OPTImal PHARMacological therapy for patients with heart failure: Rationale and design of the OPTIPHARM-HF registry
Abstract
Aims
Patients with heart failure (HF) remain often undertreated for multiple reasons, including treatment inertia, contraindications, and intolerance. The OPTIimal PHARMacological therapy for patients with Heart Failure (OPTIPHARM-HF) registry is designed to evaluate the prevalence of evidence-based medical treatment prescription and titration, as well as the causes of its underuse, in a broad real-world population of consecutive patients with HF across the whole ejection fraction spectrum and among different clinical phenotypes.
Methods
The OPTIPHARM-HF registry (NCT06192524) is a prospective, multicenter, observational, national study of adult patients with symptomatic HF, as defined by current international guidelines, regardless of ejection fraction. Both outpatients and inpatients with chronic and acute decompensated HF will be recruited. The study will enroll up to 2500 patients with chronic HF at approximately 35 Italian HF centres. Patients will be followed for a maximum duration of 24 months. The primary objective of the OPTIPHARM-HF registry is to assess prescription and adherence to evidence-based guideline-directed medical therapy (GDMT) in patients with HF. The primary outcome is to describe the prevalence of GDMT use according to target guideline recommendation. Secondary objectives include implementation of comorbidity treatment, evaluation of sequence of treatment introduction and up-titration, description of GDMT implementation in the specific HF population, main causes of GDMT underuse, and assessment of cumulative rate of cardiovascular events.
Conclusion
The OPTIPHARM-HF registry will provide important implications for improving patient care and adoption of recommended medical therapy into clinical practice among HF patients.
Introduction
Heart failure (HF) represents a global health issue affecting more than 60 million people worldwide and leads the cause of hospitalization in both the United States and Europe.1 Despite the improvement in cardiovascular (CV) outcomes, patients with HF are yet exposed to an excess risk of hospitalization for HF and CV death.1, 2
Beta-blockers (BB), mineralocorticoid receptor antagonists (MRAs), angiotensin receptor–neprilysin inhibitor (ARNI) sacubitril/valsartan, and sodium–glucose cotransporter 2 (SGLT2) inhibitors, dapagliflozin and empagliflozin, have been shown to improve survival and outcomes in patients with HF with reduced and mildly reduced ejection fraction (HFrEF and HFmrEF, respectively). Combination of these four drugs is recommended by international guidelines3, 4 for HFrEF and may be considered for patients with HFmrEF (class IIb for BB, MRA, and ARNI). SGLT2 inhibitors have also shown to improve HF outcomes in HF with preserved ejection fraction (HFpEF).5, 6 All four drug classes are now approved by international Medicines Agencies, representing the standard of care of patients with HF. Recently, vericiguat has been shown to reduce the risk of CV death or hospitalization for HF in patients with HF with maximally tolerated guideline-directed medical therapy (GDMT).7 Although scientific societies strongly recommend treatment optimization, in practice patients remain often undertreated for multiple reasons, including treatment inertia, cost/access limitations, absolute or relative contraindications, and real or perceived intolerance.8, 9 Prior registries and administrative databases have largely characterized medication use patterns prior to the introduction of the most recent GDMT, SGLT2 inhibitors, prior to latest guideline updates10-13 and mostly included HFrEF patients. There is a need to describe the prevalence of GDMT at a national level in a contemporary large ‘real-world’ HF population and to assess the extent to which current recommendations for drug combination therapy are adopted in clinical practice.
These insights can facilitate a better understanding of barriers and disparities in HF medical treatment to eventually improve efforts to translate new knowledge into clinical practice and guarantee better patient care. The OPTIimal PHARMacological therapy for patients with Heart Failure (OPTIPHARM-HF; NCT06192524) registry is designed to (i) evaluate medical care and outcomes of patients with HF, (ii) understand reasons for lack of implementation of evidence-based treatment and the impact of comorbidities on treatment tolerance and titration; (iii) assess the impact of adherence to treatment on clinical outcomes in patients with HF across the spectrum of ejection fraction; and (iv) assess management strategy differences between naïve versus chronic HF patients.
Registry objectives and outcomes
The primary objective of the OPTIPHARM-HF registry is to assess prescription and adherence to evidence-based GDMT in patients with HF. The primary outcome is to describe the prevalence of use of GDMT, both as drugs administered and their dosing, defined according to target guideline-recommended doses in patients with HFrEF, HFmrEF and HFpEF(Table 1). Secondary objectives include (i) implementation of treatment of comorbidities, including atrial fibrillation, valvular heart disease, iron deficiency, kidney dysfunction and electrolyte abnormalities, including hyperkalaemia, and telemonitoring; (ii) evaluation of sequence of introduction of the recommended GDMT, their up-titration, when needed, and maintenance of target dose during follow-up; (iii) description of GDMT implementation, dosing, and sequencing in specific HF populations including de novo HF, worsening HF, advanced HF and HF with improved ejection fraction (HFimpEF); (iv) description of the main causes of underuse and underdosing of GDMT based on identifiable clinical parameters (such as bradyarrhythmia, hypotension, kidney dysfunction, patient personal decision); (v) evaluation of variability in GDMT use, dosing, and sequencing based on clinical site/hospital; and (vi) assessment of cumulative rate of CV events (including first and recurrent hospitalizations for HF, CV mortality, arrhythmic events, ischaemic or kidney events) (Table 1).
| Primary outcome |
| Description of the prevalence of use of GDMT, both as drugs administered and their dosing, defined according to target guideline-recommended doses in patients with HFrEF, HFmrEF and HFpEF. |
| Secondary outcomes |
| Implementation of treatment of comorbidities including atrial fibrillation, valvular heart disease, iron deficiency, chronic kidney disease and electrolyte abnormalities, and telemonitoring. |
| Evaluation of sequence of introduction of the recommended GDMT, their up-titration, when needed, and maintenance of target dose during follow-up. |
| Description of the main causes of underuse and underdosing of GDMT based on identifiable clinical parameters. |
| Description of GDMT implementation, dosing, and sequencing in specific HF populations, including de novo HF, worsening HF, advanced HF and HFimpEF. |
| Evaluation of variability in GDMT use, dosing, and sequencing based on clinical site/hospital. |
| Cumulative incidence rates of clinical events: all-cause death; CV death; unplanned hospitalization for HF (including recurrent events); unplanned outpatient facility visits for HF where patient is treated with intravenous therapy; hospitalization for interventional procedures; heart transplantation or ventricular assist device implantation; non-fatal MI; non-fatal stroke; new-onset atrial fibrillation; ICD shock or hospitalization for ventricular arrhythmia; hospitalization for acute kidney injury or other kidney disease event including dialysis or end-stage renal disease defined as eGFR <15 ml/min/1.73 m2 or the need for renal replacement therapy. |
| Exploratory outcomes |
| NYHA class change. |
| Change in LVEF and reclassification of patients with HFimpEF during follow-up. |
| Change in biomarkers (NT-proBNP, troponin) and renal function during follow-up. |
- CV, cardiovascular; eGFR, estimated glomerular filtration rate; GDMT, guideline-directed medical therapy; HF, heart failure; HFimpEF, heart failure with improved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Study design
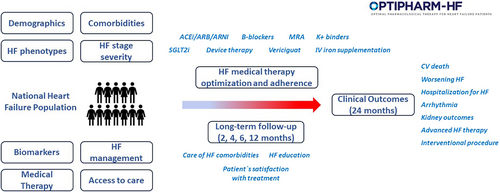
The OPTIPHARM-HF registry is a prospective, observational, national study of adult patients with HF in Italy (Figure 1). Both outpatients and inpatients with chronic and acute decompensated HF will be consecutively recruited per protocol intention. Centres will enrol patients during the course of an outpatient visit or during a hospitalization for HF. Patients will be followed for a maximum duration of 24 months or until death or study withdrawal. Because this is a prospective observational non-interventional cohort study, there will be no attempt to influence clinical practice. The study will enrol patients from approximately 35 Italian HF care centres (community, specialized and advanced care centres). Investigators' sites include facilities specialized in the management of adults with HF. Inpatient and outpatient HF practices are identified to ensure broad representation and affiliation with academic institutions. The research protocol has been approved by the ethics committee of the University Hospital of Brescia under the number NP5441 and complies with the Declaration of Helsinki. When the investigator has decided to include a patient in the registry, a signed informed consent form will be obtained from each patient, once the purposes and objectives of the registry have been illustrated. Patients may withdraw their consent at any time.
Population
The study population will consist of patients with symptomatic HF, as defined by current international guidelines,3, 4 regardless of ejection fraction, aged 18 years or older, able to give their written informed consent to participate in the registry (Table 2). Each patient will be uniquely identified in the study by a combination of his/her site number and patient number. Consecutive patients visited after the approval of each Ethics Committee centre will be included, up to the achievement of the study size. Visits will be classified as outpatient visits or at-discharge visits in patients hospitalized for HF. Outpatients visits will be further classified as planned or unplanned visits for worsening HF. Unplanned visits will be further classified based on no change in HF treatment, increase in oral diuretic doses or with ambulatory administration of intravenous diuretics or hospital admission.14-16 Exclusion criteria will be: planned participation or participation in a clinical trial; life expectancy <1 year because of non-cardiac causes; previous heart transplant or left ventricular assist device implantation; cardiac dysfunction in the absence of symptoms, i.e. pre-HF, according to the recent universal definition of HF.17
| Inclusion criteria | Exclusion criteria |
|---|---|
|
- HF, heart failure.
Study duration and study visits
The study consists of an enrolment period of approximately 2 years and a follow-up of 2 years. The overall study duration is expected to be 4 years. Each centre will collect baseline data and then data regarding medications, clinical studies, and outcomes at regular intervals (Table 3). At V1 (discharge for inpatients and ambulatory visit for outpatients) inclusion criteria and HF therapy will be evaluated. HF therapy information will be collected based on recommended HF guidelines.3, 4 At V1 along with demographic, vital signs, main echocardiographic cardiac parameters (including measures of left ventricular structure, ejection fraction, diastolic function, valve disease severity and right ventricular structure and function) and biochemistry data, medications will be entered into the database including dosage, main causes of underuse and underdosing and therapeutic changes (information will include therapy at the time of the enrolment or before hospital admission and at the end of the visit/discharge). Drug dosing will be classified into 0%, <50%, 50–99% and ≥100% target doses, according to previous studies.11 Centres will also complete a 12-month retrospective HF-related history for each patient, along with documentation of the last HF admission and duration of HF since the initial diagnosis. Two intermediate visits after V1 and before 6 months are included to re-evaluate clinical status and medical therapy modulation. At 6 months, patients will be re-evaluated for the second study visit (V2) to assess clinical status, echocardiographic parameters, adherence to therapy and main causes of underuse and underdosing of evidence-based treatment. At V2 physicians can optimize HF treatment or re-evaluate the cause of treatment underuse and underdosing. After 12 months from the V1, patients will be re-evaluated for the third study visit (V3) to assess clinical status, echocardiographic parameters, adherence to therapy and main causes of underuse and underdosing. Patients will be followed up for other 12 months to assess clinical outcomes and any interventional procedure (including valve intervention, ablation procedure for arrhythmias, or surgical intervention) (Table 3). Clinical outcomes will be ascertained during all the study period by patient or caregiver self-report during study visits or by telephone interviews. Clinical outcome reporting will also be retrieved by from electronic medical records, in-hospital charts, and the regional death registry.
| Study procedure | Baseline (V1)a | Intermediate 1 | Intermediate 2 | V2 | V3 | Clinical FU |
|---|---|---|---|---|---|---|
| Timing of visit (weeks) | 0 | 8 | 16 | 24 | 48 | 96 |
| Visit window (weeks) | 0 | ± 2 | ± 2 | ± 4 | ± 4 | ± 4 |
| Obtain informed consent | ✓ | |||||
| Basic demographic | ✓ | |||||
| Past medical history | ✓ | |||||
| Medication history | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Cardiac pharmacotherapy | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Physical examination | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Vital signs/NYHA class | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Body weight | ✓ | ✓ | ✓ | |||
| Laboratory test | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Electrocardiogram | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Echocardiogram and LVEF | ✓ | ✓ | ✓ | |||
| Safety and side-effect therapy reporting | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Clinical outcomes (as in Table 1) | ✓ | ✓ | ✓ | ✓ | ✓ |
- FU, follow-up; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
- a Ambulatory visit for outpatients/at discharge for inpatients.
Study size and statistical considerations
The size of the study is planned for its primary aim, to assess the prevalence of GDMT in a real-world outpatients HF population, and the treatment optimization/adherence during follow-up visits. Assuming each centre will recruit, on average, 70 patients during the enrolment period, the expected total sample size will be approximately 2500 patients. With the expected sample size of 2500 patients, the precision of the estimate of the proportion of patients with GDMT, and of patients who adhere to it, provided by the 95% confidence intervals calculated using the normal approximation of the binomial distribution, under different scenarios, is shown in Table 4. Statistical methods will vary according to the specific analysis performed and will be detailed in a pre-specified statistical analysis plan for each analysis. In general, continuous data will be summarized by measures that may include the mean, standard deviation, median, first and third quartiles, minimum, and maximum. Categorical data will be presented by frequencies (n and percentage) or contingency tables. Descriptive techniques that account for length of observation time (e.g. incidence rate ratios) will be used where appropriate. Multivariable analyses will be conducted using appropriate regression models (e.g. ordinary least squares, logistic regression) based on the distribution of the measure. Time-to-event data will be analysed using appropriate methods for right-censored data, e.g. Kaplan–Meier estimator, Cox proportional models, or cumulative incidence functions accounting for competing risks if necessary. Unless stated otherwise, two-sided p-values <0.05 will be considered statistically significant. Statistical analyses will be performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA) and STATA software (version 16; StataCorp, College Station, TX, USA).
| Patients with GDMT (%) | 95% CI | Optimization/ adherence (%) | 95% CI |
|---|---|---|---|
| 80 | 78.4–81.6 | 80 | 78.2–81.8 |
| 70 | 68.0–72.0 | ||
| 60 | 57.9–62.1 | ||
| 70 | 68.2–71.8 | 80 | 78.1–81.9 |
| 70 | 67.9–72.1 | ||
| 60 | 57.7–62.3 | ||
| 60 | 58.1–61.9 | 80 | 78.0–82.0 |
| 70 | 67.7–72.3 | ||
| 60 | 57.5–62.5 |
- CI, confidence interval; GDMT, guideline-directed medical therapy.
- Analysis accounts for different scenarios, given a sample size of 2500 patients, provided by the 95% CIs.
OPTIPHARM-HF organization oversight
The OPTIPHARM-HF registry is conducted by an independent steering committee including HF specialists, cardiologists, and outcome researchers. The Italian Heart Failure Association (ITA-HFA) reviewed the protocol and endorsed the study. This registry is developed in compliance with the Guidelines for Good Pharmacoepidemiology Practices (GPP) Revision 3 June 2015 and in line with the position paper ‘ENCEPP considerations on the definition of non-interventional trials under the current legislative framework’ (clinical trials directive 2001/20/EC), and Good Clinical Practice (GCP) and Helsinki Declaration, in compliance with the Italian Ministerial Decree of 30/11/2021. Given the observational nature of the registry, no additional insurance policy beyond the one in place for normal clinical practice is required. The disease registry and related documentation will be submitted to the Ethics Committee competent for each centre, pursuant to applicable law. The registry will be initiated after authorization in accordance with the in-house procedures of each institution. Patient data will be collected in an anonymous form and all data will be collected using an electronic case report form (REDCap, v11.0.3) and managed by the University Hospital ASST Spedali Civili of Brescia. The University of Brescia will serve as the data analytic centre.
Discussion
In recent years there has been a relevant breakthrough in the medical therapy management of patients with HF across the left ventricular ejection fraction (LVEF) spectrum. Along with traditional conventional medical therapies, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARB), MRAs and BB, sacubitril/valsartan and SGLT2 inhibitors have shown additional significant reduction of hard clinical endpoints on top of conventional medical therapy.2, 5, 18 Compared with conventional therapy, HFrEF quadruple pharmacological therapy was estimated to afford up to eight additional years free from CV death or HF hospitalization and to confer up to 73% relative risk reduction in all-cause mortality.2, 19, 20 Based on the results of PARAGON-HF and PARAGLIDE-HF, ARNI may be considered in patients with a LVEF up to 60% or, according to the guideline classifications, with HFmrEF and HFpEF.21 On the other hand, based on landmark trials and meta-analyses18 SGLT2 inhibitors are effective irrespective of LVEF and are recommended also in patients with HFpEF.3, 4 Recent evidence supports also treatment of iron deficiency and the use of vericiguat. A recent metanalysis showed a 25% relative risk reduction of CV death or HF hospitalization conferred by intravenous iron therapy among patients with HF with evidence of iron deficiency.22 Lastly, vericiguat showed a 10% risk reduction of CV death or HF hospitalization among worsening HFrEF on top of conventional medical therapy and is recommended in class IIb.23
Despite the profound benefits deriving by the combination of foundational HF medical therapies, yet there are unmet clinical needs related to the low prescription rate and the clinical challenge in the patterns of drug introduction, limiting a wide and consistent optimal background medical therapy. The CHAMP-HF registry showed a 27%, 33% and 67% of eligible HFrEF patients who were not prescribed ACEi/ARB/ARNI, BB and MRAs, respectively.11 Also, among patients with prescribed medications, the doses were generally low. Similar results come from other European and Asian registries, showing a substantial underutilization of GDMT, particularly with respect to achieving target doses.24, 25
There is an urgent need to understand the barriers for reaching a full drug pattern prescription, titration to target doses and persistence of GDMT. Indeed, most studies on medication use in HF show the unmet need of guideline implementation, but do not explain why this is the case. Also, available data mostly derive from studies conducted prior to the recent evidence of the efficacy of SGLT2 inhibitors, iron replacement and vericiguat. In addition, epidemiological data are mostly confined to HFrEF patients or, worse, based on administrative databases lacking details regarding mandatory characteristics of the patients enrolled such as their LVEF. In contrast, OPTIPHARM-HF is one of the first registries describing contemporary practice of management of HF with full details regarding patient characteristics.
OPTIPHARM-HF will provide important information on medication prescription and adherence in a real-world setting with broad inclusion criteria reproducing real-world clinical practice as compared to clinical trials. The registry will provide a comprehensive understanding of the contemporary management of patients with HF across the ejection fraction spectrum and across different clinical stages and phenotypes, such as those with worsening HF, advanced HF and HFimpEF. The registry will also assess medication changes, including the initiation and up-titration of new HF therapies in patients with de novo HF and after a hospitalization for acute HF. Yet, there is a lack of evidence from real-world data regarding the sequence initiation of GDMT in these patients.26-28 Compared to previous international guidelines which advocated a sequential step-by-step initiation by starting the drug classes in chronological order of entry into the guideline, new recommendations suggest a rapid initiation and up-titration of pillars drugs without delay.3, 4 Although different sequencing approaches have been proposed, no validated data are yet available. Recently, the STRONG-HF trial provided direct evidence that intensive, simultaneous and rapid sequence initiation and titration of GDMT up to 100% of the recommended doses within 2 weeks of discharge for acute HF is safe, well-tolerated, and effective for reducing death and HF hospitalization.29 However, its applicability and adoption in clinical practice, beyond the context of a clinical trial, is less known. Finally, the OPTIPHARM-HF will assess implementation of comprehensive HF management including treatment of comorbidities, such as iron deficiency, valvular heart disease, kidney dysfunction and electrolyte abnormalities, including hyperkalaemia and the use of potassium binders in optimization of GDMT.
The OPTIPHARM-HF is a national registry, potentially limiting the external generalizability of our findings, although barriers encountered on HF care are most likely exchangeable across different regions. Patients will be enrolled in HF centres with different levels of quality of care. Nevertheless, as compared to rural hospitals or general practice there will be an expected more consistent and stringent GDMT and dose up-titration. This may limit the ability to reflect real-world data across different geographic areas. Potential clinical confounding that could affect treatment implementation, response or prognosis could not be captured by available case report forms, such as socioeconomic status, lifestyle factors and quality of life.
Although data will be consecutively collected, the study population will be limited to patients providing informed consent and completing study visits. This may introduce a potential selection bias, although this limitation is common to other previous prospective registries11 and clinical trials in general. Additionally, due to the nature of this study, clinical endpoints will be reported by the investigator and not centrally adjudicated.
Conclusion
The OPTIPHARM-HF will collect essential information on the HF medical therapy guideline implementation, uptake of GDMT and new drugs, and quality of care for patients with HF in a national context. The study will assess in a real-world context the prevalence of medication treatment prescription and titration as well as the cause of underuse, in a broad HF population across the whole ejection fraction spectrum and among different clinical phenotypes. These data will have important implications for improving patient care and adoption of new evidence into clinical practice.
Acknowledgements
We thank all the investigators and contributors involved in the registry.
Conflict of interest: none declared.
Open access publishing facilitated by Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia, as part of the Wiley – SBBL agreement.
Appendix A
A.1 OPTIPHARM-HF Investigators
Marianna Adamo1, Giuseppe Armentaro43, Andrea Attanasio39,40, Roberto Badagliacca48, Natale Brunetti23,24, Francesca Bursi21,22, Martino Canonero6, Angelo Caporotondi26, Velia Cassano43, Gennaro Cice9, Giuliana Cimino13, Francesco Clemenza13, Michele Correale23,24, Emilia D'Elia44, Letizia Fiorentino1, Alberto Foà41, Paolo Fornaro1, Stefano Ghio27,28, Lucia Guerisoli6, Manuela Iseppi45, Rossella Manai16,17, Giulia E. Mandoli10, Sofia C. Marini1, Fabio Marsico42, Gabriele Masini14,15, Ciro Mauro42, Alberto Mazzoni1, Marta Mazzotta1, Marco Merlo45, Savina Nodari1, Filippo Novarese6, Chiara Oriecuia46, Matteo Pagnesi1, Stefania Paolillo38, Maria C. Pastore10, Nicola R. Pugliese47, Claudia Raineri16,17, Federica Ramani45, Mario Sabatino41, Laura Scelsi27,28, Giulia Spoto27,28, Davide Stolfo45, Roberto Tarantini27,28, Enrico Vizzardi1, Mattia Zampieri20.




