for trend no CS | |
| Age, mean, years | 83 ± 5 | 82 ± 5 | 83 ± 5 | 82 ± 5 | 83 ± 5 | 82 ± 5 | 84 ± 5 | 82 ± 5 | 0.46 | 0.195 |
| Age class (years) | ||||||||||
| 75–79 | 18 (23) | 217 (36) | 38 (41) | 283 (48) | 34 (34) | 395 (41) | 16 (25) | 339 (38) | 0.81 | 0.88 |
| 80–84 | 25 (32) | 211 (35) | 22 (24) | 140 (24) | 29 (29) | 314 (33) | 20 (31) | 308 (34) | ||
| ≥85 | 36 (46) | 176 (29) | 33 (35.5) | 168 (28) | 36 (36) | 254 (26) | 28 (44) | 249 (28) | ||
| Female, n (%) | 44 (56) | 307 (51) | 39(42) | 284 (48) | 51 (51.5) | 483 (50) | 41 (64) | 391 (44) | 0.22 | 0.01 |
| STEMI, n (%) | 59 (75) | 400 (66) | 75 (81) | (439 (74) | 50 (50.5) | 395 (41) | 38 (59) | 399 (44.5) | 0.001 | <0.001 |
| Medical history and risk factors, n (%) | ||||||||||
| Body mass kg/m2 | 24 ± 3 | 25 ± 4 | 24 ± 4 | 25 ± 4 | 25 ± 6 | 26 ± 4 | 26 ± 5 | 26 ± 4 | 0.03 | <0.001 |
| Diabetes mellitus, n (%) | 16 (21) | 122 (20.5) | 24 (26) | 138 (23) | 26 (26) | 269 (28) | 26 (41) | 260 (29) | 0.02 | <0.001 |
| Hypertension, n (%) | 47 (60) | 345 (58) | 49 (53) | 373 (63) | 74 (75) | 719 (75) | 54 (84) | 669 (75) | <0.001 | <0.001 |
| Hypercholesterolaemia, n (%) | 12 (15) | 171 (29) | 24 (26) | 202 (35) | 45 (45.5) | 426 (44) | 29 (39) | 389 (43) | <0.001 | <0.001 |
| Current smoking, n (%) | 4 (5) | 52 (9) | 5 (6) | 38 (6.5) | 8 (8) | 79 (8) | 0 | 56 (6) | 0.37 | 0.17 |
| Prior myocardial infarction, n (%) | 22 (28) | 123 (20) | 22 (24) | 137 (23) | 19 (19) | 208 (22) | 15 (23) | 203 (23) | 0.36 | 0.46 |
| Prior PCI (%) | – | – | 6 (6.5) | 42 (7) | 10 (10) | 137 (14) | 10 (16) | 180 (20) | 0.06 | <0.001 |
| Prior CABG (%) | – | – | 4 (4) | 27 (5) | 5 (5) | 71 (7) | 3 (5) | 69 (8) | 0.89 | 0.03 |
| Prior heart failure, n (%) | 20 (25.5) | 110 (18) | 23 (25) | 72 (12) | 13 (13) | 107 (11) | 11 (17) | 81 (9) | <0.001 | 0.054 |
| Presentation, n (%) | ||||||||||
| Shock on admission | – | – | 35 (38) | – | 30 (30) | – | 31 (51) | – | – | – |
| Admission SBP <90 mmHg | – | – | 22 (24) | 25 (4) | 17 (17.5) | 25 (3) | 14 (23) | 23 (3) | 0.71 | 0.10 |
| LVEF | ||||||||||
| Missing | 50 (63) | 272 (45) | 57 (61) | 179 (30) | 28 (28) | 235 (24) | 10 (16) | 139 (15.5) | <0.001 | <0.001 |
| >40% | 12 (15) | 230 (38) | 14 (15) | 39 (52) | 27 (27) | 533 (55) | 29 (45) | 557 (62) | ||
| ≤40% | 17 (21.5) | 102 (17) | 22 (24) | 103 (17) | 44 (44) | 195 (20) | 25 (39) | 200 (22) | ||
| Extent of CAD (patients with angiography) | ||||||||||
| None/missing | – | – | 2 (5) | 33 (10) | 1 (2) | 84 (13) | 3 (7) | 90 (12) | 0.70 | 0.03 |
| 1-vessel disease | – | – | 8 (20.5) | 133 (39.5) | 11 (26) | 223 (35) | 9 (20) | 226 (31) | ||
| 2-vessel disease | – | – | 13 (33) | 100 (30) | 15 (36) | 188 (29) | 20 (44) | 223 (30) | ||
| 3-vessel disease | – | – | 16 (41) | 71 (21) | 15 (36) | 148 (23) | 13 (29) | 197 (27) | ||
| Management, n (%) | ||||||||||
| Antiplatelet agents | 52 (66) | 536 (89) | 81 (87) | 547 (93) | 88 (89) | 902 (89) | 60 (94) | 872 (97) | <0.001 | <0.001 |
| Thienopyridine | – | – | – | – | 61 (62) | 751 (78) | 54 (84) | 831 (93) | 0.002 | < 0.001 |
| Unfractionated heparin | 71 (90) | 581 (96) | 81 (87) | 425 (72) | 67 (68) | 569 (59) | 38 (59) | 141 (46) | <0.001 | <0.001 |
| Low molecular weight heparin | 0 | 0 | 18 (19) | 174 (29) | 38 (38) | 553 (57) | 25 (39) | 468 (52) | <0.001 | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 0 | 0 | 12 (13) | 48 (8) | 18 (18) | 221 (23) | 12 (19) | 186 (21) | <0.001 | < 0.001 |
| Vitamin K antagonists | – | – | 0 | 11 (2) | 0 | 11 (1) | 2 (3) | 48 (5) | 0.042 | <0.001 |
| Amiodarone | – | – | 21 (23) | 97 (16) | 38 (38) | 138 (14) | 13 (20) | 111 (12) | 0.98 | 0.028 |
| Lipid-lowering agents | 1 (1) | 24 (4) | 9 (10) | 195 (33) | 49 (50) | 633 (66) | 45 (70) | 733 (82) | <0.001 | <0.001 |
| Diuretics | 60 (76) | 331 (55) | 55 (59) | 238 (40) | 75 (76) | 479 (50) | 40 (63) | 422 (47) | 0.46 | 0.15 |
| Beta-blockers | 13 (17) | 259 (43) | 25 (27) | 332 (56) | 38 (38) | 583 (61) | 26 (41) | 659 (74) | <0.001 | <0.001 |
| ACE inhibitors | 15 (19) | 299 (50) | 20 (22) | 247 (42) | 37 (37) | 420 (44) | 22(34) | 431 (48) | 0.005 | 0.975 |
| Inotropes | -- | -- | 49 (53) | 27 (5) | 46 (47) | 53 (6) | 16 (25) | 32 (4) | <0.001 | 0.26 |
| Procedures, n (%) | ||||||||||
| Reperfusion therapy in STEMI | 12 (20) | 93 (23) | 28 (37) | 153 (35) | 22 (44) | 172 (44) | 20 (57) | 250 (66.5) | <0.001 | <0.001 |
| Fibrinolysis in STEMI patients | 8 (14) | 72 (18) | 14 (19) | 86 (20) | 11 (22) | 65 (16.5) | 2 (6) | 29 (8) | – | – |
| Primary PCI | 4 (7) | 21 (5) | 14 (19) | 67 (15) | 11 (22) | 107 (27) | 18 (51) | 221 (59) | – | – |
| Pre-hospital fibrinolysis | 0 | 0 | 3 (4) | 22 (5) | 3 (6) | 43 (11) | 2 (6) | 20 (5) | – | – |
| PCI in the first 3 days | 9 (11) | 32 (5) | 26 (28) | 140 (24) | 26 (26) | 354 (37) | 31 (48) | 896 (100) | <0.001 | <0.001 |
| CABG | 0 | 2 (0) | 1 (1) | 14 (2) | 0 | 32 (3) | 2 (3) | 29 (3) | <0.001 | < 0.001 |
| Intra-aortic balloon pump | – | – | 11 (12) | 3 (0.5) | 8 (8) | 3 (0.3) | 9 (14) | 7(0.8) | 0.76 | 0.39 |
| Complications, n (%) | ||||||||||
| Atrial fibrillation | 15 (19) | 132 (22) | 27 (29) | 86 (15) | 18 (18) | 83 (9) | 13 (20) | 84 (9) | <0.001 | < 0.001 |
| Ventricular fibrillation | 8 (10) | 22 (4) | 14 (15) | 19 (3) | 6 (6) | 15 (2) | 5 (8) | 13 (2) | 0.001 | 0.25 |
| Atrioventricular block | 24 (30) | 54 (9) | 12 (13) | 33 (6) | 8 (8) | 15 (2) | 7 (11) | 29 (3) | <0.001 | <0.001 |
| Reinfarction | – | – | 3 (3) | 17 (3) | 8 (8) | 18 (2) | 1 (2) | 16 (2) | 0.81 | 0.17 |
| Stroke | – | – | 2 (2) | 9 (2) | 4 (4) | 11 (1) | 1 (2) | 4 (0.4) | 0.09 | 0.03 |
| Major bleeding | – | – | – | – | 6 (6) | 33 (3) | 1 (2) | 8 (1) | < 0.001 | < 0.001 |
| Discharge medicationsb, n (%) | ||||||||||
| Antiplatelet agents | 13 (56.5) | 493 (90) | 30 (67) | 493 (88) | 28 (38) | 858 (90) | 34 (74) | 823 (94) | 0.61 | 0.001 |
| Statins | 1 (4) | 22 (4) | 8 (18) | 250 (45) | 17 (23) | 681(71.5) | 29 (83) | 736 (87) | <0.001 | <0.001 |
| Beta-blockers | 5 (22) | 247 (45) | 15 (33) | 334 (59.5) | 17 (23) | 621 (65) | 23 (66) | 692 (81) | 0.004 | <0.001 |
| ACE inhibitors or ARBs | 9 (39) | 285 (52) | 16 (36) | 296 (53) | 18 (25) | 590 (62) | 28 (80) | 645 (76) | 0.007 | <0.001 |
| Diuretics | – | – | 18 (40) | 196 (35) | 21 (29) | 357(37.5) | 29 (83) | 367 (43) | 0.001 | 0.001 |
- CABG, coronary artery bypass grafting; CS, cardiogenic shock; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction.
- –, not available.
- a P-values are P for trends by period using χ2 tests for discrete variables. For continuous variables P-values are for two-way analysis of variance.
- b In 1995, medications prescribed in 5-day survivors instead of discharge medications.
Median CK values remained unchanged in CS patients with STEMI (from 960 IU/L in 1995 to 1097 IU/L in 2010; P = 0.73), but tended to decrease in NSTEMI patients (from 608 IU/L in 1995 to 398 IU/L in 2010, P = 0.24).
Both elderly patients with and without CS during hospitalization for AMI were more likely to be prescribed antiplatelet agents, low molecular weight heparin, lipid-lowering agents, and beta-blockers over time, whereas use of ACE inhibitors increased only in the CS group.
Use of PCI increased markedly in elderly patients with and without CS, reaching 51% and 59%, respectively, in 2010, as did reperfusion therapy in STEMI, which reached 57% and 66.5%, respectively, in 2010. In elderly patients with CS, use of PCI increased across all age groups: 75–79 years from 22% to 44%, 80–84 years from 12% to 70%, and ≥85 years from 6% to 36%.
Use of inotropes decreased markedly in elderly patients with CS, from 53% in 2000 to 25% in 2010, P < 0.001. Data on use of a IABP and assist devices were collected since 2000; IABP use remained stable from 12% in 2000 to 14% in 2010 (P for trend 0.76), and assist devices were used in 2.2% of CS patients in 2000 and 3.1% in 2010 (P for trend 0.77).
Independent predictors of development of cardiogenic shock
Age, STEMI, and history of heart failure were predictors of risk of CS common to elderly and younger patients (Table 2). A more recent time period was an independent correlate of decreased risk of CS occurrence in elderly patients, but only a trend was noted in patients <75 years.
| Age ≥75 years | Age <75 years | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age (per year) | 1.04 (1.01–1.06) | 1.05 (1.04–1.07) |
| STEMI | 1.73 (1.34–2.24) | 1.82 (1.36–2.44) |
| History of heart failure | 1.71 (1.26–2.32) | 3.73 (2.42–5.75) |
| Peripheral artery disease | 1.49 (1.08–2.05) | 1.33 (0.88–2.02) |
| Period (reference 1995) | ||
| 2000 | 1.19 (0.85–1.66) | 1.03 (0.72–1.49) |
| 2005 | 0.93 (0.67–1.29) | 1.23 (0.86–1.75) |
| 2010 | 0.63 (0.44–0.91) | 0.85 (0.58–1.24) |
| History of hypertension | 0.93 (0.72–1.21) | 0.71 (0.54–0.93) |
| Hypercholesterolaemia | 0.80 (0.62–1.04) | 0.75 (0.58–0.98) |
- CI, confidence interval; OR, odds ratio; STEMI, ST-segment elevation myocardial infarction.
Trends in 30-day and 1-year mortality in elderly patients according to the presence or absence of cardiogenic shock
Thirty-day mortality decreased from 83.5% in 1995 to 46.9% in 2010 (P for trend <0.001) (Supplementary material online, Figure S2).
One-year mortality also decreased in elderly patients with CS (−32%), but remained considerably higher at all time points compared with that in elderly patients without CS (Figure 3).
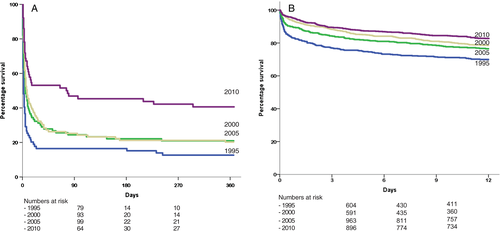
The excess of death associated with CS persisted in the 1-month survivors (adjusted HR 2.29, 95% CI 1.60–3.29, P < 0.001) (Supplementary material online, Figure S3).
In elderly patients, 30-day (Supplementary material online, Figure S4) and 1-year mortality decreased consistently over time within each age group (1-year mortality: 75–79 years from 100% to 50%, 80–84 years from 84% to 55%, and ≥85 years from 83% to 68%).
Of note, the decrease in mortality was confined to the acute stage: mortality from 30 days to 1 year remained unchanged from 1995 (23.1%) to 2010 (23.5%) (HR 2010 vs. 1995 1.09, 95% CI 0.29–4.10) (Supplementary material online, Figure S5).
Independent correlates of 1-year mortality in patients with cardiogenic shock according to age
In elderly patients with CS, STEMI and previous AMI were independent correlates of increased 1-year death, while study period was associated with decreased mortality (2010 vs. 1995: HR 0.40, 95% 0.27–0.61, P < 0.001), along with early use of PCI (Table 3). Age was not a correlate of mortality. In contrast, in younger patients developing CS, age was an independent correlate of increased 1-year death, while beta-blockers, early PCI use, and smaller infarct size assessed by maximal CK value were associated with decreased mortality in 3-day survivors (Supplementary material online, Table S1).
| HR | 95% CI | P-value | |
|---|---|---|---|
| Model 1: whole population | |||
| Period (reference 1995) | <0.001 | ||
| 2000 | 0.58 | 0.41–0.82 | |
| 2005 | 0.66 | 0.47–0.93 | |
| 2010 | 0.40 | 0.27–0.61 | |
| STEMI | 1.76 | 1.31–2.37 | <0.001 |
| Model 2: 3-day survivors | |||
| Period (reference 1995) | 0.59 | ||
| 2000 | 0.89 | 0.48–1.65 | |
| 2005 | 1.19 | 0.58–2.42 | |
| 2010 | 0.81 | 0.35–1.90 | |
| Early PCI | 0.47 | 0.29–0.75 | 0.002 |
| Maximal CK (by 10 U) | 1.001 | 1.000–1.002 | 0.004 |
| Previous MI | 1.64 | 1.03–2.60 | 0.036 |
- CI, confidence interval; CK, creatine kinase; HR, hazard ratio; MI, myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
- Model 1: including age, sex, risk factors, previous history, type of MI, and period.
- Model 2: Model 1 + early statin, beta-blockers, ACE inhibitors + early PCI (≤72 h).
Subgroup analyses in elderly patients with cardiogenic shock
One-year mortality decreased in all subsets of patients: from 91% to 61% in men and from 84% to 58% in women; from 87% to 67% in patients without early PCI and from 89% to 52% in those with early PCI; from 82% to 48% in patients with peak CK less than the median value and from 88% to 74% in those with peak CK higher than the median. Data on medications used before the index AMI were collected since 2000; mortality decreased markedly in patients receiving ACE inhibitors or ARBs before the index AMI (from 69% to 32%), but did not change in those not receiving these drugs (from 69% to 61%).
Of note, 30-day mortality decreased whatever the initial management of the patients (i.e. it decreased in patients both with and without early PCI, early ACE inhibitors, or early beta-blockers) (Supplementary material online, Figure S6).
Discussion
The results of these four nationwide registries implemented 5 years apart covering a period of 15 years provide insights into changing trends in the characteristics, management, and outcomes of elderly patients with CS complicating AMI. The prevalence of CS decreased in elderly patients but remained higher compared with younger patients at each time point. In the elderly population, older age, STEMI, and history of heart failure increased the risk of developing CS. Over the 15-year period, mean age remained constant, but the prevalence of risk factors increased. One-year mortality has declined markedly over the study period but remained much higher in patients with CS compared with patients without CS. The use of PCI increased considerably in elderly patients with CS, reflecting changes in management patterns also observed in those without CS. In addition, medical therapy also evolved, with more patients receiving early treatment with antiplatelet agents, low molecular weight heparin, lipid-lowering agents, and beta-blockers in the most recent period, whereas use of inotropes decreased.
Before 2005, the reported prevalence of CS in AMI was ∼8%,2, 5 and it has decreased to 4–5% in more contemporary cohorts.19, 20 Our results in elderly patients are consistent with these findings, showing that the decreased prevalence of CS is found both in younger and in older patients.
Development of CS remains a particular clinical concern in elderly patients with AMI, as age is a known risk factor for CS,5-7, 21, 22 and because of its very high mortality in these patients; indeed, 1-year mortality was still 59% in our most recent survey.
The previous studies assessing the outcome of elderly patients with CS complicating AMI reported a wide range of mortality rates, from 55% to 78%, reflecting both the study period and the timing of outcome assessment (in-hospital, 30-day, or 1-year mortality).4, 5, 8, 9, 22
In 20 elderly patients with CS, Samadi et al. reported an extremely high 6-month mortality (78%), which was comparable with our results during the same period (2000–2005).9 Dzavik et al. analysed the SHOCK registry between 1993 and 1997 and reported in-hospital mortality rates of 76%.8 Likewise, Jeger et al., in a large series of CS patients recruited from 1997 to 2006, found a prevalence of 8.3% for CS, and reported in-hospital death rates of 74% in patients aged 75 years or more.5
Lim et al. assessed 421 consecutive patients presenting with MI and CS who underwent PCI from the Melbourne Interventional Group registry from 2004 to 2011 and reported a 1-year mortality at 55% in 121 elderly patients with CS.4 As in our series, where survival improved notably during this 15-year period (from 13% in 1995 to 41% in 2010), most recent data report improved outcomes over the past 10–20 years.
In addition, several studies have suggested that PCI improved short-term survival in patients with CS, with survival contingent on the successful establishment of coronary reperfusion.1, 18 In all studies that assessed changing trends in the incidence and outcome of CS after AMI, an increase in the use of PCI was found, with a concomitant decline in short-term mortality.2, 5, 6, 23-25
In the SHOCK trial, however, subgroup analyses revealed an interaction between age and the impact of PCI; there was little or no effect of invasive treatment on mortality in elderly patients, in contrast to what was observed in younger patients.1 However, these findings were not corroborated in the SHOCK trial registry, in which early revascularization in the elderly was associated with better in-hospital survival.8 These latter results were concordant with other observational studies.2, 4, 10 The most recent guidelines advocate emergency PCI for patients with CS due to STEMI or NSTEMI (IB) whatever the age of patients.15
It must be kept in mind, however, that the improvement in mortality may also have been related to the use of other recommended measures and to improved overall management of the patients, as suggested by the fact that 1-year mortality significantly decreased according to the time period, independently of the use of PCI. Increasing use of early prevention medications and better overall management could have contributed to this improvement. From 1995 to 2010, we observed an increased early use of antithrombotic agents, beta-blockers, or statins, while ACE inhibitor use remained unchanged. Whether the increased use of these agents has a causal role in the improvement in mortality in this elderly population is speculative. Of note, however, mortality decreased both in patients receiving such medications and in those who did not; likewise, although mortality was higher in the patients without early PCI at all time points, it decreased over time in grossly similar proportions in both patients with or without early PCI (Supplementary material online, Figure S6).
The fact that mortality did not improve over time after the first 30 days, in spite of improved secondary prevention prescription at discharge, is surprising; it is likely that a longer observation period would have been necessary to detect a significant benefit of secondary prevention medications, in particular when considering the expected risk of competitive non-cardiovascular mortality in this elderly population.
Interestingly, the use of catecholamine therapy decreased over time. During the last period of our study, catecholamines were required in only 25% of cases. This finding may be due to the fact that CS might have been present in less severe forms of MI in the most recent period (e.g. more NSTEMI patients in the 2005 and 2010 cohorts), to a lack of evidence of the clinical benefits and the fear of potential harm with inotropes in CS, as well as to disparities in clinical practice.26 The 2012 European guidelines recommend many drugs with low levels of evidence: inotropes (e.g. dobutamine, IIaC) as first-line therapy, phosphodiesterase inhibitors (e.g. levosimendan, IIbC) to reverse the effect of beta-blockade, and vasopressors (e.g. dopamine or norepinephrine, IIbC) as second-line therapy.27 Finally, increased use of circulatory assist devices may explain the reduced catecholamine requirements.28, 29 In our population, however, the use of assist devices and IABPs was relatively marginal and did not change over time.
Beta-blockers were used in a substantial proportion of patients. In fact, beta-blocker use may have preceded the development of CS and precipitated its occurrence (40% of the patients with CS developing after admission had received beta-blockers vs. 28% of those with shock on admission). Early use of beta-blockers at the acute stage in patients with CS is associated with increased hazard; thus, Van Diepen et al. assessed 240 CS patients of whom 66 patients received either beta-blockers or renin–angiotensin–aldosterone system blockers administered within the first 24 h after the CS diagnosis.30 The observed 30-day mortality was higher in patients who received beta-blockers or renin–angiotensin–aldosterone system blockers prior to CS resolution (27.3% vs. 16.9%, P = 0.035).
Strengths and limitations
Our study is one of the largest to report long-term mortality of elderly patients presenting AMI complicated by CS. All four registries were performed using a similar methodology at institutions representing a vast majority of those taking care of patients with AMI in France during the study period. The results can therefore be considered representative of the clinical picture of these patients during the period studied.
Presence of CS was reported by the clinicians at each centre and, although the formal definition of CS did not evolve in the study period, it is possible that clinicians became more restrictive in their appraisal of the patients' conditions in the most recent periods; this, however, would probably have resulted in a population at higher risk in the most recent periods, instead of the inverse trend that we observed.
This study is a non-randomized, observational study and cannot provide a formal demonstration of the efficacy (or harm) of any management strategy. Therefore, correlations between early management and outcomes must be considered with caution. Finally, cause of death was not collected in the first two surveys; it must be kept in mind, however, that mortality in the year following an AMI is most often of cardiovascular origin.
Conclusion
In these four nationwide surveys conducted over a period of 15 years, the prevalence of CS at the acute stage of MI in elderly patients has decreased by half. Although 1-year mortality declined by 32% in these patients, mostly driven by improved early survival, it remains very high and is still a matter of clinical concern. A broader use of PCI and improved overall management of these patients are likely to explain the improvement in outcome observed.
Acknowledgements
Special thanks to Vincent Bataille, for his careful data management, to Benoît Pace (Société Française de Cardiologie) for his invaluable assistance in designing the electronic CRF, and to Geneviève Mulak (Société Française de Cardiologie) and Elodie Drouet, who supervised the patients' follow-up. Our thanks to the devoted personnel of URCEST (Hôpital St Antoine, Assistance Publique-Hôpitaux de Paris) who ensured patient follow-up in the FAST-MI 2005 and 2010 surveys. We are indebted to all patients having accepted to participate in the surveys, and to all participating physicians.
Funding
USIK 1995 was funded by Laboratoire Roussel, which was involved in the design and conduct of the study, as well as data collection and management. Laboratoire Roussel was not involved in the analysis and interpretation of the data, nor in the preparation, review, or approval of the manuscript. USIC 2000 was funded by Aventis-France, which was involved in the design and conduct of the study, as well as data collection and management. Aventis-France was not involved in the analysis and interpretation of the data, nor in the preparation, review, or approval of the manuscript. FAST-MI 2005 and FAST-MI 2010 are registries of the French Society of Cardiology. FAST-MI 2005 was supported by unrestricted grants from Pfizer and Servier, and an additional grant from the Caisse Nationale d'Assurance Maladie-Travailleurs Salariés. Pfizer, Servier, and the Caisse Nationale d'Assurance Maladie had no role in the design and conduct of the study, data collection, and management. They were not involved in the analysis and interpretation of the data, nor in the preparation, review, or approval of the manuscript. FAST-MI 2010 was supported by unrestricted grants from AstraZeneca, the Daiichi-Sankyo-Eli-Lilly alliance, GlaxoSmithKline, Merck, Novartis, and Sanofi-Aventis. None of the companies had a role in the design and conduct of the study, data collection, and management. They were not involved in the analysis and interpretation of the data, nor in the preparation, review, or approval of the manuscript.
Conflict of interest: N.A., AstraZeneca; E.P., Amgen, AstraZeneca, Bayer, BMS, Daiichi-Sankyo, Lilly, MSD, The Medicine Company, SJM, Sanofi, and Servier; N.D., research grants from Amgen, AstraZeneca, Bayer, Daiichi-Sankyo, Eli-Lilly, GSK, Merck, Novartis, Pfizer, Sanofi-Aventis, and fees for lectures or consulting for Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, Eli-Lilly, GlaxoSmithKline, MSD-Schering, Novartis, Novo-Nordisk, Pfizer, Roche, Sanofi-Aventis, Servier, and The Medicines Company. All other authors have no conflicts to declare.




