Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post-discharge period: The multinational randomized LeoDOR trial
Additional investigators in the LeoDOR trial are acknowledged in online supplementary Appendix S1.
Abstract
Aim
The LeoDOR trial explored the efficacy and safety of intermittent levosimendan therapy in the vulnerable phase following a hospitalization for acute heart failure (HF).
Methods and results
In this prospective multicentre, double-blind, two-armed trial, patients with advanced HF were randomized 2:1 at the end of an index hospitalization for acute HF to intermittent levosimendan therapy or matching placebo for 12 weeks. All patients had left ventricular ejection fraction (LVEF) ≤30% during index hospitalization. Levosimendan was administered according to centre preference either as 6 h infusion at a rate of 0.2 μg/kg/min every 2 weeks, or as 24 h infusion at a rate of 0.1 μg/kg/min every 3 weeks. The primary efficacy assessment after 14 weeks was based on a global rank score consisting of three hierarchical groups. Secondary clinical endpoints included the composite risk of tiers 1 and 2 at 14 and 26 weeks, respectively. Due to the COVID-19 pandemic, the planned number of patients could not be recruited. The final modified intention-to-treat analysis included 145 patients (93 in the combined levosimendan arm, 52 in the placebo arm), which reduced the statistical power to detect a 20% risk reduction in the primary endpoint to 60%. Compared with placebo, intermittent levosimendan had no significant effect on the primary endpoint: the mean rank score was 72.55 for the levosimendan group versus 73.81 for the placebo group (p = 0.863). However, there was a signal towards a higher incidence of the individual clinical components of the primary endpoint in the levosimendan group versus the placebo group both after 14 weeks (hazard ratio [HR] 2.94, 95% confidence interval [CI] 1.12–7.68; p = 0.021) and 26 weeks (HR 1.64, 95% CI 0.87–3.11; p = 0.122).
Conclusions
Among patients recently hospitalized with HF and reduced LVEF, intermittent levosimendan therapy did not improve post-hospitalization clinical stability.
Graphical Abstract
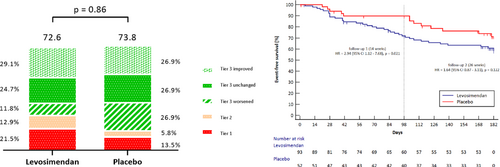
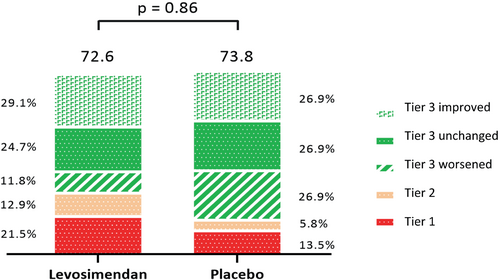
Key question: The LeoDOR trial explored the efficacy and safety of intermittent levosimendan therapy in the vulnerable phase following a hospitalization for acute HF. Key findings: Compared with placebo, intermittent levosimendan had no significant effect on the primary endpoint: the mean rank score was 72.55 for the levosimendan group vs 73.81 for the placebo group; p=0.863. However, there was a signal towards a higher incidence of the individual clinical components of the primary endpoint in the levosimendan group vs the placebo group both after 14 weeks and 26 weeks. Take home message: Among patients recently hospitalized with HF and reduced LVEF, intermittent levosimendan therapy did not improve post-hospitalization clinical stability. For the global rank score all participants were ranked across three hierarchical groups: Tier 1 = time to death or urgent heart transplantation or implantation of a ventricular assist device; Tier 2 = time to non-fatal HF requiring i.v. vasoactive therapy; and Tier 3 = time-averaged proportional change in NT-proBNP from baseline to week 14 (Tier 3 worsened/improved was defined as an increase/decrease of >25% in time-averaged proportional change in NT-proBNP, respectively).
Introduction
Patients with advanced heart failure (HF) frequently experience recurrent episodes of acute HF, which worsen an already grave prognosis.1 Readmission and mortality rates in patients with advanced HF are particularly high in the weeks immediately following discharge for such an episode2, 3: about 25% of patients are readmitted within 3 months, and about 12.5% die.4 Rehospitalizations during this early discharge period, also known as the ‘vulnerable phase’, account for a disproportionate amount of the extensive overall costs of advanced HF care. It is estimated that up to 75% of early readmissions may be preventable.5 However, effective measures to reduce event rates during this vulnerable period remain poorly defined, highlighting a critical unmet need.
The presence of haemodynamic changes and hypoperfusion even after stabilization of the acute phase of a HF hospitalization contributes to the high event rate.6, 7 Levosimendan is a calcium sensitizer and potassium channel opener with a pharmacology and clinical profile potentially relevant to this situation.8-10 Clinical studies of the repetitive use of intravenous (i.v.) levosimendan suggested that this intervention may benefit patients with advanced HF, reducing the risk of rehospitalization and death.11-13 Similar treatment has not hitherto been studied in the transition phase following an acute HF event but the possibility of clinical advantage is plausible and worthy of investigation.
The randomized LeoDOR trial (Repetitive LevosimenDan infusions fOR patients with advanced chronic heart failure in the vulnerable post-discharge period; ClinicalTrials.gov NCT03437226) was designed to evaluate the effects of intermittent levosimendan therapy on clinical stability of patients with advanced HF in the immediate post-discharge period after an acute HF hospitalization. We tested the hypothesis that levosimendan would reduce short-term risk for clinical endpoints and established surrogates for prognosis.
Methods
Study design
The design of the LeoDOR trial has been previously reported in detail.14 Briefly, LeoDOR was a multicentre, randomized, double-blind, placebo-controlled, two-arm trial conducted at 19 sites in 9 European countries in patients with acute-on-chronic HF and systolic dysfunction. After stabilization, acute HF patients were randomized 2:1 at the end of the index hospitalization to intermittent levosimendan therapy or placebo. The study drug was administered in addition to standard therapy for a period of 12 weeks either as a 6 h continuous infusion every 2 weeks or as a 24 h continuous infusion every 3 weeks.
Study population
Eligible patients were aged ≥18 years and hospitalized for an acute HF event requiring i.v. diuretics, i.v. vasodilators, i.v. inotropic therapy, or any combination of those interventions. Patients were required to have had a diagnosis of HF for ≥6 months prior to screening and to have been treated with individually optimized long-term therapies for ≥1 month preceding the index hospitalization. Further inclusion criteria were left ventricular ejection fraction (LVEF) ≤30% assessed during index hospitalization, at least one previous hospitalization or visit to an outpatient clinic for acute HF within 12 months before the index hospitalization, and either in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels (as measured by the local laboratory) after stabilization ≥2500 ng/L (B-type natriuretic peptide ≥900 ng/L) or persistent New York Heart Association (NYHA) functional class III or IV at study entry. Patients were considered recompensated when (i) a haemodynamically stable, euvolaemic state had been reached according to the treating physician; (ii) evidence-based oral medication had been established; and (iii) when renal function had remained stable for at least 24 h.15 The complete list of inclusion and exclusion criteria is provided in online supplementary Table S1. Written informed consent was obtained by all patients before any study-related procedures were performed.
Randomization and masking
Eligible patients were randomly assigned (2:1) using a secure, central, interactive, web-based response system, to receive either levosimendan or placebo. The randomization algorithm used permutated blocks specific with variable sizes for each centre. The infusion schedule (6 h or 24 h) was not part of the randomization or stratification scheme but instead selected according to centre preference. The placebo had the same appearance as levosimendan to ensure that treatment was concealed for both investigators and study patients.
Study treatment
Candidates for the study were screened and randomized after full cardiac compensation at the end of a hospitalization for acute HF. The first treatment visit was scheduled before discharge from index hospitalization or, at the latest, within 14 days after discharge from hospital. Levosimendan or matching placebo was administered as a continuous i.v. infusion. For the 6 h infusion group, the study drug was administered every 2 weeks (0.2 μg/kg/min, without bolus) for 12 weeks (i.e. a total of seven applications) in an ambulatory setting that allowed for non-invasive monitoring of vital signs. For the 24 h infusion group, the study drug was administered every 3 weeks (0.1 μg/kg/min for 24 h, without bolus) also for 12 weeks (i.e. a total of five applications). Follow-up visits were performed 14 days (± 2 days) and 180 days (± 14 days) after cessation of treatment (online supplementary Figure S1).
Study endpoints
- Tier 1 = time to death or urgent heart transplantation or implantation of a ventricular assist device (VAD).
- Tier 2 = time to non-fatal HF requiring i.v. vasoactive therapy.
- Tier 3 = time-averaged proportional change in NT-proBNP from baseline to week 14 (= sum of proportional changes in NT-proBNP from baseline at weeks 6, 12, and 14), each multiplied by the number of elapsed time in weeks, divided by the total number of 14 weeks of observation from baseline to 14 weeks.
Secondary efficacy endpoints included the composite risks of the individual clinical components of the primary endpoint at short-term (i.e. 14 weeks) and intermediate-term (i.e. 26 weeks) follow-up, as well as changes in functional status and quality of life. All clinical events were adjudicated by an endpoint adjudication committee.
Endpoint assessment
Components of the primary endpoint, which included death, urgent heart transplantation or VAD implantation, as well as non-fatal acute HF events, were recorded from the start of the first study drug application until week 26. A ‘non-fatal acute HF event’ was adjudicated if the patient presented for an urgent, unscheduled visit at a clinic/hospital or emergency care office/department with a primary diagnosis of HF. The latter was defined as a patient exhibiting new or worsening symptoms of HF, objective evidence of new or worsening HF, and receiving i.v. vasoactive therapy (i.e. defined according to Hicks et al.17 as i.v. diuretics, i.v. vasodilators, or i.v. inotropes). NT-proBNP was assessed at baseline and at week 6, 12, and 14. Biomaterials were frozen locally at −80°C, then shipped to and stored and analysed at the Interdisciplinary Bank of Biomaterials and Data, University of Würzburg.
Components of the secondary endpoint, which included NYHA functional class, 6-min walk test (6MWT), Kansas City Cardiomyopathy Questionnaire (KCCQ-23), and EQ-5D visual analogue scale (VAS), were assessed before the first study drug application and after 14 weeks.
Statistical analysis
Statistical analyses were performed according to the study protocol of the trial registered at ClinicalTrials.gov with one exception: for the primary analysis, the modified intention-to-treat (mITT) strategy was used instead of the intention-to-treat (ITT) strategy to reduce bias toward a more realistic estimate of treatment effect. Assumptions of rates of death and acute HF events in the placebo group (10% and 30%, respectively) were based on data from Gheorghiade et al.,18 Cowie et al.,1 and OPTIME-CHF.19 The assumption of a 20% risk reduction was based on experience in previous studies of levosimendan, namely LevoRep,11 LION-HEART,12 and LAICA.13 From these assumptions, the planned sample size of 264 patients provided 90% power to detect a difference in the global rank endpoint between levosimendan and placebo at a two-sided significance level of 5% using the Wilcoxon–Mann–Whitney test. For the main statistical analyses, patients with 6 h and 24 h infusions were combined in both the levosimendan and placebo group (6 h infusion group: levosimendan, n = 88; placebo, n = 44; 24 h infusion group: levosimendan, n = 88; placebo, n = 44).
For the primary efficacy analysis, a global rank endpoint within 14 weeks of follow-up was generated based on the three tiers previously described. Patients who met Tier 1 criteria were ranked lowest starting with rank 1 for the patient with the earliest endpoint. Thereafter, patients meeting Tier 2 were ranked in the same way, starting with the lowest number after Tier 1. Finally, patients who did not meet Tier 1 or Tier 2 criteria were ranked according to their decline in NT-proBNP with the patient showing the largest decline assigned the highest rank. If no information was available on Tiers 1 or 2, then the worst rank principle was applied (i.e. patients who dropped out were ranked equivalent to Tier 1 at the time of drop out). If information on Tier 3 was lacking, the average baseline NT-proBNP value was imputed and used for calculations. Similarly, if information on the week 14 NT-proBNP value was missing, a LOESS smoother was used to predict the week 14 value based on the other values for a particular patient.
The global rank score (GRS) was analysed using the two-sided non-parametric Wilcoxon–Mann–Whitney test to compare pooled levosimendan and placebo groups. Time-to-event analyses for Tier 1 and combined Tiers 1 and 2 at 14 and 26 weeks were performed using Kaplan–Meier and log-rank testing. Hazard ratios (HR) together with their 95% confidence intervals (CI) were estimated with univariate Cox proportional hazards regression analysis. Baseline characteristics, secondary efficacy endpoints and safety data were tabulated using appropriate descriptive statistics. In addition, changes from baseline KCCQ, EQ-5D VAS and 6MWT were analysed with ANOVA or, in case of non-normality, Mann–Whitney U test as appropriate. Categorical data were compared between treatment groups using Fisher's exact test. Nominal 5% significance level was used to claim efficacy.
Study administration and ethics
Membership of major LeoDOR committees is summarized in online supplementary Appendix 2.
The protocol and amendments were approved by the institutional review boards at each participating centre. The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and local and national regulations. Written informed consent was obtained by all patients before any study-related procedures were performed.
Results
Recruitment, management and follow-up of patients was affected by the COVID-19 pandemic and the trial was not able to reach the intended sample size. Between March 2018 and June 2021, 148 patients were randomly assigned to receive either levosimendan in 6 h (n = 38) or 24 h infusion schedules (n = 57), or placebo (n = 53). Recruitment was interrupted from 23 March to 11 May 2020, and the COVID-19 pandemic continued to have a major impact on recruitment in subsequent months. Statistical analysis was performed following a mITT strategy including all patients who had received at least one dose of study treatment (n = 145) and for whom at least one data point had been collected after randomization (Figure 1). Post-hoc power analysis revealed that this final sample size conferred 60.1% power to detect a 20% risk reduction and 78.6% power to detect a risk reduction of 25%.
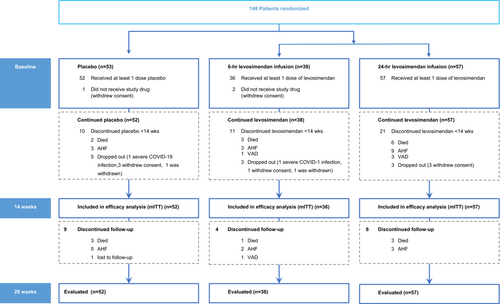
Patient characteristics and medications were similar in the two groups (Table 1).
| Levosimendan (n = 93) | Placebo (n = 52) | p-value | |
|---|---|---|---|
| Age, years | 69.3 ± 9.64 | 67.8 ± 10.1 | 0.390 |
| Female sex, n (%) | 21 (22.6) | 11 (21.2) | 0.508 |
| Body mass index, kg/m2 | 26.9 ± 4.9 | 29.6 ± 5.8 | 0.003 |
| Levo for compensation, n (%) | 21 (22.6) | 10 (19.2) | 0.402 |
| Functional measures | |||
| NYHA class, n (%) | 0.482 | ||
| II | 4 (4.3) | 5 (9.6) | |
| IIIa | 59 (63.4) | 29 (55.8) | |
| IIIb | 26 (28.0) | 17 (32.7) | |
| IV | 4 (4.3) | 1 (1.9) | |
| Kansas City Cardiomyopathy Questionnairea | |||
| Clinical summary scoreb | 44.3 [26.0–63.6] | 41.7 [23.2–66.8] | 0.475 |
| Overall summary scoreb,c | 45.6 [21.6] | 44.2 [23.7] | 0.712 |
| EQ-5D VAS | 50 [40–60] | 50 [40–60] | 0.976 |
| Six-minute walk test, m | 290 [190–360] | 274.5 [219–381] | 0.892 |
| Physical examination and echocardiographic measures | |||
| Systolic blood pressure, mmHg | 101.3 ± 28.7 | 101.4 ± 29.1 | 0.994 |
| Heart rate, bpm | 69.0 ± 20.7 | 66.4 ± 22.4 | 0.316 |
| Atrial fibrillation, n (%) | 34 (36.6) | 19 (36.5) | 0.998 |
| LVEF, % | 24 ± 5 | 24 ± 5 | 0.817 |
| Laboratory measures | |||
| NT-proBNP, ng/L | 4740 [2235–9016] | 5380 [2287–8204] | |
| eGFR (MDRD), ml/min/1.73 m2 | 58.6 ± 21.4 | 51.0 ± 19.9 | 0.086 |
| Serum sodium, mmol/L | 138.2 ± 3,7 | 137.8 ± 4,4 | 0.715 |
| Serum potassium, mmol/L | 4.4 ± 0.6 | 4.3 ± 0.50 | 0.699 |
| Medical history | |||
| Duration of heart failure >12 months, n (%) | 80 (86.0) | 43 (82.7) | 0.379 |
| Hospitalizations within the last 12 months before index hospitalization, n | 1.9 ± 1.4 | 1.7 ± 1.1 | 0.143 |
| Myocardial infarction, n (%) | 30 (32.3) | 23 (44.2) | 0.105 |
| PCI, n (%) | 31 (33.3) | 22 (42.3) | 0.185 |
| CABG, n (%) | 13 (14.0) | 8 (15.4) | 0.499 |
| Valve repair, n (%) | 9 (9.7) | 6 (11.5) | 0.464 |
| Pacemaker, n (%) | 6 (6.5) | 2 (3.8) | 0.403 |
| ICD alone, n (%) | 17 (18.3) | 17 (32.7) | 0.04 |
| CRT alone, n (%) | 7 (7.5) | 2 (3.8) | 0.301 |
| CRT-D, n (%) | 36 (38.7) | 15 (28.8) | 0.156 |
| ICD overall, n (%) | 53 (57.0) | 32 (61.5) | 0.593 |
| CRT overall, n (%) | 43 (46.2) | 17 (32.7) | 0.112 |
| Cause of heart failure and comorbidities, n (%) | |||
| Ischaemic heart disease | 36 (38.7) | 26 (50.0) | 0.127 |
| Dilated cardiomyopathyd | 65 (69.9) | 28 (53.8) | 0.04 |
| Valvular heart diseased | 12 (12.9) | 7 (13.5) | 0.557 |
| Restrictive/infiltrative cardiomyopathyd | 2 (2.2) | 0 (0.0) | 0.410 |
| Inflammatory cardiomyopathyd | 6 (6.5) | 2 (3.8) | 0.403 |
| Congenital heart diseased | 1 (1.1) | 0 (0.0) | 0.641 |
| Otherd | 4 (4.3) | 2 (3.8) | 0.631 |
| Comorbidities, n (%) | |||
| Hypertension | 59 (63.4) | 33 (63.5) | 0.572 |
| Diabetes | 38 (40.9) | 26 (50.0) | 0.187 |
| Dyslipidaemia | 54 (58.1) | 34 (65.4) | 0.246 |
| Chronic obstructive pulmonary disease or asthma | 5 (5.4) | 9 (17.3) | 0.023 |
| Stroke | 11 (11.8) | 2 (3.8) | 0.091 |
| PAD | 13 (14,0) | 13 (25.0) | 0.077 |
| eGFR <60 ml/min/1.73 m2 | 56 (60.2) | 34 (65.4) | 0.332 |
| Anaemiae | 37 (39.8) | 19 (36.5) | 0.700 |
| Heart failure medication at enrolment, n (%) | |||
| RAASi | 84 (90.3) | 49 (94.2) | 0.671 |
| ACEi | 30 (32.3) | 19 (36.5) | 0.365 |
| ARB | 12 (12.9) | 4 (7.7) | 0.251 |
| Sacubitril/valsartan | 42 (45.2) | 26 (50.0) | 0.349 |
| Beta-blocker | 85 (91.4) | 46 (88.5) | 0.382 |
| MRA | 76 (81.7) | 32 (61.5) | 0.008 |
| Diuretic | 90 (96.8) | 49 (94.2) | 0.369 |
| Digoxin | 13 (14.0) | 7 (13.5) | 0.443 |
| Amiodarone | 25 (26.9) | 8 (15.4) | 0.082 |
| Ivabradine | 6 (6.5) | 4 (7.7) | 0.512 |
| Nitrate | 13 (14.0) | 7 (13.59 | 0.572 |
| Allopurinol | 35 (36.6) | 25 (48.1) | 0.147 |
| Statin | 60 (64.5) | 36 (69.2) | 0.349 |
| Aspirin | 27 (29.0) | 17 (32.7) | 0.391 |
| Platelet inhibitor | 12 (12.9) | 10 (19.2) | 0.217 |
| Vitamin K antagonist | 22 (23.7) | 14 (26.9) | 0.403 |
| NOAC | 46 (49.5) | 18 (34.6) | 0.060 |
| Anticoagulation | 68 (73.1) | 32 (61.5) | 0.148 |
| Oral antidiabetic | 28 (30.1) | 16 (30.8) | 0.539 |
| Insulin | 13 (14.0) | 14 (26.9) | 0.055 |
- Values are given as mean ± standard deviation, or median [interquartile range], unless otherwise indicated.
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonist; NOAC, new oral anticoagulant; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RAASi, renin–angiotensin–aldosterone system inhibitor; VAS, visual analogue scale.
- a Range from 1 to 100; higher scores indicate better function.
- b A composite score for functional status, quality of life, and social limitations.
- c Derived from physical function, symptom (frequency and severity), social function, and quality-of-life domains.
- d In patients with non-ischaemic cardiomyopathy, more than one pathology is possible.
- e Female <12.0 g/L, male <13.0 g/L.
The cumulative administered dose of levosimendan per patient was higher in the 24 h versus the 6 h infusion group (44.9 [35.6–53.6] mg vs. 35.5 [26.2–40.4] mg; p < 0.001). The respective dose per cycle was also consistently higher in the 24 h group (10.9 [9.3–12.1] mg/cycle vs. 5.6 [5–6.2] mg/cycle; p < 0.001).
Primary endpoint
In the mITT analysis, there was no significant between-group difference in the GRS (where higher ranks indicate better stability) (mean rank of 72.55 [44.2] for the combined levosimendan group vs. 73.81 [38.16] for the placebo group; p = 0.863). Figure 2 shows the mean GRS in both groups, with a higher score indicating better stability, and the percentage by which each tier contributed to the GRS calculation. The larger proportion of clinical events (Tiers 1 and 2) in the levosimendan group was offset by a higher proportion of favourable or stable changes in time-averaged proportional change in NT-proBNP in that group.
Sensitivity analyses regarding the primary endpoint using: (1) the ITT population (p = 0.846), (2) centre adjustment with the van Elteren test (p = 0.623), and (3) results before and during the COVID-19 pandemic (p = 0.896) (online supplementary Table S2) did not significantly change the results.
Components of the primary endpoint
After 14 weeks, 13 events had occurred in 93 patients (14.0%) in the levosimendan group in Tier 1: 9 deaths, 0 urgent heart transplantation, 4 VAD implantations. By contrast, there were two fatalities in 52 patients (3.8%) in the placebo group resulting in HR 3.71 (95% CI 0.84–16.5, p = 0.064) (Table 2). Tier 2 events (acute HF) occurred in 12 (12.9%) and 3 (5.8%), respectively. The sum of Tier 1 and Tier 2 events at 14 weeks were 25 (26.9%) in the levosimendan group and 5 (9.6%) in the placebo group, resulting in a HR of 2.94 (95% CI 1.12–7.68, p = 0.021; Figure 3A, Table 2).
| Levosimendan (n = 93) | Placebo (n = 52) | Treatment effect HR (95% CI) | p-value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Mean global rank scorea | 72.55 ± 44.2 | 73.81 ± 38.16 | 0.863b | |
| Components of the primary endpoint | ||||
| Events from baseline to 14 weeks, n (%) | ||||
| Tier 1: Death, VAD, high-urgent heart transplant | 13 (14.0%) | 2 (3.8%) | 3.71 [0.84–16.5] | 0.064c |
| Tier 2: Acute heart failure | 12 (12.9%) | 3 (5.8%) | ||
| Tier 3: NT-proBNP level from baseline to 14 weeks | ||||
| Total change, pg/ml, median (IQR)d | −207.0 [−1958.5–475.0] | 166.0 [−2018.75–874.25] | 0.233b | |
| Median proportional change form baselined | 0.92 [0.62–1.17] | 1.02 [0.73–1.46] | 0.096b | |
| Secondary endpoints | ||||
| Events from baseline to 26 weeks, n (%) | ||||
| Death, VAD, high-urgent heart transplant | 18 (19.4%) | 5 (9.6%) | 2.08 [0.77–5.60] | |
| Acute heart failure | 17 (18.3%) | 8 (15.4%) | ||
| Composite of Tier 1 and Tier 2 from baseline to 14 weeks, n (%) | 25 (26.9%) | 5 (9.6%) | 2.94 [1.12–7.68] | |
| Composite of Tier 1 and Tier 2 from baseline to 26 weeks, n (%) | 35 (37.6%) | 13 (25.0%) | 1.64 [0.87–3.11] | |
| Change from baseline to week 14e | ||||
| KCCQ overall summary scoref ≥10 points (n = 145) | 40 (43.0%) | 15 (28.8%) | ||
| KCCQ overall summary score (n = 99) | 20.1 ± 21.4 | 12.2 ± 29.5 | ||
| KCCQ clinical summary scoreg ≥10 points (n = 145) | 40 (43.0%) | 19 (36.5%) | ||
| KCCQ clinical summary score (n = 99) | 20.0 ± 23.0 | 12.7 ± 27.8 | ||
| EQ-5D VAS ≥10 points (n = 145) | 32 (34.4%) | 15 (28.8%) | ||
| EQ-5D VAS (n = 98) | 10.0 [−6.25–20.0] | 5.0 [−5.0–20.0] | ||
| 6MWT distance >30 m (n = 145) | 39 (41.9%) | 14 (26.9%) | ||
| 6MWT distance from baseline to 14 weeks (m), (n = 81) | 75.5 [8.75–123.25] | 40.0 [−56.0–90.0] |
- 6MWT, 6-min walk test; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro-B-type natriuretic peptide; VAD, ventricular assist device; VAS, visual analogue scale.
- a Ranked across three hierarchical tiers: time to death, VAD, or high-urgent heart transplantation, time to acute decompensated heart failure, and time-averaged proportional change in NT-proBNP level from baseline to week 14. Higher values indicate better health. This informative analysis does not provide an informative estimate of variability.
- b Mann–Whitney U test.
- c Log-rank test.
- d The percentage of imputed NT-proBNP values was 7.8% at baseline and 30.4% at 14 weeks.
- e Patients who reached an endpoint or with missing data were classified as not improved.
- f A composite score for functional status, quality of life, and social limitations, complete case analysis.
- g Derived from physical function, symptom (frequency and severity), social function, and quality-of-life domains, complete case analysis.
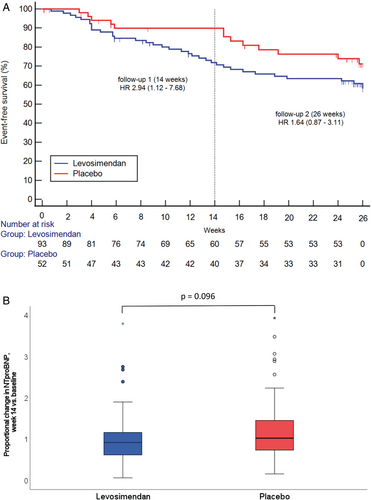
Across the entire study sample, the mortality rate after 14 weeks was 7.6%, and the rate of acute HF events was 10.3%. Among participants who were alive and not rehospitalized for HF, the median ratio of NT-proBNP at week 14 over baseline levels was 0.92 (interquartile range [IQR] 0.62–1.17) in the levosimendan group and 1.02 (IQR 0.73–1.46) in the placebo group (p = 0.096; Figure 3 and Table 2). The total change from baseline (median [IQR], in pg/ml) over the same period was −207.0 [−1958.5–475.0] in the levosimendan group and +166.0 [−2018.75–874.25] in the placebo group.
Secondary endpoints
The incidence of Tier 1 events after 26 weeks was 18 (19.4%) in the levosimendan group (13 deaths, 5 VAD implantations) and 5 (9.6%) in the placebo (5 deaths) group resulting in a HR of 2.08 (95% CI 0.77–5.60, p = 0.139) (Table 2). The incidence of Tier 2 events was 17 (18.3%) and 8 (15.4%), respectively (Table 2). The composite of Tier 1 and Tier 2 events was 35 (37.6%) in the levosimendan group and 13 (25%) in the placebo group (HR 1.64, 95% CI 0.87–3.11, p = 0.122; Figure 3 and Table 2). In the entire study cohort, the mortality rate at 26 weeks was 12.4%, entirely attributable to cardiovascular causes (including 39% deaths from sudden cardiac death, 71% of which occurred in the first 14 weeks with equal distribution between groups), and the rate of acute HF events was 19.3%.
Table 2 shows the mean differences in health status by treatment groups for the change from baseline to 14 weeks. Although there were no differences in the KCCQ overall summary score (OSS) between groups, there was a significant higher proportion of patients with a ≥10 point increase (moderate to very large) in the KCCQ-OSS in the levosimendan arm (63.5%) compared to placebo (41.7%) (p = 0.037). No similar difference was evident for the KCCQ clinical summary score (CSS) (63.5% vs. 52.8%; p = 0.298). No significant differences were found regarding changes in symptoms as assessed by physicians (NYHA class, p = 0.726) or by patients themselves (EQ-5D VAS, p = 0.684). Detailed information about these endpoints and the 6MWT is provided in supplementary Appendix 3.
Changes in background medication in patients who did not reach an endpoint after 14 weeks (n = 115) are summarized in online supplementary Table S3. Guideline-directed medical therapy prescription rates were exceptionally high at baseline, and modest improvements during the study period were comparable between groups. Diuretic dose was reduced during the study in 35% of the levosimendan group versus 25% of the placebo group. In absolute terms, however, the furosemide equivalents did not change significantly between groups.
Investigator-reported safety events
Principal adverse events associated with 705 study drug infusions are summarized in online supplementary Table S4. Hypotension (systolic blood pressure ≤80 mmHg) occurred in 43 (9.7%) infusions with levosimendan group and 29 (11.1%) in the placebo group (p = 0.564). Active measures for symptomatic infusions (e.g. administration of fluid or vasopressor) were reported in 32 (7.2%) patients treated with levosimendan and 22 (8.4%) patients in the placebo group. There was a trend for a higher frequency of tachycardia, new-onset atrial fibrillation, and non-sustained ventricular tachycardia in the levosimendan group (2.7%) than with placebo (0.8%; p = 0.070). Most applications of study medication in both groups were completed without any adverse events: levosimendan 388 courses (87.6%), placebo 231 courses (88.2%).
Discussion
The results of the LeoDOR trial indicate that intermittent levosimendan therapy, as represented using a three-component GRS, does not improve stability in patients with advanced HF during the early discharge period after hospitalization for an acute HF event. The higher rates of cardiovascular events with levosimendan observed in the underpowered LeoDOR trial do not support the use of intermittent levosimendan for clinical stabilization in the transition phase after an acute HF event (Graphical Abstract).
The lack of consistent favourable effects of levosimendan on secondary endpoints including NYHA functional class, 6MWT, or quality of life amplify the conclusion that levosimendan did not improve clinical stability. These results contrast with previous studies suggesting that intermittent treatment with levosimendan may reduce cardiovascular events in clinically stable patients with advanced HF.12
Hospitalization for the management of acute HF is a sentinel event in the trajectory of HF, and one that has important prognostic implications.1 Readmission and mortality rates are particularly high during the 3-month period immediately after discharge. LeoDOR is the first multicentre randomized clinical trial specifically designed to determine whether intermittent levosimendan benefits a high-risk subset of patients in that vulnerable post-hospitalization period. The high-risk features of patients enrolled in LeoDOR were: (i) a recent hospitalization for HF immediately prior to study start in conjunction with repeat hospital admissions in the previous year; (ii) a prolonged duration of disease in the majority of patients; (iii) a major burden of documented comorbidities; (iv) markedly reduced left ventricular ejection fraction; and (v) high baseline levels of NT-proBNP despite an excellent background therapy with HF medication.
There are several possible explanations for the disparate results of LeoDOR compared to previous studies, in particular for the failure of intermittent levosimendan to improve disease stability in critically ill patients with a very high risk of mortality and morbidity. HF is a syndrome that comprises various disease entities with unique underlying mechanisms and trajectories.20-22 Patients with different phenotypes, aetiologies, comorbidities, triggers of exacerbation, forward and backward failure, or predominant left and/or right ventricular failure were included in LeoDOR. Similarly, no distinction was made between advanced HF and end-stage HF with irreversible organ damage.23 In addition, the extent of compensation and haemodynamic stability at baseline were not uniform among the included patients. It is conceivable that within the very broad scope of patient characteristics represented in LeoDOR, certain subgroups may benefit from levosimendan therapy, while others may be harmed.24
It is notable that patients in the 24 h treatment arm with the highest cumulative levosimendan dose had the highest event rate (online supplementary Table S5). The prescription rate of guideline-directed medical therapy was exceptionally high in this study. Thus, more than 90% of patients were treated with renin–angiotensin inhibitors and beta-blockers. Although the incidence of adverse events during study drug administration was comparable between groups, it remains plausible that the combination of high-dose inodilator plus neurohumoral antagonists had an unfavourable effect on peripheral perfusion and thus on event rate. This is consistent with the observation that 71% of all events in the 24 h treatment arm occurred within 14 weeks, compared with 64% in the 6 h treatment arm and 46% in the placebo arm. In contrast, the rate of sudden cardiac death was comparable in all groups, making increased arrhythmogenicity unlikely as a major factor to explain our findings.
Attention must also be given to the possibility that the discrepant results of LeoDOR vis-a-vis previous trials of intermittent levosimendan in clinically stable patients with advanced HF, such as in the LION-HEART study,12 may have been due to chance and the circumstances in which the trial was undertaken. Recruitment, management, and follow-up of patients was substantially affected by the COVID-19 pandemic. The impact of COVID-19 cases and related changes in healthcare services provided have been acknowledged as a serious and unpredictable threat to the conduct of clinical trials.25 The LeoDOR trial was unable to reach the targeted sample size and with the 145 patients finally enrolled statistical power to detect a 20% risk reduction was reduced to 60%.
It is of interest to compare the current trial population with those of previous trials involving patients with advanced HF and a reduced LVEF at the transition from an acute event to outpatient care. In the LIFE trial26 and the VICTORIA trial,27 75% and 41% of the patients, respectively, were in either NYHA class III or IV, as compared to 97% in LeoDOR. The median NT-proBNP values in LIFE and VICTORIA were around 1900 pg/ml and 2816 pg/ml, respectively, as compared to 4920 pg/ml in LeoDOR. These differences probably account for the extrapolated 66% annualized frequency of the composite of cardiovascular death and acute HF in LeoDOR, which was nearly twice as high as observed in the aforementioned trials. There was no statistically significant difference between sacubitril/valsartan and valsartan with respect to reducing NT-proBNP levels, which was the primary endpoint in LIFE,25 while in patients in VICTORIA in the highest NT-proBNP quartile (>5314 pg/ml), no reduction was observed in the primary endpoint, which included the composite risk of death from cardiovascular causes or first hospitalization for HF.27
Possible interpretations of these overall unfavourable results, suggesting an interaction of the treatment effect with time from HF hospitalization, are that rapid intensification of therapy may not be appropriate in still highly unstable patients. It is conceivable that in the immediate period following hospital discharge such patients are more likely to benefit from close monitoring and support, such as invasive pressure monitoring28 or telemedicine-assisted transitional care,29 or should be considered early for implantation of an assist device or cardiac transplantation. This speculation is compatible with the view that this complex disease, encountered at a very vulnerable stage, needs to be looked at systemically and treated in the context of networks, rather than through selective interventions.
Potential limitations
The LeoDOR trial has several limitations in addition to its smaller-than-intended sample size.
Due to difficult recruitment some centres were able to enrol only a few patients: here the results were particularly unfavourable. However, sensitivity analyses regarding the primary endpoint using centre adjustment with the van Elteren test did not significantly change the results. Because of the small sample size, it was not possible to adjust for differences in baseline characteristics that may have influenced outcomes, such as persistent congestion or haemodynamic instability at baseline. Accordingly, subgroups that were more or less likely to benefit from levosimendan could not be identified. More precise inclusion criteria and a correspondingly stringent selection of patients might have led to different results. The primary endpoint of the study was limited to assessing the short-term effects of the intervention, which may not necessarily accurately represent its longer-term impact. This limitation was partly addressed with the inclusion of NT-proBNP as a surrogate endpoint in the composite GRS and the additional assessment of a secondary intermediate term endpoint, neither of which produced a signal of possible longer-term clinical effects.
This study was underpowered, so the results are highly unlikely to be adequately replicated or conclusive. Therefore, no definitive assumptions can be made about the efficacy of levosimendan on clinical endpoints, including measures of quality of life or functional capacity in the vulnerable transition period after an acute HF event, as futility may also be suspected in this study.
Conclusions
Among severely ill patients recently hospitalized with HF and reduced LVEF, intermittent levosimendan therapy did not improve clinical stability in the high vulnerability post-hospitalization period in this underpowered study. These findings do not support the use of intermittent levosimendan for clinical stabilization in the transition phase after an acute HF event.
Acknowledgements
The LeoDOR researchers gratefully acknowledge all patients who participated in the study and staff at the participating centres.
Funding
The LeoDOR study was supported by an unrestricted grant from Orion Pharma, Espoo, Finland, the manufacturer of levosimendan. Orion Pharma was not involved in data management, assessment of endpoints, analysis, or publication of the LeoDOR trial.
Conflict of Interest: G.P., J.A, J.C.C., J.F.D., F.F., F.G., J.M., Z.P., and G.W have received speakers fees from Orion Pharma. G.P., J.A., M.J.G.G., and E.Z. have received unrestricted grants from Orion Pharma. All other authors have nothing to disclose.