Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF)
Abstract
Aims
Skeletal muscle wasting affects 20% of patients with chronic heart failure and has serious implications for their activities of daily living. Assessment of muscle wasting is technically challenging. C-terminal agrin-fragment (CAF), a breakdown product of the synaptically located protein agrin, has shown early promise as biomarker of muscle wasting. We sought to investigate the diagnostic properties of CAF in muscle wasting among patients with heart failure.
Methods and results
We assessed serum CAF levels in 196 patients who participated in the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Muscle wasting was identified using dual-energy X-ray absorptiometry (DEXA) in 38 patients (19.4%). Patients with muscle wasting demonstrated higher CAF values than those without (125.1 ± 59.5 pmol/L vs. 103.8 ± 42.9 pmol/L, P = 0.01). Using receiver operating characteristics (ROC), we calculated the optimal CAF value to identify patients with muscle wasting as >87.5 pmol/L, which had a sensitivity of 78.9% and a specificity of 43.7%. The area under the ROC curve was 0.63 (95% confidence interval 0.56–0.70). Using simple regression, we found that serum CAF was associated with handgrip (R = − 0.17, P = 0.03) and quadriceps strength (R = − 0.31, P < 0.0001), peak oxygen consumption (R = − 0.5, P < 0.0001), 6-min walk distance (R = − 0.32, P < 0.0001), and gait speed (R = − 0.2, P = 0.001), as well as with parameters of kidney and liver function, iron metabolism and storage.
Conclusion
CAF shows good sensitivity for the detection of skeletal muscle wasting in patients with heart failure. Its assessment may be useful to identify patients who should undergo additional testing, such as detailed body composition analysis. As no other biomarker is currently available, further investigation is warranted.
Introduction
Chronic heart failure (HF) is a major public health problem and carries a poor prognosis. The majority of patients present with co-morbidities that are either present already at diagnosis or develop during the course of the disease. Co-morbidities that have received increasing clinical and research interest over the last several years include chronic kidney disease, anaemia, and diabetes mellitus, however, chronic obstructive pulmonary disease (COPD), atrial fibrillation, sleep disordered breathing, iron deficiency, depression, obesity, and cachexia are also common.1-4 Co-morbidities generally be considered to have strong impact on patients' functional capability, exercise capacity, quality of life, hospitalizations, and survival. Thus, it is worthwhile to target the co-morbidities of HF, as these may in some instances not only vary in terms of their diagnostic necessities, but also with regard to their specific treatment. Indeed, some co-morbidities have seen specific treatment development in recent years, in particular atrial fibrillation, iron deficiency, and anaemia.5-7 Undoubtedly, specific treatment is necessary in these cases, but evidence is less clear for phenomena such as obesity or cachexia, and the so-called obesity paradox has revealed beneficial effects of large body size in terms of mortality.8
To date, skeletal muscle mass in HF has received little research endeavour. This is surprising, because patients' exercise capacity is directly connected to their skeletal muscle mass and, hence, quality of life.9, 10 Our group has recently described muscle wasting as a novel co-morbidity of HF that has serious clinical consequences.11, 12 Sarcopenia is the geriatric term for muscle wasting that exceeds two standard deviations of the mean muscle mass of arms and legs combined of a young and healthy control population.13 In a geriatric population, sarcopenia is a frequent co-morbidity affecting 5–13% of patients aged 60–70 years and up to 50% in patients older than 80 years.14 As a major cause of frailty, sarcopenia leads to mobility limitations and decline in functional performance, and therefore an increase in health-care costs.15 The prevalence of muscle wasting in comparatively young patients with chronic HF has been shown to be unexpectedly high at 19.5% in a heterogeneous population of patients with a clinical diagnosis of HF.5 The accurate assessment of muscle wasting is technically challenging and includes imaging techniques such as magnetic resonance imaging or computed tomography; other well established techniques embrace dual energy X-ray absorptiometry (DEXA) and bioimpedance analysis (BIA).16 As these techniques are time-consuming, costly, and, in some cases, associated with radiation exposure, easily applicable tests are required to screen patients for the presence of muscle wasting.
A biomarker would be a useful tool in this regard, however, no biomarker for the reliable detection of muscle wasting is currently available. Agrin is a protein released by motor neurons into the synaptic gap.17 One of the physiological effects of agrin is the clustering of acetylcholine receptors (AChRRRgbrgbnr) in the postsynaptic muscle cell membrane thereby initiating and maintaining neuromuscular junctions.18 Neurotrypsin is a protease also released by motor neurons. It cleaves agrin either at an alpha or a beta site.19 Depending on the cleavage site, different breakdown products of the C-terminal fraction of agrin, called C-terminal agrin fragment (CAF), are released (Figure 1). The synaptic serine protease neurotrypsin cleaves the synapse organizer agrin at two distinct sites. The cleavage of agrin at both the alpha and the beta cleavage site results from direct proteolytic activity of neurotrypsin. The two neurotrypsin-dependent cleavage sites of agrin are homologous and highly conserved in evolution. The finding of unique substrate binding pockets of neurotrypsin proves the selectivity for agrin as substrate at strictly conserved basic amino acids, and the cleavage of agrin strictly depends on neurotrypsin.48 Cleavage of agrin at the beta-site releases a 22 kDa sized fragment called CAF22. Cleavage at the alpha-site releases a larger CAF fragment with a molecular mass of 110 kDa (CAF110).20 This fragment can be split by additional beta-cleavage into a 90 kDa middle fragment and the 22 kDa CAF fragment. TotalCAF is defined as the sum of CAF22 and CAF110. The cleavage leads to inactivation of agrin and consequently to a dispersal of neuromuscular junctions.21
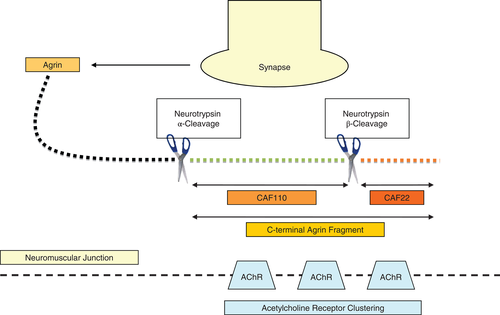
Using data from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF), a prospective, multinational, observational study into the co-morbidities of chronic HF, we aimed to study the diagnostic properties of CAF to identify skeletal muscle wasting in patients with HF.
Methods
Study population
We recruited, in a prospective manner, 200 ambulatory patients with chronic HF who participated in SICA-HF at the Charité Medical School, Campus Virchow-Klinikum, Berlin, Germany. Recruitment began in March 2010, and we included all subjects who were enrolled until April 2012. The inclusion criteria for chronic HF patients were a diagnosis of heart failure and either a left ventricular ejection fraction ≤40% or a left atrial dimension >4.0 cm (>2.5 cm/m height), or N-terminal pro-brain natriuretic peptide (NT-proBNP) >400 pg/ml or BNP >150 pg/mL. In addition, patients had to be aged >18 years and be willing to provide informed consent. Patients with previous heart transplantation, a history of unstable angina, myocardial infarction, stroke, cardiovascular revascularization, or open abdominal surgery within 6 weeks of the planned baseline visit were excluded. Also excluded were patients with known pregnancy, haemodialysis at baseline, or patients unable to understand and comply with protocol or to give informed consent.22 Hence, the study cohort is consistent with that used to describe the prevalence and clinical effects of muscle wasting for the first time.5 Four patients had to be excluded because of missing measurement of serum CAF values. The remaining 196 patients' main clinical features are detailed in Table 1. Patients' medication at study entry was documented. Functioning as an observational study, drug therapy was not changed and there was no significant difference in baseline medication between patients with or without muscle wasting.11 All subjects provided written informed consent at enrolment, and the local ethics committee approved the protocol. The study was funded by the European Commission's 7th Framework programme (FP7/2007–2013) under grant agreement number 241558 and fulfils all requirements of the Declaration of Helsinki.
| All (n = 196) | Muscle wasting (n = 38) | No muscle wasting (n = 158) | P-value | |
|---|---|---|---|---|
| Age (years) | 67.0 ± 10.4 | 70.6 ± 8.4 | 66.1 ± 10.6 | 0.01 |
| Sex (male/female, %) | 80.1/19.9 | 97.4/2.6 | 76.0/24.1 | 0.02 |
| Weight (kg) | 87.0 ± 16.9 | 77.0 ± 16.8 | 89.3 ± 16.1 | <0.0001 |
| BMI (kg/m2) | 28.9 ± 5.1 | 24.7 ± 4.4 | 29.9 ± 4.8 | <0.0001 |
| Heart failure aetiology (ischaemic/non-ischaemic (%) | 59.3/40.7 | 76.3/23.7 | 55.1/44.9 | 0.02 |
| LVEF ≤40%/LVEF >40% (%) | 68.4/31.6 | 86.8/13.2 | 63.9/36.1 | 0.006 |
| NYHA class | 2.35 ± 0.65 | 2.46 ± 0.77 | 2.32 ± 0.61 | 0.23 |
| LVEF (%) | 38.99 ± 13.42 | 35.00 ± 11.73 | 39.92 ± 13.66 | 0.04 |
| 6-min walk (<400 m), n = 180 | 37.2% | 57.6% | 32.7% | 0.007 |
| 4 m-gait speed (<0.8 m/s), n = 178 | 16.3% | 23.5% | 14.6% | 0.21 |
| TotalCAF (pM) | 600.07 ± 241.98 | 703.22 ± 274.56 | 574.46 ± 226.99 | 0.001 |
| CAF22 (pM) | 107.95 ± 47.17 | 125.06 ± 59.48 | 103.83 ± 42.92 | 0.008 |
- CAF, C-terminal agrin fragment; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
We examined a heart failure population in the broadest sense with mainly ischaemic heart failure aetiology (59.3%). According to the study protocol of SICA-HF, patients were divided according to left ventricular ejection fraction (LVEF) into HF with reduced ejection fraction with LVEF ≤40% (68.4%) and HF with LVEF >40% and a left atrial dimension >4.0 cm.22 The peak oxygen consumption (peakVO2 in mL/min.kg) was recorded using treadmill performance testing according to the modified Bruce protocol23 or, in selected patients, the modified Naughton protocol.24 Both a 6-min corridor walk test and a 4-m walk test to assess gait speed were performed according to standard protocols. All patients underwent echocardiography assessment and were stable on medication at for at least 4 weeks before being studied.
Assessment of muscle wasting
According to Morley et al.,25 we assumed the diagnostic criteria of sarcopenia to assess muscle wasting. Sarcopenia is defined as an appendicular skeletal muscle mass two standard deviations below the mean of a young healthy reference group aged 18–40 years. DEXA scanning was used with a Lunar Prodigy scanner and Lunar Encore 2002 software (GE Medical Systems, Madison, WI, USA).25, 26 DEXA measures total mass, fat mass, and fat-free mass (fat-free mass corresponds with lean mass and provides a reliable estimate of muscle mass). The muscle mass of interest for the definition of muscle wasting was defined as appendicular skeletal muscle (ASM) divided by the height in metres squared. Appendicular skeletal muscle equals lean mass of arms and legs combined.27, 28 In addition, a 6-min walk distance of less then 400 m is described as a cut-off for exercise capacity. Patients with a history of peripheral nervous system disorders were excluded.26
Assessment of C-terminal agrin fragment
Patients' blood was drawn from an antecubital vein in fasting condition and totalCAF and CAF22 were analysed from serum samples immediately centrifuged and frozen at −80 °C until analysis. A sandwich enzyme-linked immunosorbent assay (ELISA) (NTCAF ELISA and NTtotalCAF ELISA; Neurotune, Schlieren-Zurich, Switzerland) was used to detect totalCAF and CAF22. TotalCAF and CAF22 are stable for many years in serum frozen at −86 °C.29 The intra- and inter-assay coefficient of variance of the research kit is between 0.8% and 12.3%. The intra- and inter-plate variability was tested to be 5% for calibrator solution and 12% for serum/plasma. A measurement was valid if the double-measurement's % coefficient of variation (CV) value was below 20%. This was the case for all measurements. Measurements performed with reference serum on different time-points with different assay batches differed less than 20%.29 In healthy individuals, totalCAF values range from 140 to 430 pM and CAF22 values range from 20 to 100 pM. The assay has a measuring range of 40–800 pM for totalCAF and 20–400 pM for CAF22, and allows measurement of totalCAF and CAF22 in plasma and serum, as well as CAF22 in urine.29
Statistics
Data are presented as mean ± standard deviation (SD) or median with 25th and 75th percentiles. For statistical analysis, StatView 5.0 (SAS Institute, Inc. Cary, CA, USA) was used. Serum levels of CAF, high-sensitivity C-reactive protein (hsCRP), alkaline phosphatase, gamma-glutamyltranferase (gamma-GT), creatinine, and ferritin were non-normally distributed and therefore log-transformed to achieve a normal distribution. Analysis of variance (ANOVA), Student's unpaired t-test, Fisher's exact test, Pearson's simple regression, and logistic regression were used as appropriate. MedCalc for Windows version 11.2.1.0 (Broekstraat, Mariakerke, Belgium) was used to perform receiver operating characteristics (ROC) curve analysis. Areas under the ROC curve (AUCs) were constructed for sensitivity and specificity to compare different predictive values. The best prognostic cut-off for muscle wasting was defined as the highest product of sensitivity and specificity. The method for paired ROC curves described by Hanley and McNeil30 was used, performing statistical comparison of ROC curves. A value of P < 0.05 was considered to indicate statistical significance in all analyses.
Results
We analysed 196 patients with symptomatic, clinically stable chronic HF, 134 (68.4%) of whom presented with LVEF ≤40% and 62 with LVEF >40%. The study cohort consisted of 157 males and 39 females. Patients with LVEF ≤40% tended to be younger (P = 0.05) and were predominantly male (P < 0.01) with a lower body mass index (BMI, P < 0.01). The average age of all patients was 67.0 ± 10.4 years and the mean LVEF was 39.0 ± 13.4%. Most patients were in New York Heart Association (NYHA) classes II and III. Baseline characteristics are summarized in Table 1. Patients were divided according to whether or not muscle wasting was present. Thirty-eight patients (19.4%) met this criterion. Patients with muscle wasting tended to be older than patients without muscle wasting. Their weight and BMI were lower and their LVEF was lower than in patients without muscle wasting. Measurement of the distance walked during the 6-min walk revealed that more than 50% of patients with muscle wasting did not reach 400 m walk distance.22, 31 No significant difference in gait speed was detected (Table 1).
Descriptive statistics for C-terminal agrin fragments
Levels of totalCAF were significantly elevated in patients with muscle wasting compared with those without (P = 0.0014, Figure 2). Forty-four (23.0%) patients had values within the normal range of 140–430 pM, 147 (77%) patients had values above the normal range (35 with muscle wasting, 112 without muscle wasting). TotalCAF showed a minimum value of 234 pM and a maximum of 1749 pM. Its mean (± SD) value was 600 ± 241.98 pM, and the median was 550.5 pM [interquartile range (IQR) 425.5–675.5 pM].
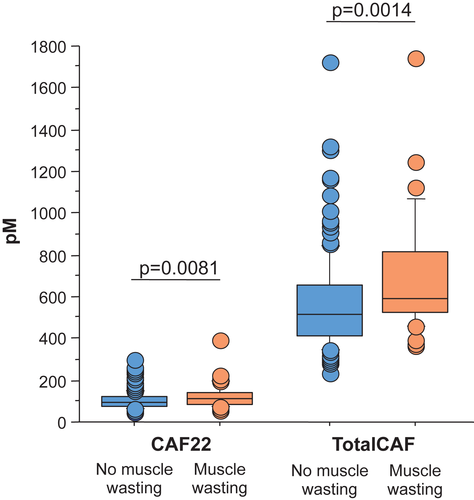
Levels of CAF22 were also significantly elevated in patients with muscle wasting (P = 0.0081, Figure 2). One hundred and three (52.6%) patients had CAF22 values within the normal range and 93 (47.5%) patients were above normal range (24 with muscle wasting, 69 without muscle wasting). CAF22 was expressed at a minimum serum value of 44 pM and a maximum of 391 pM, the mean value was 108 ± 47.17 pM, the median was 98 pM (IQR 75.57–120.43 pM, Table 2).
| TotalCAF (n = 191) | CAF22 (n = 196) | |
|---|---|---|
| Minimum | 233.8 | 43.9 |
| Maximum | 1748.0 | 390.5 |
| Mean ± SD | 600.07 ± 241.98 | 107.95 ± 47.17 |
| Median | 550.5 | 98.0 |
| Interquartile range | 250.0 | 44.9 |
| P-value (muscle wasting) | 0.003 | 0.012 |
| Optimal cut-off value to identify the presence of muscle wasting (receiver operating characteristic analysis) | 512.5 | 87.5 |
| Patients below cut-off | 82 (43%) | 77 (39%) |
| Patients above cut-off | 109 (57.1%) | 119 (60.7%) |
A higher CAF value was present in patients who did not accomplish 400 m during the 6-min walk test, with mean (± SD) values of totalCAF 694.84 ± 260.81 vs. 530.92 ± 194.11, and CAF22 values of 123.43 ± 48.81 vs. 96.14 ± 37.54 (all P < 0.0001). The also occurred in patients who did not achieve 0.8 m/s gait speed in the 4-m walk test, with mean (± SD) values of totalCAF 775.31 ± 353.93 vs. 567.59 ± 205.88, and CAF22 values of 136.73 ± 76.40 vs. 103.04 ± 39.29 (all P < 0.003). Overall, patients with LVEF ≤40% with mean LVEF of 31.27 ± 7.57% showed significantly higher levels of totalCAF (633.84 ± 261.86 vs. 524.53 ± 168.92) and CAF22 (113.69 ± 51.55 vs. 95.55 ± 33.03, all P < 0.01) than patients with LVEF >40%,(LVEF 55.53 ± 6.40%, P < 0.0001), and the prevalence of muscle wasting was increased at 24.6% compared with 8.1% (P = 0.01). These patients also had a lower body mass index (BMI) (28.2 ± 4.8 vs. 30.4 ± 5.4, P = 0.01).
Correlation analyses
Using simple regression, we found that values of all CAF fragments correlated with age (P < 0.02), NYHA class (P < 0.0001), parameters of cholestasis (alkaline phosphatase and gamma-GT, both P < 0.0001), iron metabolism parameters such as serum iron, ferritin, and transferring saturation (Figure 3) as well as with exercise time (P < 0.0001), leg strength (P < 0.001), 6-min walk distance (P < 0.0001), and kidney function (P < 0.0001, Figure 3). The CAF values are significantly correlated with renal function (all P < 0.01). We also examined patients with CAF values above the cut-off. Patients show lower glomerular filtration rate (GFR) and higher serum creatinine levels. Kidney function was evaluated by serum creatinine levels and the glomerular filtration rate, using both the Cockroft–Gault and the Modification of Diet in Renal Disease (MDRD) formula. There was no significant association between CAF and hsCRP or appendicular skeletal muscle mass (both P < 0.2, Table 3). Dividing the study cohort into an age subgroup of 65 years and above, we found no significant correlation between the levels of CAF fragments and age (n = 122 , all P < 0.05).
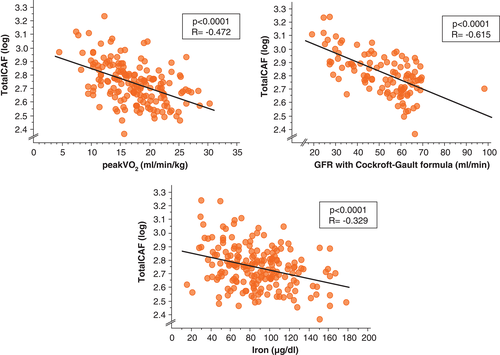
| TotalCAF (log) | CAF22 (log) | |||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| Age (years) | 0.23 | 0.01 | 0.17 | 0.02 |
| NYHA class | 0.37 | <0.0001 | 0.32 | <0.0001 |
| Albumin (g/L) | −0.18 | 0.02 | −0.18 | 0.01 |
| High-sensitivity C-reactive protein (log) | 0.10 | 0.22 | 0.16 | 0.04 |
| Alkaline phosphatase (log) | 0.30 | <0.0001 | 0.29 | <0.0001 |
| Gamma-glutamyltranferase (log) | 0.37 | <0.0001 | 0.29 | <0.0001 |
| Uric acid (mg/dL) | 0.53 | <0.0001 | 0.47 | <0.0001 |
| Creatinine (log) | 0.68 | <0.0001 | 0.62 | <0.0001 |
| GFR (ml/min) | −0.62 | <0.0001 | −0.57 | <0.0001 |
| Serum iron (µg/dl) | −0.33 | <0.0001 | −0.33 | <0.0001 |
| Ferritin (log) | −0.21 | 0.01 | −0.26 | <0.01 |
| Transferrin (mg/dl) | 0.23 | <0.01 | 0.22 | <0.01 |
| Transferrin saturation (%) | −0.32 | <0.0001 | −0.32 | <0.0001 |
| Haemoglobin (g/dL) | −0.24 | <0.01 | −0.30 | <0.0001 |
| LVEF (%) | −0.27 | <0.01 | −0.26 | <0.01 |
| Handgrip strength (kg) | −0.17 | 0.02 | −0.17 | 0.03 |
| Leg strength (kg) | −0.28 | <0.01 | −0.31 | <0.0001 |
| Exercise time (s) | −0.43 | <0.0001 | −0.43 | <0.0001 |
| Peak VO2 (mL/min/kg) | −0.47 | <0.0001 | −0.47 | <0.0001 |
| 6-min walk (m) | −0.34 | <0.0001 | −0.32 | <0.0001 |
| 4-m gait speed (m/s) | −0.26 | <0.01 | −0.25 | <0.01 |
- CAF, C-terminal agrin fragment; GFR, glomerular filtration rate (Cockroft-Gault formula); LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VO2, oxygen consumption.
Using logistic regression analysis with the presence of muscle wasting serving as the dependent variable, we found that age, sex, NYHA class, LVEF, totalCAF and CAF22, handgrip strength, and distance walked in the 6-minute walk test all predicted the presence of muscle wasting (all P < 0.05, Table 4). In multivariate analysis, totalCAF and CAF22 remained independently predictive of muscle wasting after adjusting for sex, creatinine, and age (all P < 0.05, Table 4).
| Univariate | |||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Age (years) | 1.05 | 1.01–1.10 | 0.02 |
| Sex (female) | 0.09 | 0.01–0.64 | 0.02 |
| NYHA class (I/II/III/IV) | 1.41 | 0.80–2.48 | 0.23 |
| LVEF (%) | 0.97 | 0.94–1.00 | 0.05 |
| Creatinine (mg/dL) | 1.17 | 0.52–2.61 | 0.70 |
| TotalCAF (10 pM) | 1.02 | 1.01–1.03 | 0.01 |
| CAF22 (10 pM) | 1.10 | 1.01–1.16 | 0.02 |
| Handgrip strength (kg) | 0.06 | 0.92–0.99 | 0.01 |
| 6-min-walk (per 10 m increase) | 0.96 | 0.94–0.99 | 0.01 |
| Multivariable, adjusted for sex, creatinine, and age | |||
| TotalCAF (per 10 pM increase) | 1.04 | 1.02–1.07 | 0.002 |
| CAF22 (per 10 pM increase) | 1.14 | 1.04–1.26 | 0.008 |
- CAF, C-terminal agrin fragment; CI, confidence interval; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OR, odds ration.
In logistic regression analysis with CAF levels above the cut-off, and above the median as dependent variables, we saw that the presence of muscle wasting is predictive for CAF22 levels above the cut-off value for totalCAF levels above the median and above the cut-off value. In multivariate analysis, muscle wasting remained independently predictive only for totalCAF levels above the cut-off value adjusted for NYHA class, LVEF, and leg strength (all P < 0.05, Table 5).
| Univariate | |||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Age (per year increase) | 1.05 | 1.02–1.08 | <0.01 |
| Sex (female) | 0.57 | 0.28–1.18 | 0.13 |
| NYHA class (I/II/III/IV) | 3.90 | 2.24–6.79 | <0.01 |
| LVEF (%) | 0.97 | 0.94–0.99 | <0.01 |
| Creatinine (mg/d) | 34.79 | 8.88–136.27 | <0.01 |
| GFR (ml/min) | 0.92 | 0.88–0.97 | <0.01 |
| Iron (mg/dL) | 0.99 | 0.98–0.99 | 0.02 |
| Transferrin saturation (%) | 0.96 | 0.94–0.99 | 0.01 |
| Muscle wasting (no/yes%) | 5.26 | 2.08–13.31 | <0.01 |
| Leg strength (kg) | 0.95 | 0.93–0.98 | <0.01 |
| Exercise time (s) | 0.99 | 0.99–1.00 | <0.01 |
| Peak VO2 (mL/min) | 0.83 | 0.77–0.89 | <0.01 |
| 6-min walk (m) | 0.99 | 0.99–1.00 | <0.01 |
| 4-m gait speed (m/s) | 0.05 | 0.01–0.24 | <0.01 |
| LVEF ≤40%/LVEF >40% | 2.15 | 1.15–4.01 | 0.02 |
| Multivariable, adjusted for NYHA class, LVEF, and leg strength | |||
| Presence of muscle wasting | 1.04 | 1.02–1.07 | 0.002 |
- CAF, C-terminal agrin fragment; CI, confidence interval; GFR, glomerular filtration rate (Cockroft-Gault formula); LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OR, odds ratio; VO2, oxygen consumption.
Sensitivity and specificity
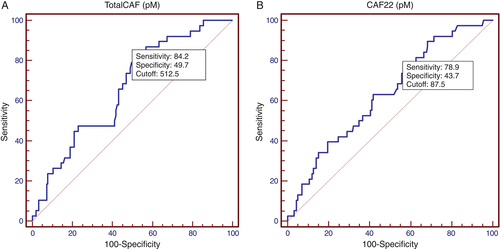
CAF22 as well as totalCAF displayed acceptable sensitivity for the correct identification of muscle wasting. At the optimal cut-off point from ROC analysis, the values for sensitivity were 0.84 [95% confidence interval (CI) 0.59–0.73] for totalCAF and 0.79 (95% CI 0.56–0.70) for CAF22 (Figure 4). Specificity remained comparatively low with values reaching 0.50 and 0.44, respectively. The corresponding AUC values were 0.66 and 0.63 The optimal cut-off value for the diagnosis muscle wasting was 513 pM for totalCAF and 88 pM for CAF22. Correspondingly, 109 patients showed levels above the cut-off for totalCAF and 120 showed levels above the cut-off for CAF22. Assuming a prevalence of 19.4% in our study population, the calculated positive predictive value at the optimal cut-off point from ROC analysis was 29.4% for totalCAF and 25.2% for CAF22. The corresponding negative predictive values were 92.7% and 89.6%, respectively.
Discussion
We have shown that the CAF fragments totalCAF and CAF22 are sensitive markers for the detection of muscle wasting in patients with chronic HF, whether those with LVEF >40% or those with LVEF ≤40%. We investigated a cohort of 200 patients with chronic HF, 19.4% of whom presented with muscle wasting, as defined using the criteria for sarcopenia. As a basis of our findings, we found CAF values significantly elevated in many, but not all patients with muscle wasting. Patients with a lower distance walked during the 6-min walk test and a lower gait speed during the 4-m walk test showed elevated CAF levels. Furthermore, we found a strong correlation between CAF values and renal function parameters. A missing association of either CAF fragments and appendicular skeletal muscle mass or CAF fragments and age cannot be explained and calls for further investigation, possibly by dividing the study population further into subsets of patients with different types of muscle wasting. Importantly, multivariable analysis revealed that CAF remains an independent predictor of muscle wasting after adjustment for sex, creatinine, and age. We also found that the presence of muscle wasting is independently predictive of totalCAF levels above the cut-off value after multivariate adjustment. Comparing totalCAF and CAF22, the fragments show similar results with regard to their association with clinical parameters, their sensitivity and specificity, and properties after multivariable adjustment. However, the major limitation of CAF as a biomarker for muscle wasting remains its low specificity.
Muscle wasting represents a recently identified co-morbidity in patients with chronic HF that affects almost 20% of ambulatory patients.11 The aetiology is assumed to be multifactorial, but an over-activation of protein degradation has been observed13 and the reduction in the number of neuromuscular junctions seems to play a role in the pathogenesis of muscle wasting.14 Several other mechanisms are also involved in the pathophysiology of sarcopenia. The reduction in muscle mass is related to a decline in muscle fibre size and number. Type I fibres are recruited for low-intensity activities while type II fibres are activated during high-intensity exercise. In aging, a selective atrophy of type II fibres is described. Changes in muscle innervation, especially reduced agonist and increased antagonist activity are also part of the multifactorial pathogenesis. In addition, a disproportionate decrease in skeletal muscle protein synthesis and an increased protein breakdown are responsible for muscle wasting. Distinct molecular pathways are part of this model with an inactivation of anabolic mechanisms such as the phosphatidyl inositol 3 kinase/serine-threonine kinase Akt system and a stimulation of catabolic proteins such as cytokines stimulating, for example, Muscle Ring Finger-1 (MuRF-1), which activates the ubiquitin–proteasome system leading to muscle protein degradation.47
Degeneration of neuromuscular junctions (NMJ) is relevant in the multifactorial aetiology of muscle wasting, even though such effects may be particularly prevalent in a yet unidentified subgroup of patients. The NMJs are built via dialogue between muscle cell and motor neuron, and neurons maintain these contacts by the secretion of agrin.32 Agrin induces postsynaptic differentiation, especially clustering of acetylcholine receptors.33 The inactivation of agrin by cleavage via neurotrypsin leads to a release of CAF, which can be detected in human serum.12, 34 With the disappearance of agrin, the NMJ is unprotected and the endplate is degraded. In a mouse model of neurotrypsin over-expression, Bütikofer et al.35 presented a full phenotype of muscle wasting. In this context it should be noted that agrin-dependent sarcopenia can be distinguished from ageing-associated muscle wasting,14 as a result, CAF seems to be primarily a marker of muscle wasting caused by degeneration of NMJs.36
Currently, CAF22 is the only agrin fragment, which has been evaluated as a potential biomarker for muscle wasting, even though a large number of different candidates has been suggested.16 Compared with other studies examining CAF as a potential biomarker for muscle wasting, this is the first study evaluating CAF22 and totalCAF. Both showed good sensitivity and negative predictive values, enabling the ruling out of the presence of muscle wasting. Both fragments showed large standard deviations, which is not an unexpected finding in studying a novel biomarker. In a population with advanced disease such as heart failure, multiple subgroups with different stages of chronic HF and co-morbidities are likely involved in this effect.
A comparison with other CAF studies has proven to be difficult. There is a lack of comparability, because studies differ in measuring units and methods. In our study, CAF was measured in pM with an ELISA. Other studies assessed CAF22 in ng/mL using western blot. Drey et al.36 examined 69 pre-frail adults (male: n = 21, 79.4 ± 7.6 years, female: n = 47, 76.0 ± 6.1 years) before and after an intervention training exercise. The pre-intervention CAF22 levels were 5.4 ± 2.9 ng/ml (which is equivalent to 270 ± 145 pM) for male participants and 4.5 ± 2.2 ng/ml (225 ± 110 pM) for female participants. Patients were not evaluated with regard to the presence or absence of muscle wasting. Levels of CAF22 were negatively correlated with appendicular lean mass and significantly reduced after physical exercise.36 A study by Hettwer et al.20 evaluated participants with muscle wasting (n = 73, 70.8 ± 5.5 years, 46.6% female) and an age-matched control group (n = 60, 71.2 ± 5.6 years, 46.7% female). The CAF22 values were significantly elevated in participants with muscle wasting (4.71 ± 2.60 vs. 2.64 ± 0.97 ng/ml or 235.5 ± 130 vs. 132 ± 48,5 pM, P < 0.001).20 Compared with this study, our CAF22 levels are much lower (see Table 1). The explanation for the gross variation in measured values might be in the differences in age or gender distribution among cohorts, but the most important aspect is probably the difference in assessment methods. In summary, other studies46 show, that CAF levels are associated with appendicular lean mass and significantly elevated in participants with muscle wasting, highlighting a subgroup of patients with increased agrin degradation as a primary mechanism of muscle wasting.46 Recently, CAF22 was reported to be a sensitive marker for the glomerular filtration rate in renal transplantation patients.46 However, in the cohort under investigation, kidney function was in the normal range, as suggested by serum creatinine values. The results obtained may therefore be a consequence of muscle function rather than of kidney function itself.
Except for CAF, no biomarkers specific for muscle wasting are currently available. Markers of inflammation such as serum levels of TNF-α, it soluble receptors, or IL-6, may give a rough estimation, but are not muscle specific. Myostatin, a regulator of muscle mass and function, is expressed in skeletal muscle and local over-expression leads to muscle reduction, but a reliable assay to quantify myostatin in patients' serum has not reached clinical use, even though a few are on the market. Endocrine release of myokines such as irisin by skeletal muscle could also be related to muscle wasting and could serve to monitor endocrine muscle function.37 Another candidate marker, N-terminal propeptide of type III procollagen (P3NP), has recently been studied by Fragala et al.;38 this is the product of proteolytic cleavage during the synthesis of skeletal muscle. P3NP is reflective of tissue remodelling and levels are reduced in older adults. Levels of P3NP have been shown to be predictive of changes in lean body mass and strength.38 Next to an easy assessment method by taking a patient's blood sample and its independent predictive value for muscle wasting, CAF stands out from other biomarkers as the only one with a high sensitivity for muscle wasting.
Patients with HF may benefit from CAF testing in order to select those for additional evaluation with regard to the presence of muscle wasting. This may be particularly true for patients who present with physical frailty and limitations in their activities of daily living. In this regard, low 4-m gait speed, low 6-min walk distance, or low values of handgrip strength could be screening tests and have been used in geriatric medicine.15 Such findings may also prompt CAF testing and further work-up. However, evaluation guidelines or even treatment advice other than intensive exercise training is not currently available. At present, targeted sarcopenia screening is currently recommended only for patients who are bedbound, non-ambulatory, unable to rise from a sitting position unassisted, have a history of weight loss (>5% of body weight), or co-morbidities associated with loss of muscle mass (e.g. diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease).39 For clinical use, a multiparametric algorithm is advisable at present to identify chronic HF patients with sarcopenia. Screening of patients with CAF, handgrip strength, and 6-min walking test appears useful. Elevated serum levels, low handgrip strength, and walking distance should lead to gold standard diagnostic tests of sarcopenia, for example DEXA scan, computed tomography (CT) or magnetic resonance imaging (MRI).
Treatment possibilities for muscle wasting are currently limited to exercise training assessed by peak oxygen consumption. According to the FITT acronym (frequency, intensity, time, and training), three different training modalities are recently prescribed for HF patients. Aerobic endurance, both continuous and interval, strength or resistance training, and respiratory muscle training have so far been evaluated and are recommended by the European Society of Cardiology.40 Both resistance and endurance training have been shown to increase muscle strength and improve function.39 Of all the proposed dietary agents, protein supplements and vitamin D optimization seem to be the most promising. In addition, growth hormone and ghrelin, an endogenous growth hormone secretagogue and appetite stimulant, increase lean body mass but induce no change in muscle function.39 The study of Candow et al.41 showed that resistance training and nutritional interventions with essential amino acids, milk-based-proteins, creatinine monohydrate, essential (omega-3) fatty acids, and vitamin D have beneficial effects on aging muscle biology. The effect of anabolic steroids was evaluated by Rosano and co-workers.42, 43 Long-acting testosterone therapy improves exercise capacity (PeakVO2) and muscle strength in men and women.42, 43 With regard to CAF levels, Drey et al.36 showed a decrease in CAF22 values of patients with muscle wasting in association with physical exercise and vitamin D supplementation. These recommendations are not yet proven for patients with chronic HF, but the European Society of Cardiology HF guidelines firmly recommend regular physical activity and structured exercise training: both endurance and resistance training.44 Thus exercise training is the only therapeutic approach to muscle wasting in HF patients at the present.45 Patients with clinically verified muscle wasting have so far not been shown to benefit from these treatments. Clinical trials are therefore required.
Conclusion
In conclusion, the multicentre observational study SICA-HF shows that chronic HF patients with muscle wasting have significantly elevated CAF levels. In multivariate analysis, the biomarker CAF has proven to be independently predictive for this common co-morbidity in chronic HF patients. Conversely, the presence of muscle wasting is an independent predictor for totalCAF levels above the cut-off value. Serum values of CAF may be useful to identify patients with HF and muscle wasting. However, CAF seems to be primarily a marker of muscle wasting caused by degeneration of NMJs and can be distinguished from age-related muscle wasting. With high sensitivity and negative predictive values, elevated CAF fragment levels should prompt further investigation using DEXA scan. Currently, CAF is the best blood biomarker for the detection of muscle wasting. While its specificity remains low, its sensitivity is high enough to warrant additional research in order to improve the assay profile and, thus, sensitivity and specificity.
Funding
This project was supported by the 7th framework programme (FP7/2007/2013) under grant agreement number 241558 of the European Commission (SICA-HF) and the Russian Ministry of Science and Education within the FTP ‘R&D in priority fields of the S&T complex of Russia 2007–2012’ under state contract number 02.527.11.0007.
Conflict of interest: Pius Dahinden and Stefan Hettwer are employees of Neurotune, Switzerland.




