Integration of implantable device therapy in patients with heart failure. A clinical consensus statement from the Heart Failure Association (HFA) and European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC)
Abstract
Implantable devices form an integral part of the management of patients with heart failure (HF) and provide adjunctive therapies in addition to cornerstone drug treatment. Although the number of these devices is growing, only few are supported by robust evidence. Current devices aim to improve haemodynamics, improve reverse remodelling, or provide electrical therapy. A number of these devices have guideline recommendations and some have been shown to improve outcomes such as cardiac resynchronization therapy, implantable cardioverter-defibrillators and long-term mechanical support. For others, more evidence is still needed before large-scale implementation can be strongly advised. Of note, devices and drugs can work synergistically in HF as improved disease control with devices can allow for further optimization of drug therapy. Therefore, some devices might already be considered early in the disease trajectory of HF patients, while others might only be reserved for advanced HF. As such, device therapy should be integrated into HF care programmes. Unfortunately, implementation of devices, including those with the greatest evidence, in clinical care pathways is still suboptimal. This clinical consensus document of the Heart Failure Association (HFA) and European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC) describes the physiological rationale behind device-provided therapy and also device-guided management, offers an overview of current implantable device options recommended by the guidelines and proposes a new integrated model of device therapy as a part of HF care.
Introduction
The treatment of heart failure (HF) has evolved tremendously in the past decades. The most important breakthrough was realized at the end of the previous century with the introduction of disease-modifying neurohormonal blockers to treat HF with reduced ejection fraction (HFrEF). These drugs improved survival and decreased HF hospitalizations significantly. In the same era, the first left ventricular assist device (LVAD) was approved by the Food and Drug Administration (FDA) to avoid patients from dying while waiting for a heart transplant. As the risk of sudden cardiac death remained high despite drug therapy, a quest for other treatment options started. This eventually led to the introduction of the first device that was used on a large scale in HF: the implantable cardioverter-defibrillator (ICD). In the subsequent decade, cardiac resynchronization therapy (CRT) was shown to improve prognosis. Since then, device therapy has become an essential part of HF treatment.
Despite the vastly improved treatment options, up to 20% of ambulatory HFrEF patients still experience a HF hospitalization or die within 2 years and 50% of hospitalized HFrEF patients have a readmission within 6 months despite optimal medical therapy.1-3 In addition, therapeutic options for patients with HF with preserved ejection fraction (HFpEF) remain limited and only sodium–glucose cotransporter 2 (SGLT2) inhibitors have been convincingly shown to reduce HF hospitalizations.4, 5 As such, there is an unmet need for more treatment options to improve the outcomes and improve quality of life of HF patients.
The last decades have seen leaps in the use of devices in HF, either to provide monitoring with the aim of improved tailoring of care, or directly providing therapy in synergistic with medical therapies. This document aims to provide an overview of guideline-recommended implantable devices currently available to treat and monitor HF, to summarize the clinical evidence and how it defines their role in an integrated HF treatment model. A selection of non-implanted as well as investigational device therapies that might be used in the future for HF management can be found in online Supporting Information.
Rationale for the use of device therapy in heart failure
Haemodynamic alterations
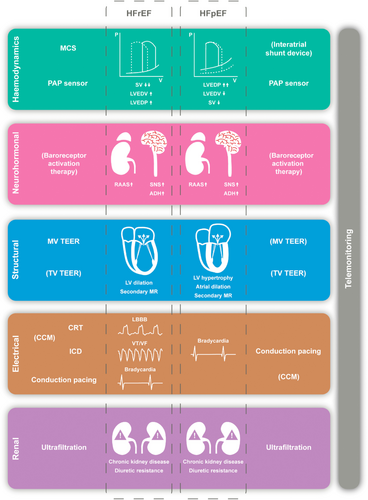
Central (cardiac) and peripheral haemodynamic alterations are characteristic of HF6 and can be due to both systolic and diastolic dysfunction. The impaired contractility of systolic dysfunction results in elevated left ventricular (LV) end-diastolic pressure which is compensated for by shifting the pressure–volume relationship towards higher volumes, initially preserving stroke volume and cardiac output at rest although in most patients higher filling pressures become a consistent part of the picture (Figure 1). In contrast, diastolic dysfunction is characterized by increased filling pressures due to impaired ventricular relaxation and compliance. Here too, cardiac output at rest is preserved in the initial stages. Of note, increased filling pressures and impaired cardiac output are common to both HFrEF and HFpEF, but both can be normal (at rest) in a ‘compensated’ state.7 Exercise can unmask haemodynamic derangements and exercise-based assessment can therefore be especially helpful in the diagnosis of HFpEF.8 Several devices have been developed to monitor or intervene upon these central haemodynamic derangements (Figure 1).
Neurohormonal activation
Heart failure is also characterized by neurohormonal activation. The most important upregulated pathways include the sympathetic nervous system (SNS), the renin–angiotensin–aldosterone system (RAAS) and antidiuretic hormone (ADH). Although neurohormonal activation can initially compensate for the impaired cardiac output by increasing vascular tone, preload and heart rate, these neurohormones become drivers of the disease process. The maladaptive neurohormonal activation is counterbalanced by natriuretic peptides as a response to atrial and ventricular stretch. In essence, these natriuretic peptides oppose the effects of RAAS and SNS activation, but cannot halt disease progression. Of note, there are several other hormones, neurotransmitters, neuromodulators and inflammatory peptides that are up- or downregulated in HF.9 Neurohormonal activation has been the target of most successful disease-modifying drug classes in HFrEF, reducing HF hospitalizations and mortality.10-13 Several devices modulate the autonomic nervous system14 (Figure 1).
Cardiac remodelling
The haemodynamic alterations and neurohormonal activation are also accompanied by important structural changes. In HFrEF, the left ventricle remodels with progressive dilatation, while HFpEF is associated with LV hypertrophy and atrial dilatation (Figure 1). The remodelling in HFrEF can impair mitral valve function and lead to secondary mitral regurgitation (MR) due to increased tethering forces, decreased closing forces and altered mitral valve function.15 In addition, atrial dilatation as seen in HFpEF can also impair mitral valve function and lead to secondary MR by annular dilatation.16 Both types of secondary MR are associated with worse outcomes.17 Several device therapies target these structural changes (Figure 1).
Arrhythmias
Heart failure patients can develop a wide range of arrhythmias during their disease course. These include tachy-arrhythmias such as atrial fibrillation (AF) and ventricular arrhythmias (VAs), and brady-arrhythmias. AF is the most prevalent supraventricular tachy-arrhythmia occurring in one third of HF patients, is associated with worse disease severity,18 and is a common precipitant of decompensation.19, 20 Although AF is a common consequence of atrial and ventricular remodelling, long standing AF with high ventricular rates can also cause LV dysfunction and HF.21 VAs can be a life-threatening complication of HF and VA-related death can be prevented by ICDs. Brady-arrhythmias and conduction abnormalities leading to a standard pacemaker indication occur more frequently in people with HF, and the presence of important LV systolic dysfunction often require a personalized approach (Figure 1).
Cardiorenal interaction
Heart failure and chronic kidney disease often coexist and complicate treatment.22 The physiological age-related decline in glomerular filtration rate (−0.5 to −1 ml/min/1.73 m2 per year) is accelerated in HF patients (up to −3 to −5 ml/min/1.73 m2 per year).23 As chronic kidney disease progresses, sodium and volume retention remain primarily driven by HF disease processes, but in severe cases the intrinsic renal dysfunction also contributes. Importantly, renal dysfunction can also lead to a reduced response to diuretic therapy.24 Of note, increased central venous pressures, observed in many acute HF cases, can drive further deteriorations in renal function.25 In cases of severe renal impairment, diuretics alone can be insufficient to achieve euvolaemia. Several devices have been developed to assist in sodium and volume homeostasis in such patients.
Devices aimed at management of electrical abnormalities
Implantable cardioverter-defibrillator
How does it work?
An ICD is an implantable electronic device that continuously monitors the ventricular rhythm and can deliver a shock should ventricular fibrillation or fast ventricular tachycardia (VT) occur. As such, ICDs can reduce the risk of sudden cardiac death caused by VAs. The defibrillator lead is placed either endovascularly in the right ventricle or subcutaneously. In contrast to subcutaneous ICDs, endovascular ICDs also provide anti-tachycardia pacing (ATP). This pacing mode, which stimulates the ventricle at a rate faster than the VT, can entrain and convert VT to normal rhythm in order to obviate shock therapy. Moreover, endovascular ICDs have all the functionalities of modern pacemakers to treat brady-arrhythmias. Potential disadvantages are inappropriate tachy-therapies (ATP or shocks), excess right ventricular (RV) pacing, or device-related complications (including lead failure and infection).
In whom to implant a cardioverter-defibrillator?
The annual risk of fatal VAs is around 4–5% in primary prevention and 10–20% in secondary prevention indications.26-28 As such, ICDs are indicated in HFrEF with LV ejection fraction (LVEF) <35% without prior VAs on optimal medical HF therapy in primary prevention (as recommended in ischaemic and should be considered in non-ischaemic, according to guidelines) and in secondary prevention to reduce risk of all-cause mortality.29, 30 In the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality) trial, ICDs significantly reduced the rate of sudden cardiac death, but did not reduce all-cause mortality in non-ischaemic cardiomyopathy, except in the subgroup of patients younger than 70 years.31 Importantly, disease-modifying drugs also reduce all-cause mortality and sudden cardiac death.26, 28
How to implement implantable cardioverter-defibrillator therapy?
Despite the robust evidence and clear guideline recommendations, ICDs are underutilized in HF, especially in primary prevention settings. While the proportion of the implanted patients is increasing in recent large trials in HFrEF,13, 32, 33 registry data indicate there is still a substantial underuse. For example, only 10% of HF patients with a guideline indication for a primary prevention ICD actually have one in a large national Swedish registry.34 While physician inertia likely plays a role, another possible explanation for the low uptake of ICDs in clinical practice is the misconception of low sudden cardiac death risk in HFrEF patients.26 Importantly, while current HF therapies prolong life significantly,35 life-time risk of sudden cardiac death accumulates over time, especially in younger patients.36 The lack of all-cause mortality benefit of ICDs in non-ischaemic cardiomyopathy in the DANISH trial31 has increased the scepticism about primary prevention ICDs in such patients. However, the ICD did reduce all-cause mortality in non-ischaemic patients below 70 years (with competing risk for mortality in those >70 years) and reduced sudden cardiac death overall.31 It is likely that in the future multiparametric risk stratification beyond LVEF, incorporating factors associated with an increased sudden cardiac death risk such as the presence of scar on cardiac magnetic resonance37, 38 and specific gene mutations39 may improve patient selection. Prediction models such as the MADIT-ICD benefit score40 and the Seattle prediction model for sudden cardiac death41 may also be helpful for risk stratification and discussion with patients and their referring clinicians. Better organization of HF (multidisciplinary teams) care and access to cardiology specialists is essential to ensure more appropriate implementation of ICDs. Finally, the decision to implant an ICD should be a shared decision with the patient,42, 43 also taking into account the patient's life expectancy and quality of life.
Cardiac resynchronization therapy
How does it work?
Cardiac resynchronization therapy is a treatment for selected HFrEF patients with prolonged QRS duration on the surface electrocardiogram (ECG), indicating electrical dyssynchrony. As a consequence of disturbed conduction, the activation of the left ventricle is delayed, leading to inefficient and dyssynchronous contraction, triggering structural, electrical, and contractile remodelling processes.44 In contrast to standard RV pacing, CRT requires two ventricular leads. In addition to the RV lead, an LV lead is implanted in a coronary sinus vein branch or alternatively a surgically placed epicardial lead is used. By pacing both ventricular leads, electrical activation resynchronizes and ventricular contraction and relaxation become more effective, improving haemodynamics.45 As dyssynchrony is a driver of remodelling, CRT can halt and often reverse HF progression.46 CRT can be delivered using a device capable of pacing only (CRT-P) or one that can also deliver ICD function (CRT-D).
In whom to implant a cardiac resynchronization therapy device?
QRS prolongation (QRS >120 ms) is present in 35–40% of HFrEF patients and 20–30% have a left bundle branch block (LBBB).43 CRT has unequivocally been shown to improve symptoms, reduce HF hospitalizations and mortality in HFrEF patients with LVEF ≤35%, sinus rhythm and a wide QRS (>130 ms) in several randomized trials.47-49 The largest benefit is seen in patients with a LBBB and those with a QRS >150 ms.50, 51 In patients with AF, evidence of benefit is less robust and adequate rhythm control (e.g. by performing AF ablation52) or, ultimately, atrioventricular junction ablation is required to ensure effective biventricular pacing.53-55 CRT-P rather than RV pacing is recommended for patients with HFrEF (<40%) who have an indication for RV pacing and high-degree atrioventricular block.4, 56 CRT is not indicated in patients with mechanical dyssynchrony and narrow QRS as it is associated with increased mortality.57
How to implement cardiac resynchronization therapy?
In spite of the strongest class of recommendation in all guidelines backed by solid evidence of benefit in selected patients, recent European data suggest that only one in three eligible patients actually receive a CRT device.58 The Heart Failure Association (HFA), European Heart Rhythm Association (EHRA) and European Association of Cardiovascular Imaging (EACVI) recently published a joint position paper with a call for action for referral and optimization of care in CRT.43 Greater penetration of the therapy requires education of both primary care and secondary care physicians, nurses and allied professionals. A common misconception that might hamper referral is the definition of ‘response’. The success of CRT should not be defined as the degree of reverse remodelling it induces, but rather as the grade of disease modification it provides. As HF is a progressive disease, stabilization of the LV function and the patient's clinical condition can already be considered as a treatment success.43 However, the best way to assess response is to show a decrease in hospitalization rate, an improvement in quality of life and improved survival. Another inappropriate barrier to referral and acceptance for implantation is the presence of comorbidities. CRT is effective in people with a wide range of comorbidities.59 Moreover, the implantation of the device should not be seen as the end of the pathway: post-CRT care is also essential to make the most of the opportunity and drug titration and device optimization is best delivered by a multidisciplinary ‘post-CRT’ team. CRT requires sufficient expertise as inappropriate patient selection, inadequate device implantation, or lack of optimization of device settings, exposes patients to periprocedural risks without any long-term benefit. Of note, it is very important that the LV lead is adequately positioned to ensure effective resynchronization.
Conduction system pacing
How does it work?
In conventional pacing, ventricular stimulation is provided in the RV apex or septum, in addition to right atrial pacing if indicated. However, RV pacing induces a dyssynchronous activation of both ventricles and a high pacing burden is associated with a decrease in LVEF.60 This might especially be particularly deleterious in patients with HF. Therefore, interest has grown in more physiological pacing methods. Firstly, His bundle pacing can be performed by implanting a pacing lead directly in the His bundle. Stimulation at this point can activate the ventricles over the conduction system even in a proportion of people with atrioventricular block, inducing a ‘physiological’ activation as evidenced by narrow QRS complexes on the surface ECG. His bundle pacing requires sufficient training, is not successful in all patients, often requires a back-up RV lead because of unstable pacing thresholds with the risk of loss of capture, and battery longevity may be reduced due to higher pacing thresholds. A similar approach that does not have these two limitations but also aims to specifically capture the conduction system is left bundle branch (LBB) pacing where, in order to capture the left bundle, the lead must be deployed deeply into the interventricular septum. Pacing activates the LBB directly inducing at least a more ‘physiological’ activation of the left ventricle with narrower QRS complexes than conventional RV pacing.61
In whom to implant a conduction system pacing lead?
The role of conduction system pacing in HF is yet to be determined. According to guidelines, His bundle pacing may be considered to prevent pacing-induced cardiomyopathy in patients with a high pacing burden.6, 62 Further, His bundle pacing should be considered as a treatment option in CRT candidates in whom a coronary sinus lead implantation is unsuccessful.6, 62-64 However, to date, large randomized data demonstrating efficacy, safety and long-term durability are lacking. The experience with LBB area pacing is still limited and randomized outcome trials are equally missing. A large observational prospective European registry indicated success percentages of 91% of LBB area pacing in narrow QRS and only 76% in wide QRS patients with complication rates up to 8.2%.65 Lastly, His bundle pacing or LBB area pacing may not be able to induce narrower QRS complexes in HF patients with diffuse conduction disease. Based upon the available data, European guidelines only make recommendations about His bundle pacing.
How to implement conduction system pacing?
Further data are needed to guide the use of conduction pacing in HF patients, beyond a bail-out strategy. If conduction system pacing proves to be a valid and better alternative to current pacing techniques, training of physicians will be needed to ensure wide adoption. Conduction system pacing often requires more guiding by an electrophysiological mapping system, but LBB area pacing can be performed without. As such, conduction system pacing could be implemented in many centres experienced in pacemaker implantations, once clear indications have been established.
Cardiac contractility modulation
How does it work?
A cardiac contractility modulation (CCM) device is comparable to a pacemaker with similar implantation technique and has two leads positioned in the RV septum. CCM provides high voltage (± 7.5 V), long duration (∼20 ms), biphasic stimulation to the RV septum during the absolute refractory period (∼30 ms after the beginning of the QRS complex). In contrast to regular pacing, these signals are non-excitatory and therefore do not generate an action potential. However, they improve the deficient calcium handling in the diseased cardiomyocytes of HF patients and therefore increase contractility without increasing myocardial oxygen consumption.66 In addition, CCM may be beneficial on other disease processes at the cellular level such as improvement of calcium handling and reversal the foetal myocyte gene programme.67 Currently, only the OPTIMIZER device (Impulse Dynamics, Orangeburg, NY, USA) is commercially available and has a battery that requires weekly recharging via a transcutaneous system.
In whom to implant cardiac contractility modulation?
Cardiac contractility modulation improved the quality of life and exercise capacity in symptomatic patients in sinus rhythm with LVEF <45% and QRS <130 ms in three open-label randomized trials,68-70 but the effect was rather small. There are no blinded, sham-controlled trials limiting the robustness of the data to influence guidelines.6 However, the AIM HIGHer clinical trial is a prospective, multicentre, randomized, quadruple-blind, sham-controlled, trial in subjects with HF and an LVEF ≥40% and ≤60% (NCT05064709).
How to implement cardiac contractility modulation?
Further evidence is needed to guide the role of CCM in routine practice, but in general CCM is only advised in selected patients by experienced operators working within a multidisciplinary HF service capable of follow-up and trouble shooting.
Telemonitoring
Telemonitoring via implantable cardiac rhythm management devices
How does it work?
All current cardiac implantable electronic devices (CIED) can be connected with a wireless telemonitoring system that enables follow-up of device and lead functioning and monitors arrhythmias. Different manufacturers also provide additional data such as thoracic impedance and patient activity or multiparametric integrated monitor systems aimed at early detection of worsening HF. If a certain threshold is crossed, the monitoring institution receives an alert upon which an action can follow.
In whom to use cardiac implantable electronic device telemonitoring?
Several randomized studies investigating the early detection of HF decompensation have been conducted. In the Diagnostic Outcome Trial in Heart Failure (DOT-HF), the use of an implantable diagnostic tool to measure intrathoracic impedance with an audible patient alert did not improve the composite of all-cause mortality and HF hospitalizations.71 Moreover, the system increased HF hospitalizations and outpatient visits. The results were similar in a later trial in higher risk ICD patients when no audible alert was given.72 In contrast, in the Influence of Home Monitoring on Mortality and Morbidity in Heart Failure Patients with Impaired Left Ventricular Function (IN-TIME) trial, automatic, daily, implant-based, multiparameter telemonitoring improved a composite clinical score combining all-cause death, overnight hospital admission for HF, change in New York Heart Association (NYHA) class, and change in patient global self-assessment in ICD and CRT-D patients after 1 year.73 Of note, there was also a clear reduction in all-cause mortality. Lastly, the Remote Management of heart Failure Using Implantable Electronic Devices (REM-HF) trial tested a remote monitoring strategy across three different manufacturers in 1650 patients with a CIED.74 In REM-HF, telemonitoring did not use manufacturer specific alerts, but monitored trends in measured parameters. Telemonitoring with weekly transmissions did not improve death from any cause or unplanned hospitalization for cardiovascular reasons. The discrepancy in trial results might be explained by the differences in the parameters that were used (multiparameter probably vs. single parameter, e.g. thoracic impedance), frequency of transmissions (daily vs. weekly) and actions taken by the treating centre.
How to implement cardiac implantable electronic device telemonitoring?
Guidelines recommend remote device management to reduce the number of in-office follow-up visits, and to enable early detection of actionable events in increased risk patients.6, 62 They also clearly state that the patient and device should be treated as a single entity and that the underlying cardiac disease should not be overlooked. Importantly, a structured organization of a remote monitoring ‘unit’ is essential with clear distribution of tasks between all stakeholders (including doctors, nurses, administration, etc.) and clearly defined actionable items for different occurring scenarios. More evidence is needed from randomized trials before large scale implementation should be organized to also monitor HF status beyond remotely monitoring device/lead integrity and arrhythmias.
Remote pulmonary artery pressure monitoring
How does it work?
Pulmonary artery pressure sensors are small implantable devices without a battery that enable remote monitoring of pulmonary artery pressures. The device is implanted percutaneously via the femoral or jugular vein. The delivery catheter with the device on the tip is advanced up to a side branch of the right or left pulmonary artery. Next, the device is fixed in position by releasing the self-expandable side anchors. The device is energized through an external source and contains an inductor coil and pressure-sensitive capacitor that translate pressures in resonance frequency. Pulmonary artery pressures can be estimated using a patient electronic system, which detects the resonance frequency and translates this back to pressures, creating real-time estimates of pulmonary artery pressure waves. Measurements are saved to a secured server, accessible to healthcare providers so that pulmonary artery pressures can be monitored remotely. As increases in filling pressures precede symptoms and signs of decompensation,75 monitoring pulmonary artery pressures enables early detection of congestion.
In whom to use remote pulmonary artery pressure monitoring?
Two large randomized trials have studied the CardioMEMS system (Abbott, Chicago, IL, USA). In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, haemodynamic-guided therapy with remote pulmonary artery pressure monitoring reduced the total number of HF hospitalizations by 28% after 6 months in NYHA class III patients with a HF hospitalization in the previous year.76 The beneficial effect persisted up to 18 months in a longer follow-up study.77 The Haemodynamic-Guided Management of Heart Failure (GUIDE-HF) trial investigated the CardioMEMS system in a broader patient population, including NYHA class II–IV patients with either a previous HF hospitalization or elevated natriuretic peptides.78 Haemodynamic management did not reduce the composite of all-cause mortality and total HF events after 12 months. Of note, in a prespecified pre-COVID-19 sensitivity analysis, haemodynamic-guided management was beneficial in the period before the pandemic. Very recently, the Remote Haemodynamic Monitoring of Pulmonary Artery Pressures in Patients with Chronic Heart Failure (MONITOR-HF) trial showed a significant improvement in quality of life and a 44% reduction in HF hospitalizations with the CardioMEMS system in NYHA class III patients with a previous HF hospitalization.79 A second pulmonary artery pressure monitoring device (Cordella, Endotronix Inc., Woodridge, IL, USA) has shown to also provide reliable pulmonary artery pressure data, and is designed to be more user friendly.80 Current guidelines recommend that using these pulmonary artery pressure sensors in selected HF patients may be considered, independent of ejection fraction.6
How to implement remote pulmonary artery pressure monitoring?
A key for successful implementation of haemodynamic-guided management is proper patient selection. The expected risk of HF hospitalization should be high enough, so that the remote monitoring might be able to reduce the event rate. However, the benefit of monitoring in very high-risk patients with advanced disease and few treatment options is likely to be less. In addition, these patients also often suffer from advanced renal dysfunction, which might also hamper diuretic response. In such patients other technologies such as mechanical circulatory support (MCS) might be more appropriate. Furthermore, just as is the case with other remote monitoring tools, patient compliance is essential, as well as adequate reaction of the HF care team to the observed changes in haemodynamics. Observational European studies suggest a reduction in HF hospitalizations81-83 as well as cost benefits for the CardioMEMS device.83 Currently, most studies used the mean pulmonary artery pressure to guide therapies (mostly changes in diuretics and/or neurohumoral blockers), but it is still unclear what action should be taken upon these pressures. The existing trials did not mandate any specific treatment adjustment, although most activity centred around diuretic dose adjustments. Information beyond mean pulmonary artery pressure can be collected with these sensors and it is likely that in the future a multiparametric approach integrating different metrics and using trends rather than ‘cut-offs’ is likely to not only improve sensitivity and specificity but also to encourage a particular treatment strategy.
Devices aimed at cardiac reverse remodelling
Mitral valve transcatheter therapies
How does it work?
Mitral valve transcatheter edge-to-edge repair (TEER) is a percutaneous technique aimed at reducing MR, using specially designed devices. Currently, both the Mitraclip (Abbott, Chicago, IL, USA) and PASCAL (Edwards Lifesciences, Irvine, CA, USA) devices are available while many others are under investigation. Both devices require access through the femoral vein, with a transseptal puncture offering access for a steerable guiding catheter to the left ventricle on which the device is loaded. The posterior and anterior mitral leaflets are grasped, pulled together and fixed with the device locked in place, creating an artificial double orifice valve, reducing the mitral valve orifice, thus reducing MR. The devices are available in different sizes and often more than one is needed to achieve a satisfactory result. The procedure is performed (typically under general anaesthesia) with transoesophageal echocardiographic guidance. Excessive reduction in effective orifice area should be avoided to prevent mitral valve stenosis. Although initially developed to treat primary MR, the technique can also treat secondary MR which even when mild, is increasingly recognized as a key driver of deterioration.
Other percutaneous techniques to reduce MR include direct and indirect annuloplasty. The Carillon device (Cardiac Dimensions, Kirkland, WA, USA) is inserted in the coronary sinus and externally clinches the posterior mitral valve annulus84 to reduce annular size and MR. It reliably reduces LV dimensions even in patients with marked LV dilatation, in whom a TEER procedure does not.85 The Cardioband Mitral System (Edwards Lifesciences, Irvine, CA, USA) was a direct annuloplasty technique with a ring system, that is currently not being marketed anymore, attached to the atrial side of the posterior annulus.86
In whom to use mitral valve transcatheter therapies?
Mitral valve TEER in the setting of secondary MR has been investigated in two large trials using the Mitraclip device. In the Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR) trial, 304 symptomatic HF patients with severe secondary MR (regurgitant volume of >30 ml per beat or an effective regurgitant orifice area of >20 mm2), LVEF 15–40% and who were no candidates for mitral valve surgery were randomized to Mitraclip plus medical therapy versus medical therapy alone.87 After 12 months, there was no difference in the composite of death from any cause or unplanned hospitalization for HF. The endpoint remained neutral after an extension up to 24 months of follow-up.88 In the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial,89 614 symptomatic HF patients with moderate to severe secondary MR (defined according to the American Society of Echocardiography guidelines,90 including effective regurgitant orifice area of >30 mm2), LVEF 20–50%, LV end-systolic diameter ≤70 mm who were no candidates for mitral valve surgery were randomized to Mitraclip plus medical therapy versus medical therapy alone. After 24 months, there was a 47% reduction in the annual rate of total HF hospitalizations. In addition, there was a reduction in all-cause mortality and an increase in exercise capacity and quality of life. The incongruent findings between MITRA-FR and COAPT have been attributed to differences in patient selection, concomitant medical and device therapy, echocardiographic criteria for MR and technical procedural factors.15 MitraClip has gained considerable popularity despite the diverging results of the two main studies and evidence from the RESHAPE-HF2 (NCT02444338) and MATTERHORN (NCT02371512) trials is awaited. Mitral valve TEER should be considered in carefully selected HF patients fulfilling the COAPT criteria.6 In those not fulfilling COAPT criteria, the guidelines suggest that a TEER or an annular approach may be considered and that the choice should be determined by local skills and patient comorbidities (the indirect annular approach of the Carillon device can be done under local anaesthetic without transoesophageal echocardiography).
How to implement mitral valve transcatheter therapies?
Secondary MR is a consequence of LV/atrial remodelling and should therefore be viewed as a part of the HF disease process. As such, optimal medical and device treatment of this disease process is a prerequisite before any intervention can be considered. Hence, a critical first step is the optimization of volume status since congestion and high filling pressures increase MR severity.91 Second, it is advised that all guideline-indicated disease-modifying drugs are uptitrated to the maximal tolerated dose. Neurohormonal blockers improve survival, lead to reverse remodelling and can reduce secondary MR.92-94 Third, patients eligible for CRT should be implanted with a CRT device according to guidelines.4 Dyssynchrony not only contributes to the HFrEF disease process and progressive remodelling, but also directly impairs mitral valve function.95 CRT induces reverse remodelling and reduces secondary MR, both at rest as well as during exercise.96 According to guidelines, only patients with persisting severe secondary MR after medical and device optimization should be considered for mitral valve TEER.4 Relatively young patients with severe LV dysfunction and severe MR may also be suitable candidate for advanced HF therapy, including LVAD or heart transplant, and should be considered according to guidelines before mitral valve TEER is performed.4 Every patient should be discussed in the Heart Team, including a HF specialist, to ensure optimal treatment and to evaluate eligibility for surgery and potential indication for additional revascularization. A more in-depth overview on the treatment of secondary MR in HF was recently published.15
Tricuspid valve transcatheter therapies
How does it work?
The technique of tricuspid valve TEER is comparable with mitral valve TEER. However, in contrast to the mitral valve, the tricuspid valve consists of three leaflets (anterior, posterior and septal). This implies that with TEER different strategies to place the device are possible. In general, either anteroseptal devices (‘bicuspidalization technique’) or a combination of anteroseptal and posteroseptal devices (‘triple orifice technique’) are used.97 Currently, both the TriClip (Abbott, Chicago, IL, USA) and PASCAL (Edwards Lifesciences, Irvine, CA, USA) systems are available. Via a femoral venous approach and using a long sheath, a catheter with the device loaded at the tip is guided into the right atrium and right ventricle. The two leaflets are grasped and approximated by closing the two arms of the device. Multiple devices are generally needed to achieve a satisfactory result. The procedure is performed under fluoroscopy and transoesophageal echocardiographic guidance. Other percutaneous techniques such as annuloplasty, valve replacement, or implanting bicaval valves are under investigation.98
In whom to use tricuspid valve transcatheter therapies?
Severe tricuspid regurgitation (TR) is associated with worse survival in HF irrespective of LVEF, MR, pulmonary hypertension or RV dysfunction.99, 100 In addition, surgery for isolated tricuspid valve disease has been associated with high mortality.101 As such, percutaneous techniques to reduce TR with lower procedural risk have gained attention. Despite growing experience with these percutaneous techniques, especially with TEER, randomized data on outcomes in general and in HF patients in specific are currently lacking. In non-randomized multicentre feasibility studies, the TriClip system and the PASCAL system reduced TR, were safe,102, 103 were associated with an improved quality of life and improved exercise capacity. However, both trials had a small sample size, had a short follow-up time, included very few patients with LV dysfunction and had no control arm. For the TriClip system, the TR reduction persisted up to 1 year during extended follow-up.104 A propensity-matched registry study suggested tricuspid valve TEER could improve outcomes in patients otherwise treated with medical therapy alone.105 The recently published Trial to Evaluate Cardiovascular Outcomes in Patients Treated with the Tricuspid Valve Repair System Pivotal (TRILUMINATE Pivotal) trial demonstrated that tricuspid valve TEER was safe for patients with severe TR, reduced the severity of TR, and was associated with an improvement in quality of life.106 In addition, TEER with the TriClip system resulted in substantial and sustained health status improvement assessed by the Kansas City Cardiomyopathy Questionnaire in patients with severe TR compared with medical therapy alone.107
How to implement tricuspid valve transcatheter therapies?
Before guideline recommendations can be made on how to implement tricuspid valve TEER or other percutaneous techniques in HF patients, prospective data on relevant outcomes from randomized controlled trials with long follow-up are needed. These trials would also need to report on clinical outcomes in HF patients specifically. Importantly, surgical tricuspid repair of isolated severe TR has also not been associated with improved survival compared with medical therapy.108 Therefore, medical therapy currently remains the cornerstone of treatment, primarily consisting of diuretic therapy to treat volume overload and treatment of any underlying LV disease or pulmonary hypertension.
Devices aimed to directly improve haemodynamics
Short-term mechanical circulatory support
How does it work?
Short-term MCS devices are designed to temporarily unload the failing ventricle and/or to increase cardiac output. Current available devices include the intra-aortic balloon pump (IABP) Impella (Abiomed, Danvers, MA, USA), TandemHeart (LivaNova, London, UK), iVAC 2L (PulseCath BV, Amsterdam, The Netherlands) and venoarterial extracorporeal membrane oxygenation (VA-ECMO). An overview of the device characteristics is provided in Table 1.
| Device | IABP | Impella | TandemHeart | iVAC 2L | VA-ECMO |
|---|---|---|---|---|---|
| Pump system | Pulsatile | Continuous, axial flow | Continuous, centrifugal flow | Pulsatile | Continuous, centrifugal flow |
| Catheter size (Fr) | 7–8 | 12–22 |
Venous: 21 Arterial: 15–19 |
17 |
Venous: 18–29 Arterial: 15–21 |
| Access site | Femoral artery | Femoral/axillary artery |
Venous: femoral vein Arterial: femoral artery |
Femoral artery |
Venous: femoral vein Arterial: femoral artery |
| Fluoroscopic guiding mandatory | Yes | Yes | Yes | Yes | No |
| Location pump | Thoracic descending aorta | Transvalvular aortic valve | Extracorporeal | Extracorporeal | Extracorporeal |
| Location driver system | Extracorporeal | Extracorporeal | Extracorporeal | Extracorporeal | Extracorporeal |
| Inflow | – | Left ventricle | Left atrium | Left ventricle | Right atrium |
| Outflow | – | Ascending aorta | Iliac artery | Ascending aorta | Descending aorta |
| Aortic flow direction | Antegrade | Antegrade | Retrograde | Antegrade | Retrograde |
| Increase cardiac output | 0.5–1 L/min | 1–6 L/min | 2.5–5 L/min | 1–2 L/min | 3–7 L/min |
| LV load | ↓ afterload | ↓↓↓ preload and afterload | ↓↓ preload and afterload | ↓↓ preload and afterload |
↓ preload ↑↑ afterload |
| Maximal duration | Weeks |
5–30 daysa |
30 days | 24 h | Weeks |
| Implantation time | + | ++/++++b | +++ | + | ++ |
| Cost | + | ++++ | ++++ | ++ | +++ |
| Availability | ++++ | + | + | + | ++ |
| Complexity | − | + / ++ | ++ | + | +++ |
- IABP, intra-aortic balloon pump; VA-ECMO, venoarterial extracorporeal oxygenation.
- a 5 days for Impella 2.5 and CP; 14 days for Impella 5.0; 30 days for Impella 5.5.
- b Shorter implantation time for percutaneous insertion (Impella 2.5 and CP) vs. surgical insertion (Impella 5.0 and 5.5).
The IABP is an inflatable balloon that is percutaneously inserted mostly via the femoral artery in the descending thoracic aorta. During diastole, the balloon inflates, which increases the diastolic pressure proximal to the balloon and increases coronary perfusion. During systole, the balloon is actively deflated, creating a vacuum suction effect, which decreases LV afterload and increases cardiac output slightly. The Impella device is an axial rotatory pump that is percutaneously (femoral artery) or surgically (axillary or femoral artery) inserted retrogradely across the aortic valve. The tip with the inlet resides in the left ventricle, while the outlet is in the proximal ascending aorta. The turbine system, contained within the catheter between the inlet and outlet, continuously ejects blood into the aorta unloading the left ventricle and directly increasing forward flow over the aortic valve. The speed of the turbine is regulated by an externally attached driver system. Different Impella devices with increasing grades of haemodynamic support are available. Whilst most can be inserted percutaneously, the largest, which can provide full cardiac support, require surgical access. Haemodynamically, the Impella device unloads the left ventricle and directly increases forward flow over the aortic valve.
The TandemHeart device is an extracorporeal pump system with an inflow cannula inserted trans-septally into the left atrium and an outflow cannula in the iliac artery. Both cannulas can be inserted percutaneously. The cannulas are connected to a centrifugal rotator pump with an adjustable pump speed. If needed, a membrane oxygenator can be added to the circuit to allow extracorporeal oxygenation. The TandemHeart reduces ventricular preload by reducing the blood volume in the left atrium, but increases afterload because of the retrograde flow in the aorta. Nevertheless, the TandemHeart reduces myocardial wall stress and oxygen demand109 and increases blood flow to the organs.
The iVAC 2L device is a relatively new device that provides pulsatile flow support. It consists of a two-way valve pump system that is inserted via the femoral artery with its inlet in the left ventricle and its outlet in the ascending aorta. The bidirectional flow catheter is connected to an extracorporeal membrane pump, that is driven by an IABP console. The pump aspirates blood from the left ventricle during systole and ejects blood in the ascending aorta during diastole, decreasing preload and afterload and increasing forward flow modestly.
Venoarterial ECMO provides both oxygenation and full circulatory support. The circuit is composed of an inflow cannula at the right atrium, a centrifugal rotator pump, a membrane oxygenator with heater and an outflow cannula at the descending aorta. The most used cannulas (arterial 15–17 Fr; venous 25 Fr) can be inserted percutaneously or surgically depending upon local experience In peripheral ECMO, the venous inflow cannula is mostly inserted in the femoral vein and advanced into the right atrium. Likewise, the arterial inflow cannula is most frequently inserted in the femoral artery. To decrease the risk of limb ischaemia, an additional small cannula is often inserted in the distal femoral artery to secure antegrade flow of the limb. Alternatively, the VA-ECMO system can be inserted centrally in the right atrium and ascending aorta via sternotomy (‘central VA-ECMO’). The VA-ECMO is the only device that can provide biventricular support with one configuration as it bypasses both the left and right ventricle. VA-ECMO increases blood flow to the organs, decreases LV preload, but like TandemHeart, increases LV afterload because of the retrograde aortic flow that raises aortic pressure without supporting the left ventricle directly. At high retrograde flow, the rise in aortic pressure might hamper opening of the aortic valve, especially in patients with severely impaired LV systolic function, which increases LV volumes, myocardial wall stress, myocardial oxygen consumption, filling pressures and can induce pulmonary oedema. Therefore, VA-ECMO requires close monitoring of LV emptying with echocardiography and often an LV unloading strategy is needed which can be inotropes to improve LV emptying, another MCS device (e.g. IABP or Impella), atrial septostomy, or surgical venting with a cannula inserted in the left ventricle or the left atrium.
Several devices are available to support the right ventricle, but experience with their use is limited. The Impella RP is an axial rotator pump device that is inserted in the femoral vein and advanced such that the inlet is at the height of the right atrium and the outlet in the main pulmonary artery. MCS for RV support may be provided by a single dual lumen cannula or two single-lumen cannulas connected to an extracorporeal pump device. The ProtekDuo (LivaNova, London, UK) provides a dual flow circuit driven by an extracorporeal pump system in a single catheter inserted through the jugular vein with its tip and outflow at the main pulmonary artery and multiple inflow ports at the right atrium. Both the Impella RP and ProtekDuo can only be used in case of isolated RV failure or need to be combined with an LV support device otherwise. In contrast, VA-ECMO can be used in patients with both LV and/or RV failure and is currently the most used MCS in the setting of RV failure.
All short-term MCS devices require anticoagulation (mostly done with unfractionated heparin), exposing the patient to an increased bleeding risk. In the setting of acute coronary syndromes and dual antiplatelet therapy, clopidogrel is the preferred P2Y12 inhibitor because of the lower bleeding risk than ticagrelor and prasugrel.110 Access site complications, limb ischaemia, haemolysis and thromboembolic complications are persistent risks despite anticoagulation.
In whom to use short-term mechanical circulatory support?
Although short-term MCS devices are widely available, the evidence supporting their use is limited. Currently, they are mainly used to treat cardiogenic shock awaiting either recovery or a long-term solution (i.e. long-term MCS or transplant). In addition, short-term MCS is sometimes also used in high-risk percutaneous coronary interventions and in patients who present with high-risk myocardial infarction without cardiogenic shock. The Intra-Aortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial showed that routine use of IABP in patients with myocardial infarction complicated by cardiogenic shock did not improve 30-day mortality for whom an early revascularization strategy was planned111 and its routine use is not recommended according to guidelines.6 However, MCS may be considered as bridge to transplant or decision in cardiogenic shock. In both observational studies112-114 as well as small randomized trials115-118 Impella and TandemHeart devices improved haemodynamics, but did not improve 30-day mortality in patients with myocardial infarction and cardiogenic shock. Bleeding risk and vascular access complications were higher compared with IABP. On the other hand, a propensity matched meta-analysis of four observational studies in patients with cardiogenic shock after acute myocardial infarction suggested a lower 30-day mortality with ECMO compared with IABP, but similar outcomes to patients managed with Impella and TandemHeart.119 There are currently no prospective data to support the use of iVAC 2 L. Data on outcome in cardiogenic shock outside the myocardial infarction setting are lacking for all short-term MCS devices. Short-term MCS therefore should be considered in cardiogenic shock patients, according to guidelines.6 Patients with mechanical aortic valves and severe aortic regurgitation are not suited for Impella or iVAC 2L.
How to implement short-term mechanical circulatory support?
The limited data and experience means that the use of MCS, patient selection and outcomes vary widely across Europe and depend on local expertise. Cardiogenic shock patients should be cared for in a multidisciplinary setting in expert centres by shock teams. This arrangement improves appropriate use of short-term MCS and is associated with lower mortality.120, 121 A shock team should at least consists of intensivists, interventional cardiologists, HF specialists and cardiac surgeons, who should together weigh the risk and benefit of interventions. All datasets suggest that early interventions by such a team are associated with better survival, such that early referral is a key determinant of outcome. Regions should develop a pathway of referral and discussion between smaller hospitals and larger centres that is available 24 h a day. The decision to initiate short-term MCS is challenging: the therapy is advised in patients with a reasonable prognosis who are anticipated to recovery sufficiently to wean off the MCS or who are candidates for advanced therapies (i.e. long-term MCS or transplant). A detailed overview of the contemporary management of cardiogenic shock including MCS was described in a recent position paper.122
Long-term mechanical circulatory support
How does it work?
Long-term MCS mainly concerns LVADs. These are surgically implanted devices that have an inflow cannula in the LV apex and an outflow cannula at the ascending aorta. Current LVAD systems are fully implantable axial (HeartMate II, Abbott, Abbott Park, IL, USA) or magnetically levitated centrifugal rotator pumps (HeartMate 3, Abbott, Abbott Park, IL, USA and HVAD, Medtronic, Minneapolis, MN, USA) which provide a continuous flow. The HVAD has been taken off market recently because of numerous reports of patient injuries and deaths associated with the device, but there still patients currently being supported by this device. In contrast to the HeartMate II and the HVAD, the HeartMate 3 also provides intrinsic pulsatility by intermittent short-lived changes in pump speed every 2 s as a way to reduce the risk of thrombosis. Whereas the body of the HeartMate II is implanted in an abdominal pocket, the HeartMate 3 and the HVAD are fully implanted intrapericardially. For all devices, a driveline is tunnelled subcutaneously and connected to the extracorporeal controller and batteries that can provide power for more than 12 h. LVADs have an adjustable pump speed, which is patient tailored to maximally optimize haemodynamics. As such, the LVAD unloads the left ventricle and can increase forward flow up to 10 L/min. At present, the use of anticoagulation in combination with antiplatelet therapy is advised in all LVADs. With the currently most used HeartMate 3 device most common complications are bleeding (0.71 per patient-year), driveline infection (0.21 per patient-year) and stroke (0.07 per patient-year), whereas the risk of pump thrombosis has decreased substantially as compared with other devices (0.01 per patient-year).123
For patients with biventricular dysfunction, biventricular assist devices (BiVAD) and total artificial heart devices are available. In BiVAD, two independently working ventricular assist device systems are implanted. The right-sided ventricular assist device has its inflow at the right atrium and outflow at the pulmonary artery and is combined with a ‘classic’ LVAD. The right-sided system makes use of the same pump devices as the left-sided system, but two separated pump devices need to be used. Total artificial hearts are pump systems that replace the patient's heart and are implanted in an orthotopic position (‘mechanical transplantation’). Similar to ventricular assist devices, the total artificial heart requires drivelines and is connected to an extracorporeal controller. As both BiVAD and total artificial heart come with high complication rates, limited improvements in quality of life and are only supported by observational data, they are only used as a bridge to transplant in very selected cases.124, 125
In whom to implant long-term mechanical circulatory support?
Left ventricular assist devices were initially intended and designed to support very advanced HF patients with or without cardiogenic shock for a short period before transplantation. As the newer devices have lower complication rates and the shortage of donors is increasing, the use of LVADs has grown in the past decades. LVADs are currently approved as a bridge to transplant, a bridge to recovery (i.e. awaiting anticipated recovery of the left ventricle), bridge to decision (i.e. in case of uncertainty of transplant candidacy) but practically most are currently used as destination therapy (i.e. patients who are not eligible for heart transplantation).6 The current generation LVADs have been studied both as bridge to transplant as well as destination therapy. Overall, the newer continuous flow devices increase exercise capacity, improve quality of life and have lower complication rates in comparison with older pulsatile devices.126-130 Survival was reported to be around 70% after 2 years for HeartMate II and HVAD,131 but has further increased to around 80% after 2 years123, 132 and to around 60% after 5 years133 in HeartMate 3. Proposed criteria for LVAD eligibility are shown in Table 2.6 As LVADs can only support the left ventricle, they are not suitable for patients with concomitant severe RV failure. In patients with significant aortic regurgitation, implanting an LVAD might exacerbate the preexisting valve disease and therefore additional suture repair or LV outflow tract closure is often performed, increasing the operative risk of the procedure.134 In addition, mechanical aortic valves need to be replaced by a bioprosthetic valve or the valve has to be excluded from the circulation to allow for LVAD therapy.134 Therefore, the presence of aortic valve pathology and their associated operative risks needs to be taken into account when selecting patients for LVAD.
| Patients with persistence of severe symptoms despite optimal medical and device therapy, without severe right ventricular dysfunction and/or severe TR, with a stable psychosocial background and absence of major contraindicationsa, and who have at least one of the following: |
|
|
|
|
- HF, heart failure; i.v., intravenous; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; TR, tricuspid regurgitation; VO2, oxygen consumption.
- a Stable psychosocial background includes demonstrated understanding of the technology and patient living in the same household with a caregiver that will help the patient (i.e. living alone and poor psychosocial background is an LVAD contraindication). Major contraindications include contraindication to long-term oral anticoagulation, infection, severe renal dysfunction, ventricular arrhythmias.
- Adopted from6
How to implement long-term mechanical circulatory support?
Despite the survival benefits of LVADs in advanced HF patients, only a minority of eligible patients ultimately receive an LVAD.124 Financial constraints, the need for referral, and underappreciation of the prognostic and quality of life benefits might be some important reasons for the low uptake of LVADs in clinical practice. Patients with HFrEF, without comorbidities resulting in a life expectancy <1 year, but with persistent severe symptoms (NYHA class III–IV) despite optimal medical and device therapy, as well as patients with less severe symptoms (NYHA class II) and risk factors for progressive pump failure are advised for referral.124 Although early implantation prior to shock is associated with improved survival,135 most patients receive an LVAD only after they develop cardiogenic shock and require short-term MCS or inotropes.136 Hence increased awareness of referral criteria and a 24-h pathway of referral to the local tertiary centre is required for those working in secondary care hospitals.
Strategies to improve the care of advanced HF have been outlined in another position paper.124 A multidisciplinary evaluation by the Heart Team at the advanced HF centre is needed to select candidacy for LVAD, taking into account the patient's wishes, cardiac disease, comorbidities, and psychosocial background. Outpatient follow-up is advised in a multidisciplinary setting including cardiac surgeons, HF specialists, specialized nurses, nutritionists, pharmacologists, physiotherapists and psychologists. Currently, regulatory and reimbursement issues limit the implementation of LVAD as destination therapy in several European countries. More outcome data from Europe may enhance and support the implementation of LVADs.
Other device therapies
Regulatory issues on device therapy and selected device therapies like interatrial shunt devices, non-implantable devices for telemonitoring, to treat hypertension, sleep apnoea, and renal dysfunction as well as some devices for autonomic modulation are discussed in the online Supporting Information. While some of them are already mentioned in the guidelines, many are still under investigation.
Integration of implantable device therapies in heart failure care
Device therapy is currently only considered in HF patients after drug therapies have been optimized. As such, patients are often considered only after the period during which the devices could have their greatest effect in synergy with the medical therapy has passed. A particular example is CRT. While medical therapy is clearly linked to improvement in LVEF in HFrEF patients, the effects on remodelling are significantly less in patients with wide QRS.137, 138 Delaying the CRT implant has been associated with less reverse remodelling, more HF hospitalizations and increased all-cause mortality.139-141 Importantly, early use of CRT might improve the adverse haemodynamics (low cardiac output, low blood pressure, brady-arrhythmias) seen in HF and facilitate the optimization of medical therapy.
Therefore, a position statement endorsed by HFA/EHRA/EACVI and the latest European Society of Cardiology (ESC) guidelines on pacing encourage clinicians not to postpone CRT, particularly in patients with LBBB and QRS ≥150 ms.43, 62 As a consequence, physicians treating HF patients should not only be aware of indicated drug treatments, but also have knowledge about available devices with their indications, expected benefit and limitations. We propose to move from a commonly used sequential model to a more integrated model. The evaluation for potential device therapies is advised at diagnosis to facilitate a clear treatment plan that initially focusses on drug therapy but incorporates devices and interventions tailored to the patient phenotype with a clear strategy on their timing (Figure 2). Such a multi-modality treatment plan also should allow to initiate drugs and devices simultaneously as indicated, allowing them to work synergistically. Also, maximal efforts should be undertaken to further optimize medical therapy after device placement. Of note, such a treatment plan needs to be re-evaluated and updated continuously as conditions can change during the HF disease course.
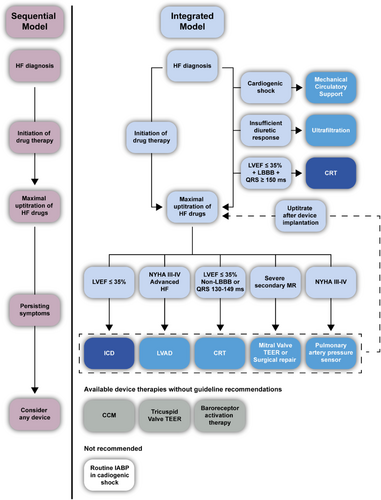
Before initiating any device therapy, a multidisciplinary team should discuss all available options to ensure adequate implementation of the device. The members of such a multidisciplinary team depend on the type of device being considered, but should always involve a HF specialist and HF nurse. Other members to be considered are imaging specialists, interventional cardiologist, cardiac intensivists, cardiac surgeons, nephrologists, psychologists, physiotherapists, nutritionists and primary care physicians. In order to ensure that the next generation of medically-qualified HF specialists will receive high-quality training, a recent consensus statement of the HFA outlined the requirements for a European training and certification programme for such specialists.142 The primary goal of such a new comprehensive educational programme is to increase the quality of patient-centred care related to HF including high-quality device care. Importantly, patient empowerment for device therapy is extremely important allowing shared decision-making process.
To facilitate a paradigm shift, which would allow better implementation of devices in routine care, it is important to raise awareness of device therapy options among cardiologists, general practitioners, nurses, allied professionals and patients. Early referral (or at least advice) and collaboration between primary and expert centres are of utmost importance to overcome the current inadequate or delayed care faced by many patients. Therefore, hospital referral networks should be created to ensure all patients have timely access to device therapies.
Follow-up of devices should be done by trained healthcare providers working in an integrated way and embedded in a larger multidisciplinary HF care programme, led by a HF specialist team with appropriate support networks for all members of that team.
Role of heart failure nurses and other allied professionals in device care
Nurses and allied professionals should be involved in daily device management in patients with HF. Who exactly is involved depends on the type of device, but also on national and local resources. Specialized HF nurses have roles in the different phases of device management. As such, HF nurses should be familiar with the available devices, their effects, indications and potential risks.143 As part of the multidisciplinary HF team, HF nurses play an important role in screening patients for device eligibility. In addition, HF nurses can help patients and their families to prepare for the device implantation by providing information about implantation procedures, working mechanisms of the device and the associated risks. Importantly, this information can help patients cope with the device after implant, set realistic expectations and prevent possible fears and misconceptions, enabling shared decision-making.144 After implantation, HF nurses can assist in monitoring for effects and side-effects/adverse events related to device function as well as further optimize HF therapies where appropriate. They should integrate results from remote monitoring or device readings in their assessment and treatment and discuss consequences of the device for daily life, e.g. handling alarms, driving restrictions, changes in body image, sexual function, pregnancy planning, social activities or self-care.145, 146 HF nurses should also identify changes in physical and emotional functioning resulting from device implantation and take appropriate action to optimize quality of life.143 During the further HF trajectory, patients and caregivers might need reassessment of the need for a device, advise on coping with the device on the long term, the need for continuing telemonitoring, or they might require further discussion about deactivation and the consequences of having a device in the end-of-life. HF nurses can play an important role in all of these issues and in advanced care planning.
In addition, allied professionals and healthcare scientists can have an important role as part of the HF multidisciplinary team, although these professions currently only exist in a small number of European countries. Some healthcare systems allow for pharmacist-led HF clinics that provide opportunities to screen for device eligibility, optimize medical therapies and provide patient information.147 Cardiac device technicians can have an important role in device optimization including recognizing patients who require escalation of care. HF nurses, allied professionals and healthcare scientists involved in the management of HF should have the appropriate level of training and competence to improve patient care and appropriate access to HF therapies.143, 148, 149
Conclusion
An increasing number of medical devices have been added to the HF management armamentarium. Some of these are supported by robust clinical evidence, while others are currently undergoing testing in clinical trials. Devices and drugs work synergistically but due to intrinsic risks associated with the procedure and permanence of implantation, a device ‘prescription’ requires careful and well-documented multidisciplinary decision-making and a coordinated follow-up process embedded into a combined HF-device care programme.
Conflict of interest: none declared.




