Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review
Abstract
The ‘epidemic’ of heart failure seems to be changing, but precise prevalence estimates of heart failure and left ventricular dysfunction (LVD) in older adults, based on adequate echocardiographic assessment, are scarce. Systematic reviews including recent studies on the prevalence of heart failure and LVD are lacking. We aimed to assess the trends in the prevalence of LVD, and heart failure with reduced (HFrEF) and preserved ejection fraction (HFpEF) in the older population at large. A systematic electronic search of the databases Medline and Embase was performed. Studies that reported prevalence estimates in community-dwelling people ≥60 years old were included if echocardiography was used to establish the diagnosis. In total, 28 articles from 25 different study populations were included. The median prevalence of systolic and ‘isolated’ diastolic LVD was 5.5% (range 3.3–9.2%) and 36.0% (range 15.8–52.8%), respectively. A peak in systolic dysfunction prevalence seems to have occurred between 1995 and 2000. ‘All type’ heart failure had a median prevalence rate of 11.8% (range 4.7–13.3%), with fairly stable rates in the last decade and with HFpEF being more common than HFrEF [median prevalence 4.9% (range 3.8–7.4%) and 3.3% (range 2.4–5.8%), respectively]. Both LVD and heart failure remain common in the older population at large. The prevalence of diastolic dysfunction is on the rise and currently higher than that of systolic dysfunction. The prevalence of the latter seems to have decreased in the 21st century.
Introduction
Heart failure is a leading cause of morbidity and mortality, and causes high health-care-related costs, posing a great burden on both patient and society.1 It mainly affects older people, and incidence and prevalence rise steeply with age in those aged over 60 years.2 The most often mentioned prevalence estimate for the adult population at large is 2% (1–3%), and 5–9% selectively in those aged 65 years and over.3-8
The syndrome of heart failure has been compared with an iceberg. The visible section represents established heart failure cases in the community: the majority managed in the primary care setting and the top managed by the cardiologists. The larger invisible part ‘below the water level’ represents cases of undetected heart failure, and those with asymptomatic left ventricular dysfunction considered prone to developing heart failure.9
Left ventricular dysfunction encompasses systolic and diastolic dysfunction. Systolic dysfunction is commonly defined in terms of reduced left ventricular ejection fraction (LVEF). A LVEF above 50% is generally considered normal and below 40% as reduced, leaving a ‘grey zone’ from 40 to 50%.10 Diastolic dysfunction refers to an abnormality in the filling properties or suction capacity of the left ventricle, and is only separately classified in those with a LVEF >50%. Importantly, no single echocardiographic parameter on its own is considered sufficiently accurate and reproducible to establish the diagnosis of diastolic dysfunction. Both structural (left ventricular hypertrophy, enlarged left atrium) and functional abnormalities should be evaluated. For the latter, tissue Doppler imaging (TDI) with measurements of the velocity of mitral annular motion is now considered crucial.3, 11
The epidemiology of ventricular dysfunction and heart failure has been studied in different settings. An overview that includes recent studies on precise prevalence data in the older population at large, based on adequate echocardiographic assessment, is lacking. Moreover, with the aging of the population, improved survival of acute coronary disease, better treatment of heart failure, and the increase of comorbidities, the epidemic of heart failure seems to be changing. We aimed to assess the trends in the prevalence of ventricular dysfunction and heart failure with reduced (HFrEF) and preserved ejection fraction (HFpEF) in the older population at large.
Methods
Literature search
We performed a systematic electronic search of the databases Medline and Embase. The entire time-scale was used, up to September 2014. Search terms used were synonyms of ‘heart failure’ and ‘ventricular dysfunction’ combined with synonyms of epidemiology and older people. The exact search strategy is presented in Box 1. Of the studies retrieved for full text assessment, reference lists were screened for other relevant studies. Review publications on associated topics were checked in order not to miss any important related articles.
Selection of publications
We screened title and abstract of all studies for eligibility. If more information was needed on abstracts we also screened the full text. Studies published in English, Dutch, and German were considered. The following predefined inclusion criteria were applied: (i) the study reported the prevalence of ventricular dysfunction or heart failure in those aged 60 years or over, or prevalence could be calculated for this age category; (ii) the study population was derived from the older population at large; and (iii) echocardiography was used to establish the diagnosis. If more than one article was based on the same cohort, the report with the greatest number of participants was selected for data extraction to obtain prevalence estimates.
Box 1. Search terms used
(“heart failure” OR “cardiac decompensation” OR “heart decompensation” OR “cardiac dysfunction” OR “heart dysfunction” OR “systolic dysfunction” OR “diastolic dysfunction” OR “ventricular dysfunction” OR “hf ref” OR “hf nef” OR “hf pef” OR“hf”) AND (“prevalence” OR “prevalent”) AND (“elderly” OR “elder” OR “elders” OR “old” OR “older” OR “geriatric” OR “geriatrics” OR “sexagenarian” OR “sexagenarians” OR “septuagenarian” OR “septuagenarians” OR “octogenarian” OR “octogenarians” OR “nonagenarian” OR “nonagenarians” OR “aged”)
Quality assessment
We performed a methodological quality assessment of each of the studies included. As there is no established checklist available to appraise the risk of bias of prevalence studies, we based our assessment on the approach of the QUADAS-2 (Tool for the Quality Assessment of Diagnostic Accuracy Studies).12 Signalling questions were used to identify potential problems in the design, conduct, and analysis of a study that might introduce bias (Box 2). The following items were covered: purpose and design of data collection, method of sampling of the participants, selection criteria used, response rate, performance of the same data collection pathway in all participants, and handling of missing data.
Signalling questions on the items a–c and e in Box 2 were answered with yes, no or unclear. Extra categories were used for Box 2d to score whether or not an individual study with a response <70% included a description of non-responders, and for Box 2f to score the handling of missing data. Scores on the different items, with a maximum of one point each, were added. Overall risk of bias was considered high (≤3 points), medium (between 3 and 5 points), or low (≥5 points). Two of three reviewers (E.vR. and F.H. or K.W.) independently scored the studies. Consensus was used to resolve disagreement. If consensus could not be reached, the third reviewer was consulted.
Box 2. Risk of bias assessment
| a. Are the data prospectively collected with the purpose of measuring the prevalence of ventricular dysfunction or heart failure? |
| • Yes |
| ° No |
| * Unclear |
| b. Was an unselected (random/consecutive) sample of patients invited to participate? |
| • Yes |
| ° No |
| * Unclear |
| c. Is it unlikely that the selection criteria introduced bias? |
| • Yes |
| ° No |
| * Unclear |
| d. Is the response rate ≥70%? |
| • Yes |
| Θ No, but information on non-responders is described |
| ° No, and information on non-responders is not described |
| * Unclear |
| e. Are the data collection methods standardized and identical for all respondents? |
| • Yes |
| ° No |
| * Unclear |
| f. Is it unlikely that the handling of missing (endpoint) data introduced bias? |
| • Yes |
| Θ No, complete case analysis |
| ° No, missings included in analysis as non-diseased |
| * Unclear |
Data extraction and analysis
Information on study characteristics was collected with a standardized data extraction form that included the participation rate, the number and type of participants, and the echocardiographic measurements that were used.
Prevalence estimates were taken directly from the reports when stated for (a subgroup of) subjects aged 60 years or over, specified by gender if possible. If only data were given for a broader age range, we calculated the prevalence for the relevant subgroup when sufficient information was provided. We decided not to pool the prevalence estimates because of the large variability in design and case definitions between studies. Median prevalence estimates were calculated.
When we mention ventricular (systolic or diastolic) dysfunction, we consider both asymptomatic and symptomatic left ventricular dysfunction. Symptomatic left ventricular dysfunction can be considered as heart failure, in accordance with the European Society of Cardiology (ESC) guidelines on heart failure.3
We defined systolic dysfunction as a LVEF <50%. If studies on systolic dysfunction gave multiple prevalence estimates for different cut-points for LVEF, only the prevalence from the highest threshold was considered for calculating the median. Because almost all patients with systolic dysfunction also have some degree of diastolic dysfunction, and systolic dysfunction may be present in preserved global LVEF,13 we did not consider the combination of systolic and diastolic dysfunction as a separate entity.
Data on heart failure were divided into HFrEF and HFpEF, if possible. We considered ‘all type’ heart failure as HFrEF and HFpEF taken together. However, some studies, explicitly included heart failure caused by valvular disease and pericardial disease in their estimates, and two studies also mentioned right-sided heart failure as a separate entity of the heart failure spectrum. These separate types of heart failure were all included in our prevalence estimates of ‘all type’ heart failure.
In summarizing the results, a distinction was made between studies that included the total older population at large (e.g. all subjects aged ≥60 or ≥65 years) and studies including specific age groups only.
Forest plots were formed to visualize the percentages of persons with systolic dysfunction and ‘all type’ heart failure with corresponding 95% confidence intervals. In the other categories, given the limited amount of data, forest plots were not constructed.
Results
Search, selection and quality assessment
A flowchart of the literature search and the selection of articles are shown in Figure 1. The search resulted in 6167 citations. In total, 28 articles from 25 study populations (three studies wrote separate reports on systolic and diastolic ventricular dysfunction) were included in our systematic review.4, 14-40 Two articles were found by screening of the reference lists. One lacked ‘older’ in title and abstract,18 and the other was primarily about valvular heart disease.20
Study characteristics and quality parameters are shown in Table 1. Most articles had a low (n = 13) or medium (n = 12) risk of bias. There were two articles with an unclear risk of bias, and one with a high risk of bias. Most studies did not receive the maximum score on the handling of missing data (Box 2f).
| First author, Country and Publication year | Source population and time of assessment | Participation rate | Age | N (male) | Echocardiographic measurements (method) | Risk of bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | Overall | ||||||
|
Pandhi, USA, 2011 |
Randomly sampled independent living individuals from Medicare lists and age-eligible persons from the same household in 1989–1993 | 61% | ≥65 | 5649 (42%) |
Systolic: EF (qualitative), LV fractional shortening Diastolic: not assessed |
• | • | • | Θ | • | Θ | Low |
| Kupari, Finland, 1997 | Random residents of Helsinki born in 1904, 1909 and 1914 selected in 1990–1991 | 77% | 75–86 | 476 (27%) |
Systolic: LV fractional shortening Diastolic: not assessed |
• | * | • | • | • | Θ | Medium |
| Mosterd, Netherlands, 1999 | All inhabitants of Ommoord, a suburb of Rotterdam in 1990–1993 | NS | 65–94 | 1037 (46%) |
Systolic: LV fractional shortening Diastolic: not assessed |
• | • | • | • | • | Θ | Low |
| Morgan, UK, 1999 | Random sample of general practices in Poole, Dorset in 1992 | 77% | 70–84 | 817 (46%) |
Systolic: qualitative and where possible EF (Simpson's rule) Diastolic: not assessed |
• | • | • | • | • | • | Low |
| McDonagh, UK, 1997 | Attendees of the third Glasgow MONICA coronary-risk-factor survey in 1992 | 83% | 65–75 | 319 (49%) |
Systolic: EF (Simpson's rule) Diastolic: not assessed |
• | • | • | • | • | Θ | Low |
| Fischer, Germany, 2003 | Random subjects from Augsburg in 1994–1995 | NS |
>60 > 65 |
372 (47%) NS |
Systolic: EF (Teichholz's formula) Diastolic: E/A ratio, isovolumetric relaxation time |
• | • | • | * | • | Θ | Medium |
| Di Bari, Italy, 2004 | Entire unselected non-institutionalized community-dwelling elderly population in Dicomano in 1995 | 71% | ≥65 | 553 (42%) |
Systolic: EF (area-length formula or Teichholz's formula) Diastolic: E/A ratio, isovolumetric relaxation time |
○ | • | • | Θ | • | Θ | Medium |
| Davies, UK, 2001 | Random sampling from primary care practices in 1995–1999 | 63% | ≥65 | 1452 (49%) |
Systolic: EF (single plane ellipse formula) or qualitative Diastolic: not assessed |
• | • | • | Θ | • | • | Low |
| Hedberg, Sweden, 2001 | Random 75-year-old residents living in Västerås in 1997 | 70% | 75 | 412 (49%) |
Systolic: EF (Simpson's rule) or wall motion index Diastolic: not assessed |
• | • | • | • | • | Θ | Low |
| Redfield, USA, 2003 | Random residents of Olmsted County in 1997–2000 | 47% | ≥65 |
815 (45%) 668 (43%) |
Systolic: EF (modified Quinones formula, Simpson's rule, semiquantitative 2-D visual method) Diastolic: E/A ratio, deceleration time, S/D, MV Adur/PV Adur, E/e' ratio |
• | • | • | Θ | • | Θ | Low |
| Raymond, Denmark, 2003 | Individuals from randomly selected general practices living at home in 1997–2000 | 70% | 60–89 | 524 (41%) |
Systolic: EF (multiplying 9th segment wall motion index score by 30) Diastolic: not assessed |
• | • | • | • | • | Θ | Low |
| Ceia, Portugal, 2002 | Systematic sampling of subjects attending randomly selected primary health care centres in 1998 | 98% | ≥60 | 4964 (NS) |
Systolic: LV shortening fraction Diastolic: LA dilatation, LV mass, LV posterior wall and inter-ventricular septum thickness |
• | • | ○ | • | ○ | Θ | Medium |
| Alehagen, Sweden, 2009 | Entire unselected elderly population of a rural municipality in 1998 | 75% | 70–80 | 876 (49%) |
Systolic: EF (semiquantitative) Diastolic: E/A ratio, deceleration time |
• | • | • | • | • | * | Low |
|
Ceia, Madeira 2005 |
Systematic sampling of subjects attending randomly selected primary health care centres in 2000–2001 | NS | ≥60 | 456 (NS) |
Systolic: LV shortening fraction Diastolic: LA dilatation, LV mass, LV posterior wall and inter-ventricular septum thickness |
• | • | ○ | • | ○ | Θ | Medium |
| Galasko, UK, 2005 | Random selected people from general practices in Harrow in 2000–2001 | 53% | ≥65 | NS |
Systolic: EF (Simpson's rule) |
• | • | • | Θ | • | Θ | Low |
| Azevedo/Goncalves, Portugal, 2006/2010 | Sample of non-institutionalized participants of a population health survey in 2001–2003 | 70% | ≥65 |
294 (45%) 244 |
Diastolic: not assessed Systolic: EF (Simpson's rule or visual estimate) Diastolic: E/A ratio, deceleration time, isovolumetric relaxation time |
• • | • • | * ○ | • • | • • | * Θ | Unclear/Medium |
| Abhayaratna, Australia, 2006 | Random residents of Canberra living at home in 2002–2003 | 75% | 60–86 | 1275 (50%) |
Systolic: EF (Simpson's rule) Diastolic: E/A ratio, deceleration time, S/D ratio, MV Adur/PV Adur, E/e′ ratio |
• | • | • | • | • | Θ | Low |
| van Bemmel, the Netherlands, 2010 | Members of the 1912–1914-birth cohort in Leiden who survived up to age 90 years in 2002–2004 | 87% | 90 | 81 (33%) |
Systolic: EF (Teichholz's formula) Diastolic: E/A ratio |
○ | • | • | • | • | * | Medium |
| Tiller, Germany, 2013 | Randomly sampled independent living inhabitants of the city of Halle in 2002–2006 | 64% | 60–83 | 1075 (54%) |
Systolic: EF (Teichholz's formula) and LV diameter index Diastolic: E/A ratio, deceleration time, left atrial diameter index, LV mass index |
• | • | • | Θ | • | Θ | Low |
| Anguita Sanchez, Spain, 2008 | Random individuals registered to participating centres in 2004–2005 | 66% | ≥65 | 824 (43%) |
Systolic: EF (NS) Diastolic: NS |
• | • | • | ○ | ○ | * | High |
| Leibowitz, Israel, 2011 | Random sample of individuals born in 1920 and 1921 and living in Jerusalem in 2005 | NS | 85 | 432 (49%) |
Systolic: EF (area-length formula) Diastolic: E/A ratio, deceleration time, isovolumetric relaxation time, E/e′ ratio |
• | * | • | * | • | Θ | Medium |
| Yousaf, UK, 2012 | People living in Newcastle or North Tyneside listed on GP patient lists in 2006 | 81% | 87–89 | 376 (38%) |
Systolic: EF (semiquantitative 2-D visual method) Diastolic: E/e′ ratio, E/A ratio, deceleration time |
• | * | • | • | • | Θ | Medium |
| Zhou, China, 2014 | Community residents of Shanghai in 2007–2008 | NS | ≥65 | 1166 (40%) |
Systolic: EF (Simpson's rule) Diastolic: E/e′ ratio, E/A ratio, deceleration time, left atrial volume index, LV mass index |
• | * | * | * | • | Θ | Unclear |
| Mureddu, Italy, 2012 | Random residents in the Lazio Region living nearby eight Community Hospitals in 2007–2010 | 34% | 65–84 |
2001 (52%) 1720 |
Systolic: EF (modified Simpson's rule) or fractional shortening (modified ellipsoidal model) Diastolic: E/A ratio, deceleration time, S/D, MV Adur/PV Adur, E/e′ ratio |
• | * | • | Θ | • | ○ | Medium |
| Vaes, Belgium, 2012 | Subjects from 29 general practices in three areas of Belgium in 2008–2009 | NS | >80 | 548 (37%) |
Systolic: EF (Simpson's rule) Diastolic: septal and lateral e′, E/A ratio |
• | * | • | * | • | Θ | Medium |
- N, number of participants analysed; EF, ejection fraction; LV, left ventricular; E, peak early mitral inflow filling velocity; A, peak mitral filling velocity at atrial contraction; S, peak velocity pulmonary venous forward flow during systole; D, peak velocity of pulmonary venous forward flow during diastole; MV Adur, duration of mitral A wave; PV Adur, duration of pulmonary venous reversal wave at atrial contraction; e′, peak velocity of mitral annulus motion during early diastole; NS, not stated; 2-D, two-dimensional.
- Score of points for risk of bias assessment: * or ○ zero points, Θ half a point, • one point. Articles with more than two * were not rated on risk of bias because not enough information was provided.
Study populations and echocardiographic measurements
Twelve studies used cross-sectional data from community-based longitudinal cohorts, six studies were based on samples of population registries or the total unselected population of a certain municipality, and seven studies used registries or attending records from primary care.
The size of the study population ranged from 81 to 5649 participants [median 815, interquartile range (IQR) 432–1166]. The proportion of males ranged from 27% to 54% (median 46%, IQR 41–49%).
Sixteen study populations were representative for older people aged ≥60 years or ≥65 years. In five studies the population consisted of a subgroup of older people (e.g. 65- to 75-year-olds), and four study populations were of a specific age (e.g. 90-year-olds).
Nineteen studies used the LVEF to define systolic function, and most of these used the (modified) Simpson's rule or Teichholz's formula. One study did not state how systolic function was assessed. The cut-point for LVEF to define systolic dysfunction ranged from 30% to 50%. Fractional shortening was used in five, mostly older, studies. Thresholds of 0.25 or 0.28 were applied, corresponding to a LVEF of around 42.5% and 47.6%, respectively.41
Diastolic parameters were assessed in sixteen studies. Eight studies explicitly reported prevalence data on ‘isolated’ diastolic dysfunction or HFpEF. Of these, three studies solely used the ratio between the early (E) and late (A) ventricular filling velocity over the mitral valve (E/A ratio) and deceleration and/or isovolumetric relaxation time. One study added left atrial diameter index and left ventricular mass index to these measurements. Four studies used TDI to measure mitral early annular lengthening velocities (e′) of the septal and/or lateral wall, and calculated the ratio of mitral early diastolic inflow velocity to mitral early annular lengthening velocity (E/e′ ratio), a surrogate measure for filling pressures, this in combination with other diastolic measurements (Table 1). For the E/A ratio the lower threshold ranged from 0.5 to 1, and the upper threshold was either 1.5 or 2. E/e′ values >8, ≥10, or >13 were applied to define abnormal E/e′.
Prevalence of ventricular dysfunction
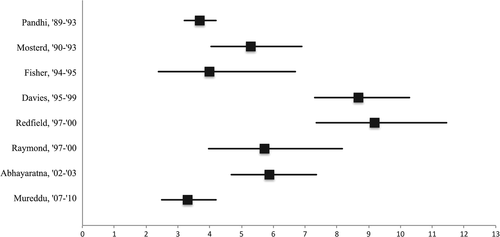
The median prevalence estimate for systolic dysfunction calculated from eight studies (n = 13,125) in the older population at large was 5.5% (range 3.3–9.2%; Table 2, Figure 2). The prevalence of systolic dysfunction was generally highest in the very old (aged ≥80 years) (Table 3). Systolic dysfunction was more common in men than women (Tables 2 and 3). We observed a peak in the prevalence of systolic dysfunction between the years 1995 and 2000.
| Author, inclusion period | Cut-point LVEF to separate systolic from diastolic dysfunction | Systolic dysfunction | Isolated diastolic dysfunction | ||
|---|---|---|---|---|---|
| All | Male | Female | All | ||
| Pandhi35, 1989–1993 | 45 | 3.7 | – | – | – |
| Mosterd33, 1990–1993 | 45 | 5.3 | 7.5 | 3.4 | – |
| Fischer25, 26, 1994–1995 | 50 | 4.0 | – | – | 15.8 |
| Davies23, 1995–1999 | 40 | 3.2 | 4.9 | 1.5 | – |
| 50 | 8.7 | 11.7 | 5.8 | – | |
| Redfield4, 1997–2000 | 40 | 3.3 | 5.7 | 1.4 | – |
| 50 | 9.2 | 14.3 | 4.9 | – | |
| Raymond36, 1997–2000 | 40 | 5.7 | 9.2 | 3.3 | – |
| Goncalves19, 2001–2003 | 45 | – | – | – | 52.8 |
| Abhayaratna14, 15, 2002–2003 | 40 | 2.1 | 3.3 | 0.9 | 29.1 |
| 50 | 5.9 | 8.6 | 3.1 | – | |
| Zhou40, 2007–2008 | 50 | – | – | – | – |
| Mureddu34, 2007–2010 | 40 | – | 2.5 | 0.5 | – |
| 50 | 3.3 | 5.1 | 1.4 | 42.8 | |
- LVEF, left ventricular ejection fraction.
| Author, Inclusion period | Cut-point LVEF to separate systolic from diastolic dysfunction | Age | Systolic dysfunction | Isolated diastolic dysfunction | ||
|---|---|---|---|---|---|---|
| All | Male | Female | All | |||
| Kupari29, 1990–1991 | 45 | 75–86 | 11.3 | – | – | – |
| Morgan32, 1992 | 40 | 70–84 | 2.4 | 4.5 | 0.7 | – |
| 50 | 70–84 | 7.5 | 12.8 | 2.9 | – | |
| McDonagh31, 1992 | 30 | 65–75 | 5.6 | 6.5 | 4.9 | – |
| Hedberg28, 1997 | 45 | 75 | 6.8 | 10.2 | 3.4 | – |
| Galasko27, 2000–2001 | 45 |
65–74 ≥75 |
6.3 10.0 |
10.8 13.2 |
0.0 7.1 |
– |
| 50 |
65–74 ≥75 |
7.0 13.7 |
12.0 21.1 |
0.0 7.1 |
– | |
| van Bemmel20, 2002–2004 | 50 | 90 | 8.6 | – | – | – |
| Leibowitz30, 2005 | 45 | 85 | 13.9 | – | – | – |
| Yousaf39, 2006 | 40 | 87–89 | 9.0 | – | – | - |
| 50 | 87–89 | 31.7 | – | – | 60.6 | |
| Vaes38, 2008–2009 | 40 | >80 | 1.6 | – | – | - |
| 50 | >80 | 5.8 | – | – | 51.3 | |
- LVEF, left ventricular ejection fraction.
Studies on diastolic function were mainly performed in the 21st century. Four studies (n = 3892) evaluated ‘isolated’ diastolic dysfunction in the total older population at large resulting in a median prevalence estimate of 36.0%, with a range of 15.8–52.8% (Table 2). Two studies (n = 924) in the very old reported that more than half of people aged ≥80 years had ‘isolated’ diastolic dysfunction (Table 3).
Prevalence of heart failure
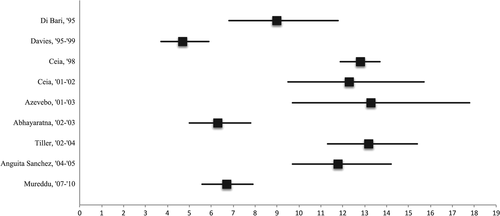
The median prevalence estimate for ‘all type’ heart failure from nine studies (n = 12,894) in the older population at large was 11.8% (range 4.7–13.3; Table 4, Figure 3). Few studies examined the prevalence of heart failure in specific age groups (Table 5). ‘All type’ heart failure prevalence estimates seem fairly ‘stable’ from 1998 onward, with estimates varying between 6.3% and 13.3%.
| Author, inclusion period | Cut-point LVEF to separate systolic from diastolic dysfunction | All-type heart failure | HFrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | ||
| Di Bari24, 1995 | 50 | 9.0 | 8.2 | 9.7 | – | – | – | – | – | – |
| Davies23, 1995–1999 | 40 | 4.7 | 5.7 | 3.8 | – | – | – | – | – | – |
| Raymond36, 1997–2000 | 40 | – | – | – | 4.0 | 5.5 | 2.9 | – | – | – |
| Ceia ‘0221, 1998 | 45 | 12.8 | – | – | – | – | – | – | – | – |
| Ceia ‘0522, 2000–2001 | 45 | 12.3 | – | – | – | – | – | – | – | – |
| Azevedo18, 2001–2003 | 45 | 13.3 | 9.8 | 16.0 | – | – | – | – | – | – |
| Abhayaratna14, 15, 2002–2003 | 50 | 6.3 | 8.2 | 4.4 | 2.5 | – | – | 3.8 | – | – |
| Tiller37, 2002–2004 | 50 | 13.2 | 12.8 | 13.6 | 5.8 | 7.2 | 4.1 | 7.4 | 5.7 | 9.5 |
| Anguita17, 2004–2005 | 45 | 11.8 | 12.3 | 11.0 | – | – | – | – | – | – |
| Mureddu34, 2007–2010 | 50 | 6.7 | 7.4 | 6.0 | 2.4 | 3.3 | 1.4 | 4.9 | 4.7 | 5.1 |
- LVEF, left ventricular ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFpEf, heart failure with preserved ejection fraction.
| Author, Inclusion period | Cut-point LVEF to separate systolic from diastolic dysfunction | Age | All-type heart failure | HFrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |||
| McDonagh31, 1992 | 30 | 65–75 | – | – | – | 3.5 | 3.2 | 3.7 | – | – | – |
| Hedberg28, 1997 | 45 | 75 | – | – | – | 3.6 | 4.9 | 2.4 | – | – | – |
| Alehagen16, 1998 | 40 | 70–80 | 17.6 | 18.9 | 16.3 | 4.5 | 6.5 | 2.5 | – | – | – |
| 50 | 70–80 | 24.5 | 29.3 | 19.9 | 11.4 | 16.9 | 6.1 | 13.1 | 12.5 | 13.8 | |
- LVEF, left ventricular ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFpEf, heart failure with preserved ejection fraction.
Median prevalence estimates of HFrEF and HFpEF were mentioned separately in four (n = 4,875) and in three (n = 4351) studies, and were 3.3% (range 2.4–5.8%) and 4.9% (range 3.8–7.4%), respectively. Men more often had HFrEF and women more often had HFpEF (Table 4). As almost all studies reporting HFpEF were performed in the 21st century, a trend in prevalence over a longer period remains unclear.
Discussion
Our systematic review shows that in community-dwelling people aged ≥60 years, diastolic left ventricular dysfunction is very common, with a median prevalence of 36.0% (range 15.8–52.8%), and systolic dysfunction is less common with a median of 5.5% (range 3.3–9.2%). A peak in prevalence of systolic dysfunction seems to have occurred between the years 1995 and 2000. ‘All type’ heart failure is prevalent in 11.8% (range 4.7–13.3%), with fairly stable estimates in the last decade, and with HFpEF being more common than HFrEF (median prevalence 4.9%, range 3.8–7.4%, and 3.3%, range 2.4–5.8%, respectively).
Because the large majority of studies was performed in Western Countries, our estimates apply to developed countries only.
Some of the studies included showed that heart failure is uncommon under the age of 60 years. Ceia et al.21 reported a prevalence of 1.2% in community-dwelling persons aged 25–49 years, and others reported a prevalence of 0.7–1.3% in people aged 45–54 years.4, 17, 23, 31 Considering that 30% of the adult population is aged 60 years or over in developed countries,42 extrapolation of the findings of our review would result in an estimated prevalence of heart failure of 11.8 × 0.30 + 1.0 × 0.70 = 4.2% for this adult population. This is substantially higher than the often mentioned estimate of 2% for heart failure in the adult population at large.3 By selectively including studies that were performed in the population at large in the review, cases of previously unrecognized heart failure were also included in the prevalence estimates.
Overall, the risk of bias of the studies included was limited. There were two articles with an overall unclear risk of bias and one with a high risk of bias according to our quality assessment. Their reported prevalence estimates, however, fall within the range of the other studies. Remarkably, most studies preferred complete case analysis, and excluded participants without an (interpretable) echocardiogram from the analysis. This might introduce bias because data are not missing completely at random.43 Reasons for absence or uninterpretability of an echocardiogram may be related to a worse physical state. Excluding those participants from the analysis without applying techniques to impute missing data, will have resulted in over-representation of the relatively fit older adults and thus underestimation of the prevalence.
Differences in prevalence estimates between studies may partly be caused by differences in the case-mix of the studies. Variation in age and gender distributions, and comorbidities of the participants could have influenced the results. Other important contributors to the observed variability in the reported prevalence are differences in case definition and echocardiographic measurements. The latter presumably played an important role in the wide range of prevalence estimates of diastolic dysfunction and HFpEF. While the earliest studies included in this review did not measure diastolic dysfunction, the more recent studies almost all used TDI, a capability that has been widely available since the year 2002 and is now considered crucial to help establish diastolic function. Nevertheless, a uniform agreement on the definition of diastolic dysfunction is still lacking. Controversies remain about the exact echocardiographic parameters required to define diastolic dysfunction. Echocardiographic evaluation of diastolic function includes both structural (left atrial volume and left ventricular mass) and functional properties (ventricular relaxation, filling, and compliance). Schemes for a practical approach to establish diastolic dysfunction and integrating multiple echocardiographic measurements have been formulated but are not yet generally accepted, nor validated.11, 44, 45 According to the recommendations of the American Society of Cardiology, TDI parameter e′ the ratio E/e′, and left atrial volume are key components for evaluating diastolic function.44 While other parameters are load dependent, E/e′ is less sensitive to loading conditions and permits more accurate estimations of filling pressures.46
For the diagnosis of HFpEF, in addition to echocardiographically observable diastolic dysfunction, signs and symptoms suggestive of heart failure are also required.3 Interestingly, the range in the estimate of the prevalence of HFpEF is smaller than for echocardiographic diastolic dysfunction, which suggests that irrespective of the non-specific character of the symptoms and signs of heart failure, they do seem to allow researchers to better put into perspective the diastolic parameters measured with echocardiography.
The definition or the measurement of systolic dysfunction (i.e. ejection fraction <40–50%) did not change over time. Therefore, the peak in prevalence of left ventricular systolic dysfunction between the years 1995 and 2000 is most likely true, with ‘chance’ as the only alternative explanation. This finding, however, fits very well with the reduction in HFrEF relative to HFpEF over the past decade, while prevalence estimates for ‘all type’ heart failure remained stable. In all four studies that provided estimates on the prevalence of both HFrEF and HFpEF, the latter was more common. Similar results were obtained in hospital populations.47, 48 Recent data from the USA suggest that the incidence of heart failure decreased from the years 2000–2010, at least in patients diagnosed with heart failure in the hospital; this is especially true for HFrEF but less so for HFpEF.49 This ‘changing epidemic’ is at least partly attributable to an increase in cardiometabolic risk factors and comorbidities such as obesity, type 2 diabetes, hyperlipidaemia, and hypertension, which may fuel the development of HFpEF in particular, whereas smoking, exposure to second-hand smoke, and the incidence of ischaemic heart disease, the major risk factors for HFrEF, are on the decline.50
Gender differences in the prevalence and age of occurrence of ischaemic heart disease likely caused the finding that systolic dysfunction and HFrEF were more common in men, while HFpEF was more common in women (a finding described earlier).51
Many practising cardiologists use 40% as cut-point below which LVEF is considered reduced, a threshold also used as an inclusion criterion in many large trials of heart failure drugs. Epidemiological studies more often prefer 45% or 50% as the cut-point below which LVEF is considered reduced. Discussion continues on what the best cut-point is.
Conclusions
Prevalence estimates between studies varied because of differences in study population, case definition, and echocardiographic measurements applied, especially for estimates of diastolic dysfunction, and should be thus interpreted with caution. Nonetheless, it is clear that left ventricular dysfunction and heart failure remain very common among older people. The prevalence of diastolic dysfunction is increasing and now higher than that of systolic dysfunction. The latter prevalence seems to have decreased in the 21st century.
Funding
This work was supported by a research grant from the Dutch Heart Foundation (Nederlandse Hartstichting, grant number 2009B048).
Conflict of interest: none declared.




